Exendin-4 Caused Growth Arrest by Regulating Sugar Metabolism in Hyphantria cunea (Lepidoptera: Erebidae) Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Exendin-4 Treatment
2.2. LC-MS-Based Metabolomics Analysis
2.2.1. Metabolite Extraction
2.2.2. LC-MS/MS Analysis
2.2.3. Data Analysis
2.3. Enzyme Activity Assay
2.4. Measurements of Carbohydrates, PA, and ATP
2.5. Quantitative RT-PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. The Toxicity Test of Exendin-4
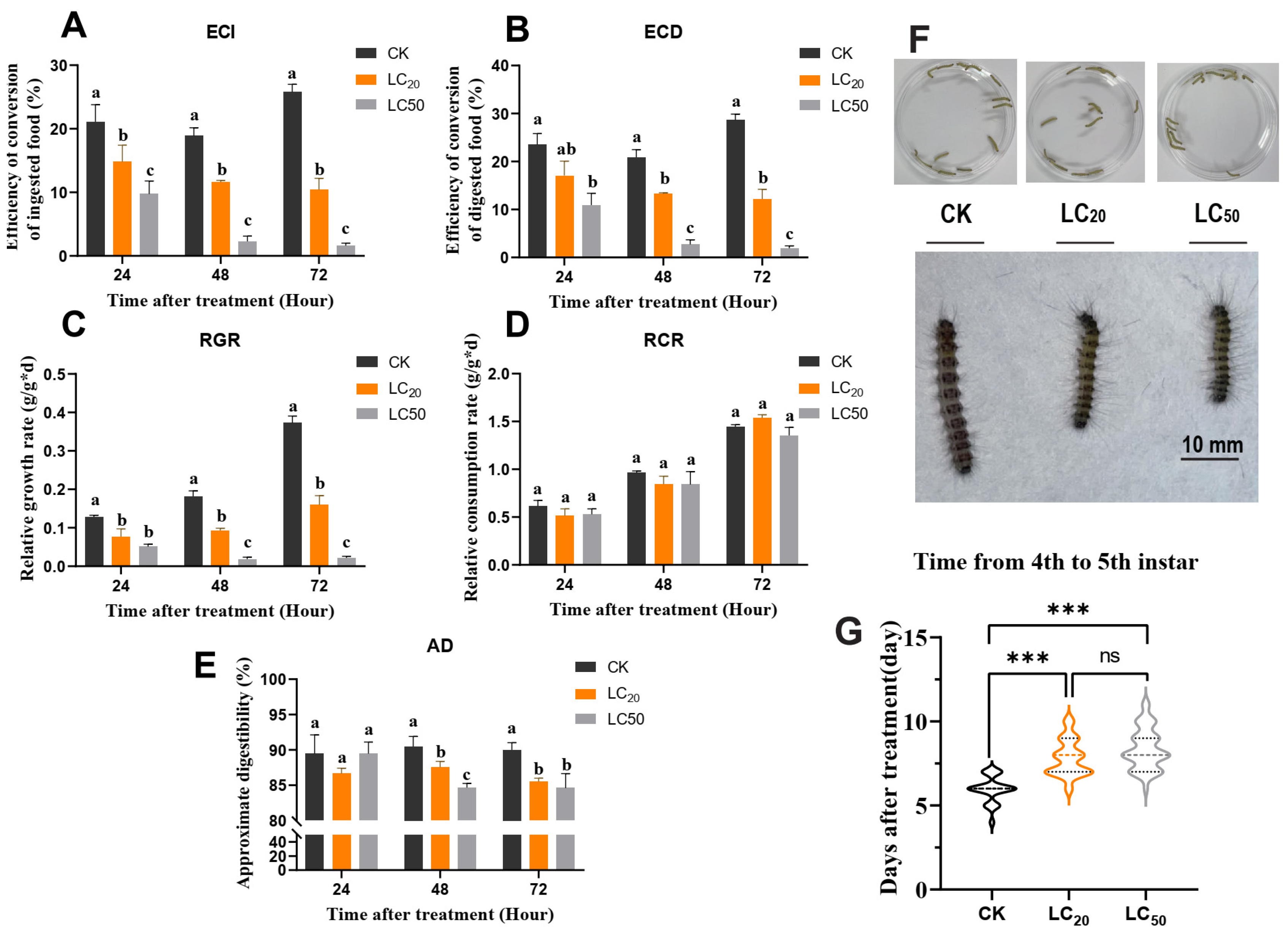
3.2. Exendin-4 Arrested Growth and Nutritional Indices
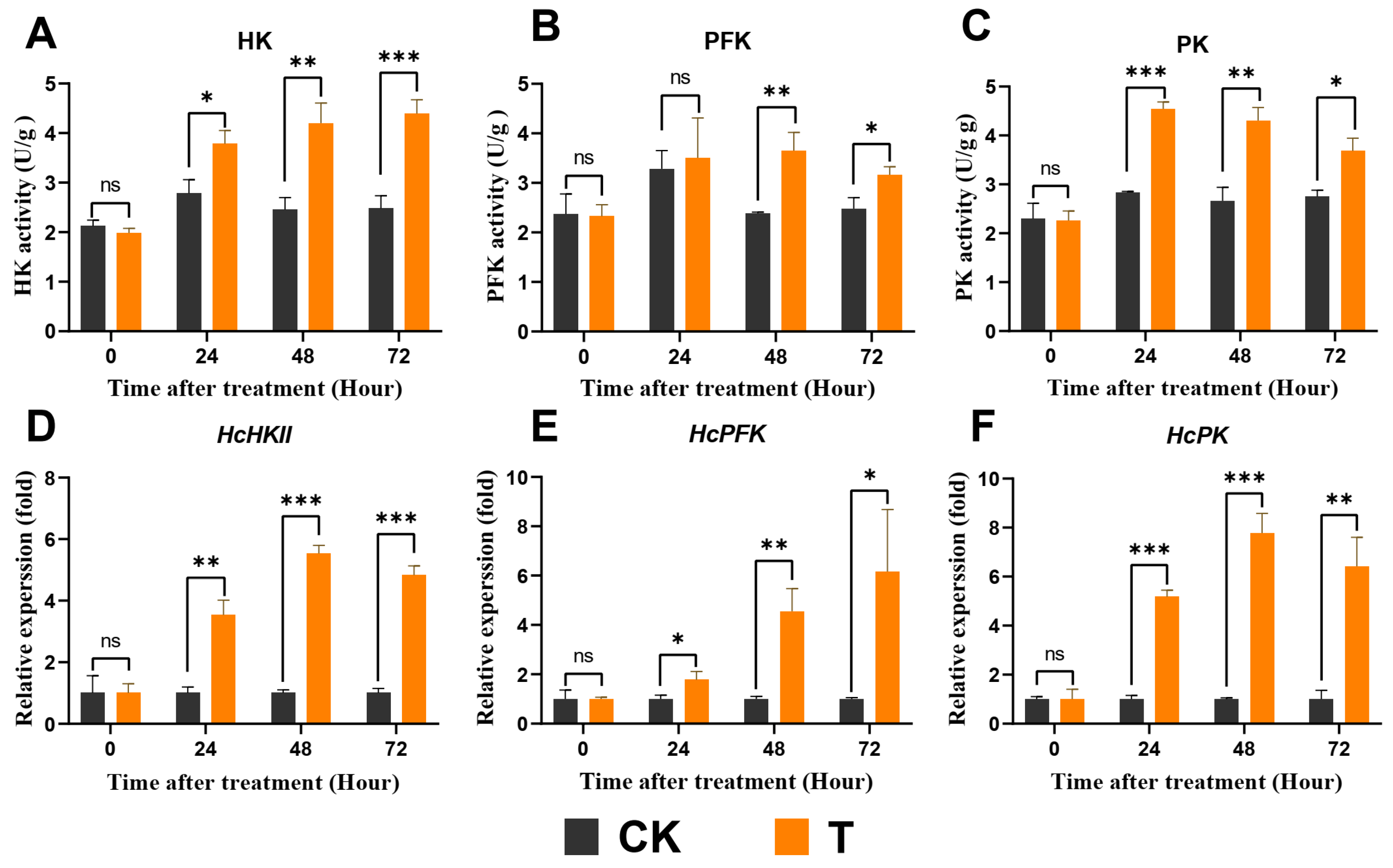
3.3. Exendin-4 Affected the Activities of Digestive Enzymes
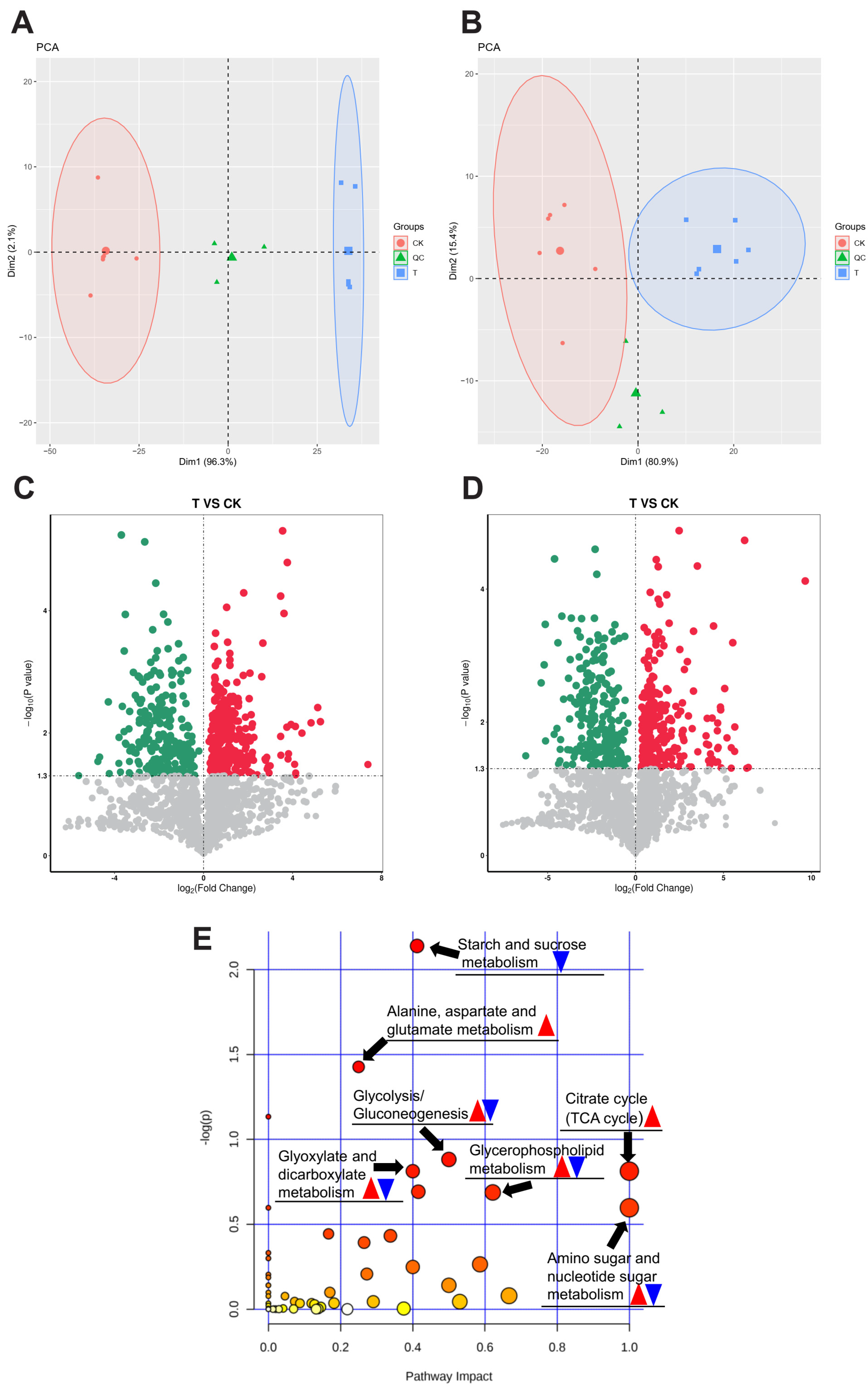
3.4. Metabolomics Analysis of H. cunea Larvae’s Response to Exendin-4 Treatment
3.5. Exendin-4 Affected Carbohydrate Levels
3.6. Exendin-4 Promoted Glycolysis
3.7. Exendin-4 Affected PA and ATP Levels
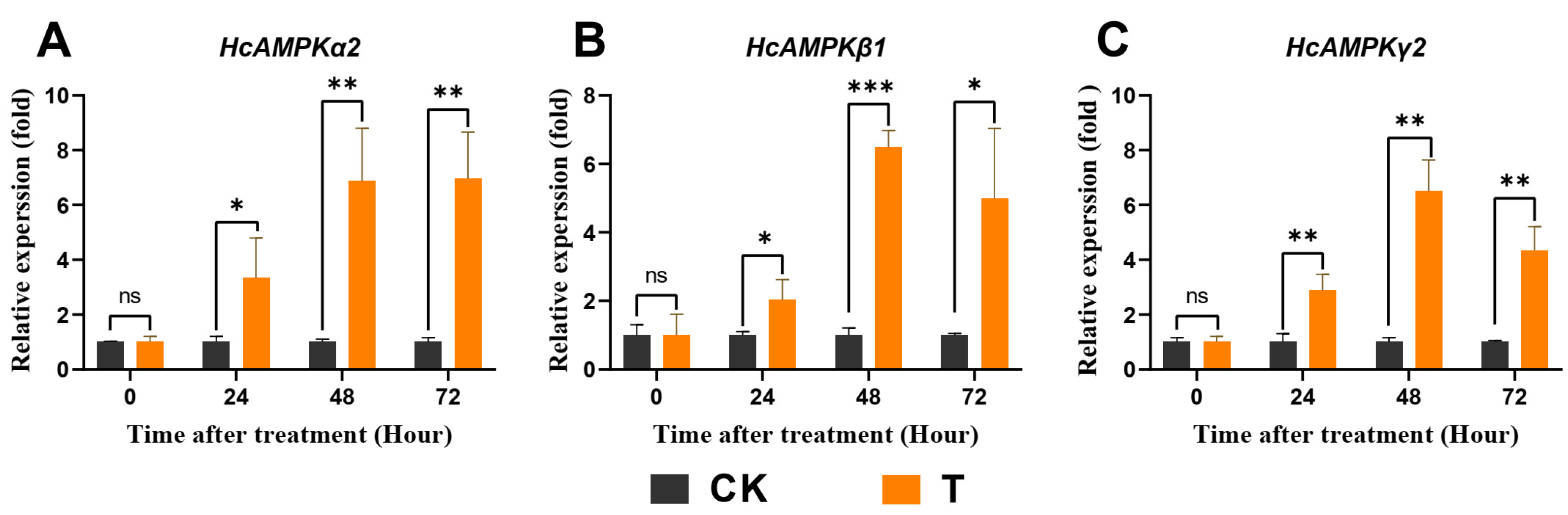
3.8. Exendin-4 Regulated Expressions of Key Genes Related to AMPK and ILP Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. B Comp. Biochem. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Dadd, R.H. Insect nutrition: Current developments and metabolic implications. Annu. Rev. Entomol. 1973, 18, 381–420. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Derridj, S.; Fiala, V.; Jolivet, E. Increase of European corn borer (Ostrinia nubilalis) oviposition induced by a treatment of maize plants with maleic hydrazide: Role of leaf carbohydrate content. Entomol. Exp. Appl. 1986, 41, 305–310. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamada, T.; Yoshida, M.; Nishimura, T. Flies without trehalose. J. Biol. Chem. 2015, 290, 1244–1255. [Google Scholar] [CrossRef]
- Guo, Q.; Hao, Y.-J.; Li, Y.; Zhang, Y.-J.; Ren, S.; Si, F.-L.; Chen, B. Gene cloning, characterization and expression and enzymatic activities related to trehalose metabolism during diapause of the onion maggot Delia antiqua (Diptera: Anthomyiidae). Gene 2015, 565, 106–115. [Google Scholar] [CrossRef]
