Simple Summary
The subfamily Aphidiinae, comprising over 500 known species globally, consists of obligatory endoparasitoids of aphids. Recognised for their role as biological control agents of various aphid pests, this group is considered relatively well-studied, particularly in Europe. However, Norway’s documented Aphidiinae diversity is notably low, with only 33 reported species. Our study aimed to assess the true biodiversity of aphid parasitoids in Norway using malaise traps. The results of morphological and molecular analyses identified several specimens distinct from any known species. In this paper, we describe four species new to science across three genera, provide an identification key for species of the subgenus Fovephedrus, and discuss their phylogenetic relationships.
Abstract
With only 33 reported species, Norway ranks among the European countries with the lowest documented diversity of parasitoids from the subfamily Aphidiinae. The “MUST Malaise” project, carried out by Museum Stavanger in Norway, aimed to assess insect abundance and biodiversity and create a reference base for future studies. The preliminary results of our study revealed four species new to science, indicating that the current number of recorded species in Norway is significantly lower than the actual diversity. All species possess unique combinations of morphological characters, distinguishing them from other known Aphidiinae species. Molecular analysis of the barcoding region confirmed that these specimens all belong to the previously undescribed species. In this study, we describe Aphidius norvegicus sp.n., Praon breviantennalis sp.n., Ephedrus gardenforsi sp.n., and Ephedrus borealis sp.n., all collected in Norway. We also provide an identification key and discuss the phylogenetic relationships within the subgenus Fovephedrus Chen, 1986.
1. Introduction
Recent reports on insect biodiversity and overall biomass numbers have drawn attention over the past decades, highlighting the consequences if this declining trend continues. Anthropogenic influence through agricultural intensification (i.e., habitat loss and use of pesticides) and climate change are now recognised as the main drivers of biodiversity loss. It is estimated that around 40% of the world’s insect species are threatened with extinction within the next several decades [1]. As this study reported, in addition to Lepidoptera and some Coleoptera (dung beetles), Hymenoptera are among the most affected terrestrial insect groups [1]. Furthermore, the global biodiversity loss is accompanied by a decline in insect biomass. Hallmann et al. [2] reported a staggering 76% reduction in insect biomass in several protected reserves in Germany. Due to these alarming projections, there is a growing intent to record the diversity of insect species globally.
In Europe, the subfamily Aphidiinae, comprising koinobiont endoparasitoids of aphids, is considered a relatively well-studied group. Extensive research efforts by experts have resulted in a high number of reported species in some regions, especially central, western, and southeastern Europe (Czech Republic, France, Serbia, Montenegro, Greece). For comparison, there are currently 121 recorded Aphidiinae species in Serbia, 135 in the Czech Republic, and 85 in Montenegro [3]. On the other hand, some areas in Europe remained unexplored, including Norway, which has only 33 recorded species [4]. This number is surprisingly low, especially considering that 119 species are recorded in neighbouring Sweden [5]. The “MUST Malaise” project, carried out by Museum Stavanger in Norway, aimed to assess insect abundance and the biodiversity of different insect groups to create a reference base for future studies. Preliminary analysis of the collected material revealed that the species richness of the subfamily Aphidiinae in Norway is high and comparable to that of other European countries (unpublished data). Among this material, multiple specimens from three different genera exhibited unique combinations of morphological characters, distinguishing them from other known Aphidiinae species. Molecular analysis of the cytochrome oxidase I (COI) barcoding region confirmed that these specimens all belong to previously undescribed species. In this manuscript, we describe four species new to science, collected in Norway. We also provide an identification key and discuss the phylogenetic relationships within the subgenus Fovephedrus Chen, 1986.
2. Material and Methods
Sampling and Morphological Analysis
The material was collected during 2020–2022 using malaise traps in Norway (Figure 1), from different localities in Rogaland county (Table 1). One additional specimen was collected as an aphid mummy in Northern Ireland, UK. In both cases, specimens were transferred to and stored in 96% ethanol.

Figure 1.