- Becker, A.; Schlöder, P.; Steele, J.; Wegener, G. The regulation of trehalose metabolism in insects. Experientia 1996, 52, 433–439. [Google Scholar] [CrossRef]
- Lin, X.; Smagghe, G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides 2019, 122, 169923. [Google Scholar] [CrossRef]
- Nässel, D.R.; Broeck, J.V. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Neufeld, T.P. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat. Commun. 2015, 6, 6846. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Okamoto, N. Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: Discovery, structure, secretion, and function. Front. Physiol. 2013, 4, 60896. [Google Scholar] [CrossRef]
- Van der Horst, D.J. Insect adipokinetic hormones: Release and integration of flight energy metabolism. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Edgar, B.A. How flies get their size: Genetics meets physiology. Nat. Rev. Genet. 2006, 7, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Du, H.; Li, Y.; Li, X.; Sun, L.; Cao, C. Identification and characterization of key genes in insulin signaling pathway as molecular targets for controlling the fall webworm, Hyphantria cunea. Pest. Manag. Sci. 2023, 79, 899–908. [Google Scholar] [CrossRef]
- Tian, L.; Guo, E.; Wang, S.; Liu, S.; Jiang, R.-J.; Cao, Y.; Ling, E.; Li, S. Developmental regulation of glycolysis by 20-hydroxyecdysone and juvenile hormone in fat body tissues of the silkworm, Bombyx mori. J. Mol. Cell Biol. 2010, 2, 255–263. [Google Scholar] [CrossRef]
- Gunn, A.; Gatehouse, A. The development of enzymes involved in flight muscle metabolism in Spodoptera exempta and Mythimna separata. Comp. Biochem. Physiol. B Comp. Biochem. 1988, 91, 315–324. [Google Scholar] [CrossRef]
- Bommer, G.T.; Van Schaftingen, E.; Veiga-da-Cunha, M. Metabolite repair enzymes control metabolic damage in glycolysis. Trends Biochem. Sci. 2020, 45, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nat. Chem. Biol. 2012, 8, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kishton, R.J.; Barnes, C.E.; Nichols, A.G.; Cohen, S.; Gerriets, V.A.; Siska, P.J.; Macintyre, A.N.; Goraksha-Hicks, P.; De Cubas, A.A.; Liu, T.; et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016, 23, 649–662. [Google Scholar] [CrossRef]
- Unterman, T.G. Regulation of hepatic glucose metabolism by FoxO proteins, an integrated approach. Curr. Top. Dev. Biol. 2018, 127, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hayano, A.; Yamanaka, R. Metabolome analysis reveals excessive glycolysis via PI3K/AKT/mTOR and RAS/MAPK signaling in methotrexate-resistant primary CNS lymphoma–derived cells. Clin. Cancer Res. 2020, 26, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Lebenzon, J.E.; Overgaard, J.; Jørgensen, L.B. Chilled, starved or frozen: Insect mitochondrial adaptations to overcome the cold. Curr. Opin. Insect Sci. 2023, 58, 101076. [Google Scholar] [CrossRef]
- du Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.; Nicolson, S.W. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef]
- Lv, N.; Ma, K.; Li, R.; Liang, P.; Liang, P.; Gao, X. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2021, 212, 111969. [Google Scholar] [CrossRef]
- Roed, N.K.; Viola, C.M.; Kristensen, O.; Schluckebier, G.; Norrman, M.; Sajid, W.; Wade, J.D.; Andersen, A.S.; Kristensen, C.; Ganderton, T.R.; et al. Structures of insect Imp-L2 suggest an alternative strategy for regulating the bioavailability of insulin-like hormones. Nat. Commun. 2018, 9, 3860. [Google Scholar] [CrossRef]
- Padmanabha, D.; Baker, K.D. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol. Metab. 2014, 25, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.J.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Erdogdu, Ö.; Nathanson, D.; Sjöholm, Å.; Nyström, T.; Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA-and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35. [Google Scholar] [CrossRef]