Malaise trap Vårvik, Suldal, Rogaland, Norway, 2021. Photo: Rune Roalkvam.

Table 1.
Malaise trap localities in Rogaland, Norway, with time periods, relevant to this study.
The material was identified to the genus level. Three females were designated as Aphidius sp., ten as Praon sp., 20 (males and females) as Ephedrus sp. 1 and one as Ephedrus sp. 2. Specimens were dissected and slide mounted in Berlese medium. Photographs of the dissected specimens were taken with a Leica DM LS phase contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany). The obtained photographs were stacked in Helicon Focus software (version 7.6.1; HeliconSoft, Kharkiv, Ukraine). ImageJ software [6] was used to measure all important taxonomical characters. Morphological terminology follows Sharkey and Wharton [7]. All material is deposited in the collection of the Institute of Zoology, Faculty of Biology, University of Belgrade (Serbia).
After the initial examination under the ZEISS Discovery V8 stereomicroscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) and prior to slide mounting, the non-destructive extraction of the total DNA was performed with the Qiagen DNeasy® Blood & Tissue Kit (Qiagen Inc., Valencia, CA, USA). Universal primers LCO1490 and HCO2198 [8] were used for PCR amplification of the barcode region (COI). The final volume of the amplification mixture (50 µL) contained 32.6 µL of nuclease-free water, 10 µL of buffer, 1 µL of each primer pair, 1 µL of nucleotides, 0.4 µL of polymerase, and finally 4 µL of extracted DNA. The following PCR temperature profile was used: 60 s of initial denaturation, 35 cycles of 60 s denaturation (94 °C), 60 s annealing (54 °C), 90 s extension (72 °C), and 7 min of final extension (72 °C). Amplification products were purified and sequenced by Macrogen Europe (Netherlands). Obtained sequences were visualised, trimmed and aligned in BioEdit software [9]. The analysis of evolutionary divergence was conducted using MEGA 6 [10] software using the Kimura 2-parameter distance model [11]. In all molecular analyses, the reconstruction of phylogenetic relationships was conducted with the Maximum Likelihood method. The Tamura–Nei [12] parameter model with discrete Gama distribution was proposed as the best-fitting model and was conducted with 2000 bootstrap