- Zhao, L.; Li, A.Q.; Zhou, T.F.; Zhang, M.Q.; Qin, X.M. Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway. Am. J. Physiol.-Cell Physiol. 2014, 307, C1130–C1141. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, Y.Z.; Salehi, A.; Ward, C.E.; Zhang, Q.; Abdulkader, F.; Bengtsson, M.; Braha, O.; Braun, M.; Ramracheya, R.; Amisten, S.; et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N-and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010, 11, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Zhong, X.; Guo, Q.; Wang, H.; Gao, J.; Bai, T.; Ren, L.; Guo, Y.; Jiao, X.; et al. Geniposide acutely stimulates insulin secretion in pancreatic β-cells by regulating GLP-1 receptor/cAMP signaling and ion channels. Mol. Cell. Endocrinol. 2016, 430, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Ahn, I.S. Exendin-4 and exercise improve hepatic glucose homeostasis by promoting insulin signaling in diabetic rats. Metabolism 2010, 59, 123–133. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Alquicer, G.; Kodrík, D.; Krishnan, N.; Večeřa, J.; Socha, R. Activation of insect anti-oxidative mechanisms by mammalian glucagon. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 226–233. [Google Scholar] [CrossRef]
- Šula, J.; Socha, R.; Zemek, R. Wing morph-related physiological differences in adults of temperate population of Pyrrhocoris apterus (L.) (Heteroptera: Pyrrhocoridae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 121, 365–373. [Google Scholar] [CrossRef]
- Hughson, B.N. The glucagon-like adipokinetic hormone in Drosophila melanogaster–Biosynthesis and Secretion. Front. Physiol. 2021, 12, 710652. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, A.; Kodrík, D.; Krishnan, N. Unique roles of glucagon and glucagon-like peptides: Parallels in understanding the functions of adipokinetic hormones in stress responses in insects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zou, H.; Li, X.; Qiu, Q.; Geng, N.; Zhang, B.; Yan, G.; Zhang, Z.; Zhang, S.; Yao, B.; et al. Metformin-induced AMPK activation suppresses larval growth and molting probably by disrupting 20E synthesis and glycometabolism in fall webworm, Hyphantria cunea Drury. Pestic. Biochem. Physiol. 2022, 183, 105083. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea chitin synthesis inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elghar, G.E.; Fujiyoshi, P.; Matsumura, F. Significance of the sulfonylurea receptor (SUR) as the target of diflubenzuron in chitin synthesis inhibition in Drosophila melanogaster and Blattella germanica. Insect Biochem. Mol. Biol. 2004, 34, 743–752. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, L.; Zuo, T.; Zhang, K.; Chen, Y.; Li, C.; Li, X. Effects of Chlorbenzuron on Cuticle Formation of Hyphantria cunea (Lepidoptera: Erebidae) and Its Detoxification Metabolism. Appl. Ecol. Environ. Res. 2023, 21, 3025–3044. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, C.; Zhang, L.; Li, C.; Li, X.; Song, L. Chlorbenzuron caused growth arrest through interference of glycolysis and energy metabolism in Hyphantria cunea (Lepidoptera: Erebidae) larvae. Pestic. Biochem. Physiol. 2023, 193, 105466. [Google Scholar] [CrossRef] [PubMed]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar] [CrossRef]
- Shaurub, E.-S.H.; Abdel Aal, A.E.; Emara, S.A. Suppressive effects of insect growth regulators on development, reproduction and nutritional indices of the Egyptian cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 2020, 64, 178–187. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Shen, X.; Liu, J.; Zhao, D.; Sun, Y.; Wang, L.; Liu, Y.; Gong, X.; Liu, Y.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Tatun, N.; Singtripop, T.; Tungjitwitayakul, J.; Sakurai, S. Regulation of soluble and membrane-bound trehalase activity and expression of the enzyme in the larval midgut of the bamboo borer Omphisa fuscidentalis. Insect Biochem. Mol. Biol. 2008, 38, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Iwami, M. Sequential changes in the regulatory mechanism of carbohydrate digestion in larvae of the silkworm, Bombyx mori. J. Comp. Physiol. B 2021, 191, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, J.E.; Magan, N. A rapid HPLC protocol for detection of polyols and trehalose. J. Microbiol. Methods 1997, 29, 7–13. [Google Scholar] [CrossRef]
- Chen, J.; Tang, B.; Chen, H.; Yao, Q.; Huang, X.; Chen, J.; Zhang, D.; Zhang, W. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.S.; Choi, M.-Y.; Paik, C.-H.; Seo, H.-Y. Food consumption, utilization, and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum triterpenes. Pestic. Biochem. Physiol. 2007, 88, 260–267. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Albrechtsen, R.; Bremholm, L.; Svendsen, B.; Kuhre, R.E.; Poulsen, S.S.; Christiansen, C.B.; Jensen, E.P.; Janus, C.; Hilsted, L.; et al. Glucagon-like peptide 1 receptor signaling in acinar cells causes growth-dependent release of pancreatic enzymes. Cell Rep. 2016, 17, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Al Batran, R.; Almutairi, M.; Ussher, J.R. Glucagon-like peptide-1 receptor mediated control of cardiac energy metabolism. Peptides 2018, 100, 94–100. [Google Scholar] [CrossRef]
- Pirro, V.; Roth, K.D.; Lin, Y.; Willency, J.A.; Milligan, P.L.; Wilson, J.M.; Ruotolo, G.; Haupt, A.; Newgard, C.B.; Duffin, K.L. Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Xue, J.; Lu, B.; Zhang, X.-C.; Zhuo, J.-C.; He, S.-F.; Ma, X.-F.; Jiang, Y.-Q.; Fan, H.-W.; Xu, J.-Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, J.-J.; Li, Y.; Gou, Y.; Quandahor, P.; Liu, C. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, L.; Bai, J.; Zhang, Y.; Min, M.; Ma, W.; Ma, L. Transcriptome analysis of emamectin benzoate caused midgut damage by inducing oxidative stress, energy metabolism disorder and apoptosis in gypsy moth (Lymantria dispar). Pest. Manag. Sci. 2022, 78, 4628–4637. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.J.; Elthon, T.E.; Cerny, R.L.; Siegfried, B.D. Proteomic analysis of atrazine exposure in Drosophila melanogaster (Diptera: Drosophilidae). Chemosphere 2010, 81, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.-B.; Zhang, D.-D.; Cao, X.-F.; Shi, H.-J.; Li, X.-F. Interactions between dietary carbohydrate and metformin: Implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture 2018, 483, 183–195. [Google Scholar] [CrossRef]
- Rogacka, D.; Audzeyenka, I.; Piwkowska, A. Regulation of podocytes function by AMP-activated protein kinase. Arch. Biochem. Biophys. 2020, 692, 108541. [Google Scholar] [CrossRef]
- Xu, W.-H.; Lu, Y.-X.; Denlinger, D.L. Cross-talk between the fat body and brain regulates insect developmental arrest. Proc. Natl. Acad. Sci. USA 2012, 109, 14687–14692. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-binding cassette (ABC) transporters: Roles in xenobiotic detoxification and Bt insecticidal activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef]
- Wallner, M.; Kolesnik, E.; Ablasser, K.; Khafaga, M.; Wakula, P.; Ljubojevic, S.; Thon-Gutschi, E.M.; Sourij, H.; Kapl, M.; Edmunds, N.J.; et al. Exenatide exerts a PKA-dependent positive inotropic effect in human atrial myocardium: GLP-1R mediated effects in human myocardium. J. Mol. Cell. Cardiol. 2015, 89, 365–375. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, F.; Raciti, G.A.; Nigro, C.; Mannino, G.C.; Procopio, T.; Davalli, A.M.; Beguinot, F.; Sesti, G.; Miele, C.; Folli, F. The GLP-1 receptor agonists exenatide and liraglutide activate Glucose transport by an AMPK-dependent mechanism. J. Transl. Med. 2016, 14, 229. [Google Scholar] [CrossRef] [PubMed]






| Exposure Time (h) | LC20 (ppm) | 95% Confidence Interval | LC50 (ppm) | 95% Confidence Interval | R2 | Linear Equation |
|---|---|---|---|---|---|---|
| 72 | 5.526 | 1.969–12.084 | 55.744 | 48.398–64.686 | 0.644 | Y = −0.934 + 0.017X |
| Metabolic Pathways | Differentially Expressed Metabolites (DEMs) | |
|---|---|---|
| Up | Down | |
| Citrate cycle (TCA cycle) | Dihydrolipoamide-E Citrate Fumaric acid Pyruvate Oxaloacetate Succinate Thiamin diphosphate Phosphoenolpyruvate 2-oxoglutarate | |
| Amino sugar and nucleotide sugar metabolism | N-acetylchitosamine N-acetyl-D-mannosamine α-D-glucose GDP-L-fucose | D-fructose 6-phosphate D-fructose D-mannose ADP-glucose Glucose 1-phosphate Glucose 6-phosphate |
| Starch and sucrose metabolism | Trehalose Sucrose D-glucose 1-phosphate D-fructose 6-phosphate D-fructose ADP-glucose | |
| Glycolysis/gluconeogenesis | Pyruvate Oxaloacetate α-D-glucose Dihydrolipoamide-E Thiamin diphosphate Phosphoenolpyruvate | D-glucose 6-phosphate D-glucose 1-phosphate D-fructose 6-phosphate |
| Glycerophospholipid metabolism | Choline Acetylcholine Ethanolamine | Phosphatidylethanolamine Phosphatidylcholine O-phosphocholine |
| Alanine, aspartate, and glutamate metabolism | Pyruvate Fumarate Succinate Oxaloacetate 2-oxoglutarate Citrate | |
| Glyoxylate and dicarboxylate metabolism | Formate Oxalate | Glycolaldehyde Hydroxypyruvate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Zhang, L.; Zhao, Y.; Li, X. Exendin-4 Caused Growth Arrest by Regulating Sugar Metabolism in Hyphantria cunea (Lepidoptera: Erebidae) Larvae. Insects 2024, 15, 503. https://doi.org/10.3390/insects15070503
Shi W, Zhang L, Zhao Y, Li X. Exendin-4 Caused Growth Arrest by Regulating Sugar Metabolism in Hyphantria cunea (Lepidoptera: Erebidae) Larvae. Insects. 2024; 15(7):503. https://doi.org/10.3390/insects15070503
Chicago/Turabian StyleShi, Wenhui, Lu Zhang, Yuecheng Zhao, and Xingpeng Li. 2024. "Exendin-4 Caused Growth Arrest by Regulating Sugar Metabolism in Hyphantria cunea (Lepidoptera: Erebidae) Larvae" Insects 15, no. 7: 503. https://doi.org/10.3390/insects15070503
APA StyleShi, W., Zhang, L., Zhao, Y., & Li, X. (2024). Exendin-4 Caused Growth Arrest by Regulating Sugar Metabolism in Hyphantria cunea (Lepidoptera: Erebidae) Larvae. Insects, 15(7), 503. https://doi.org/10.3390/insects15070503