replications. Seven sequences from the Aphidius urticae sensu stricto group were acquired from GenBank, as well as one sequence of Ephedrus persicae Froggatt (outgroup). For the molecular analysis of Praon sp. sequences, seven sequences of the Praon abjectum species group were acquired from the GenBank as well as one sequence of Ichneumon picticollis Holmgren, 1864. An additional 13 sequences of the subgenus Fovephedrus were acquired from GenBank. As the outgroup, six sequences of the subgenus Ephedrus were used, as well as one sequence of I. picticollis.
3. Results
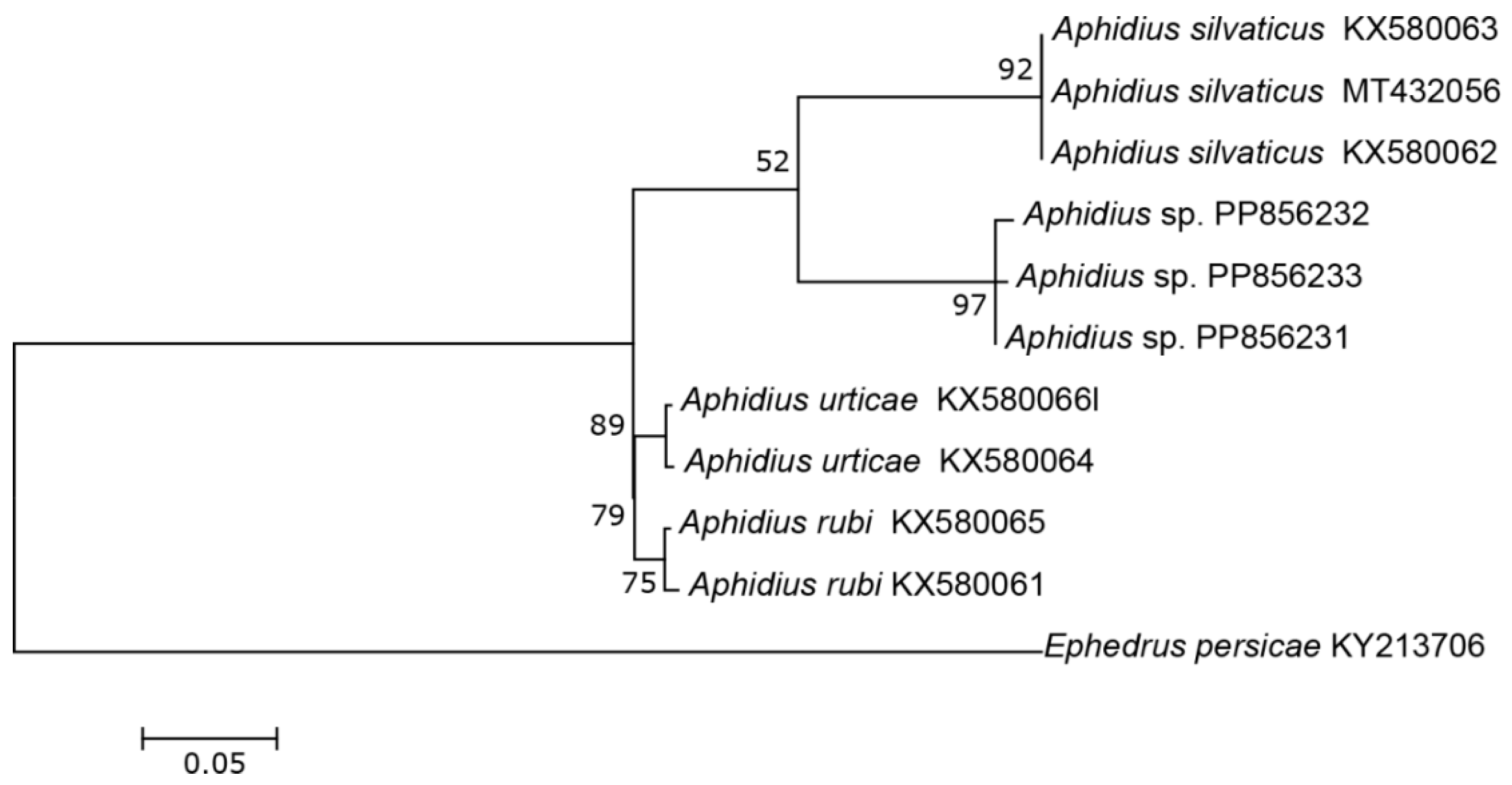
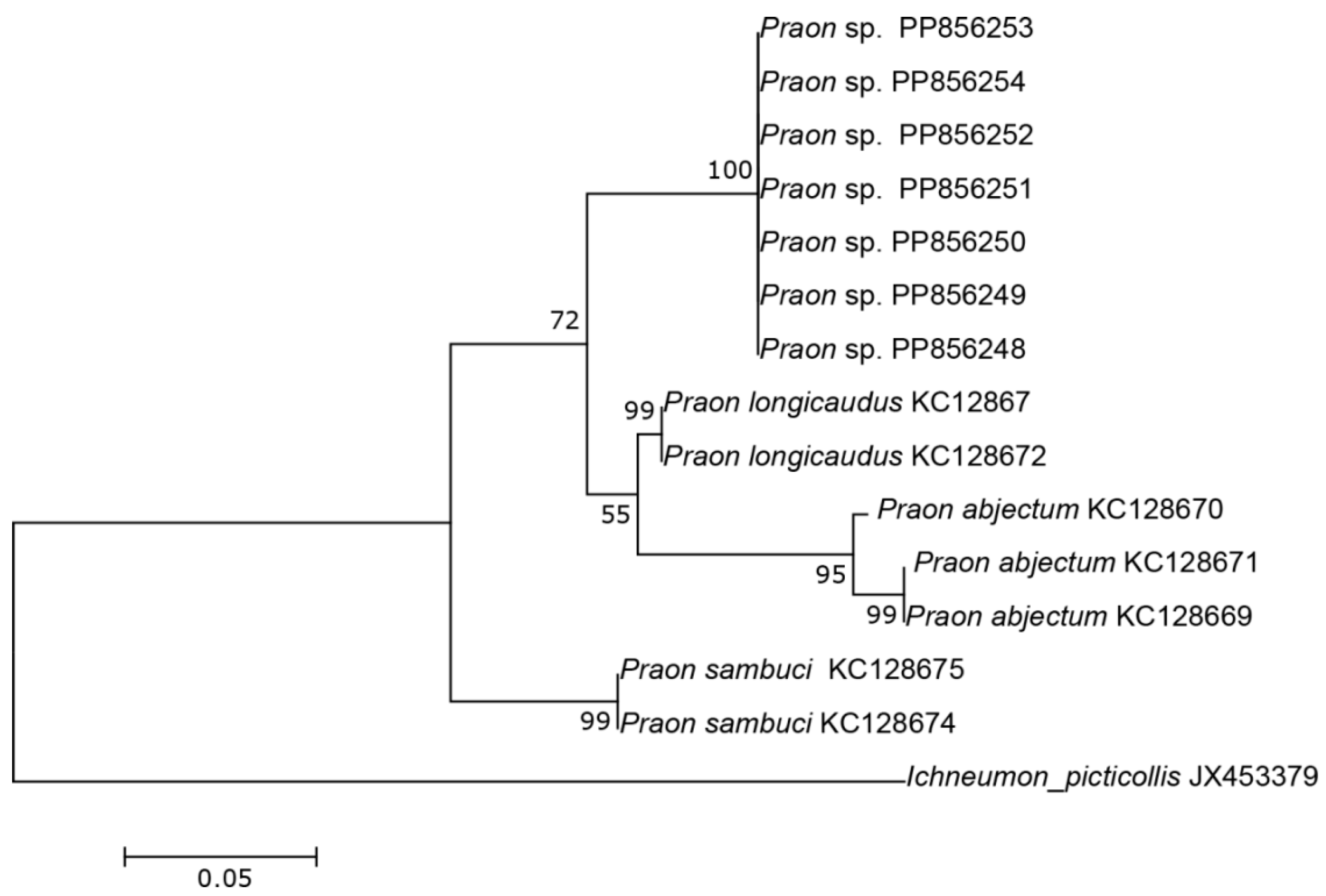
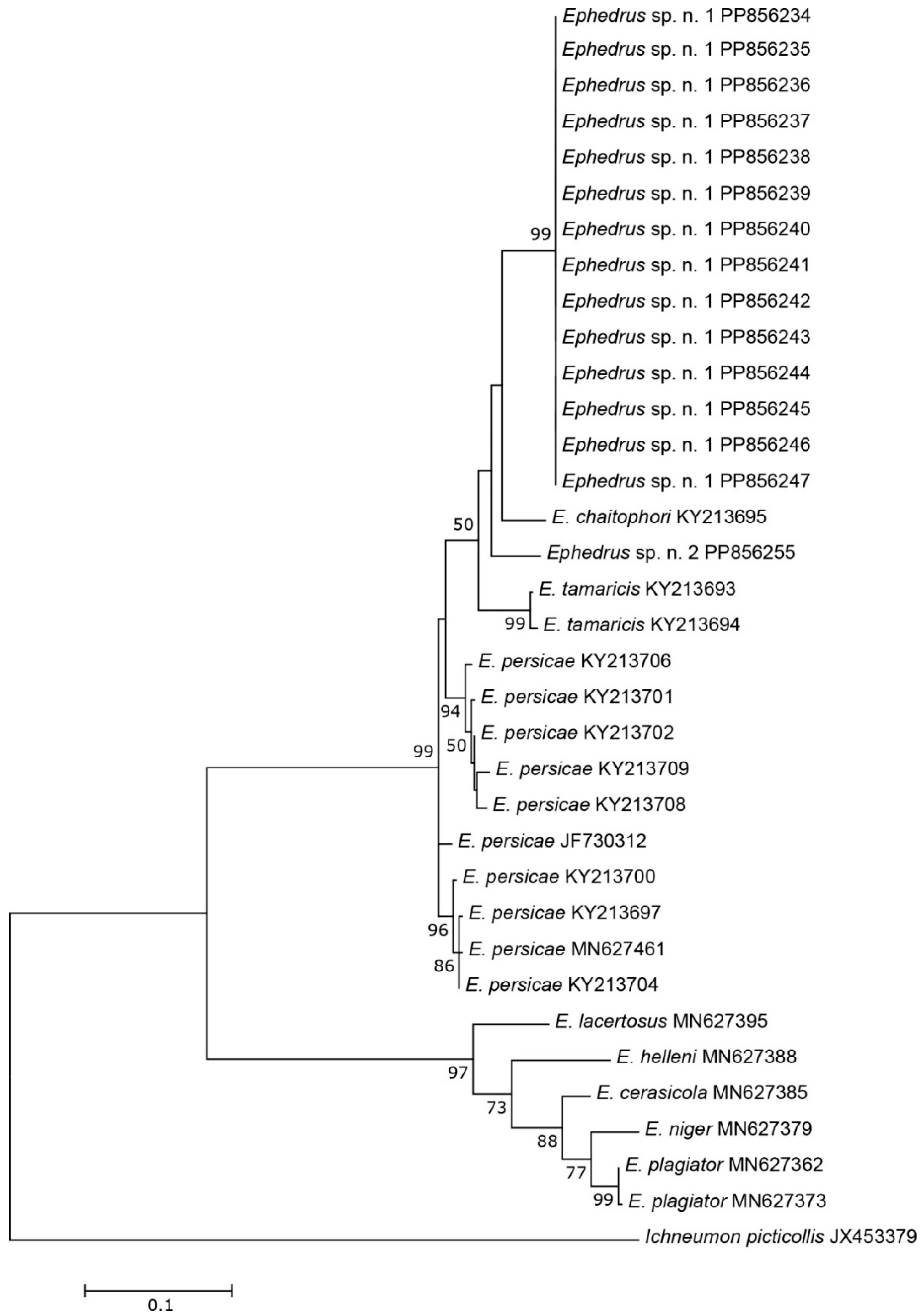
The three COI sequences recovered from specimens designated as Aphidius sp. separated from other Aphidius sequences with the mean between species evolutionary divergence ranging from 6.8% to 11.1%. Within the Aphidius urticae s. str. group, the mean between species genetic distances ranged from 8.0% to 9.7% (Figure 2). With three sequences representing three different haplotypes, the intraspecific genetic distance was 0.3–0.7% (GenBank accession numbers PP856231–PP856233). Seven sequences of the specimens designated as Praon sp. were separated from other sequences of the genus Praon with genetic distances ranging from 5.9% to 9.8%, representing a single haplotype (GenBank accession numbers PP856248–PP856254). On the phylogenetic tree of the Praon abjectum species group, these sequences clustered separately with the mean between species genetic distance ranging from 5.9% (P. longicaudus) to 9.5% (P. sambuci) (Figure 3). A total of 14 recovered sequences of the specimens designated as Ephedrus sp. 1 showed no genetic variability, all belonging to the single haplotype (GenBank accession numbers PP856234–PP856247). In the phylogenetic tree, all Ephedrus sp. 1 sequences clustered separately within subgenus Fovephedrus (Ephedrus persicae species group) with genetic distances ranging from 5.1% (Ephedrus chaitophori Gärdenfors, 1986) to 6.3% (Ephedrus persicae Froggatt, 1904). A single specimen sequence, designated as Ephedrus sp. 2 (GenBank accession number PP856255), also grouped within the subgenus Fovephedrus, with an evolutionary divergence of 5.1%–6.4% compared to other members of the subgenus (Figure 4).
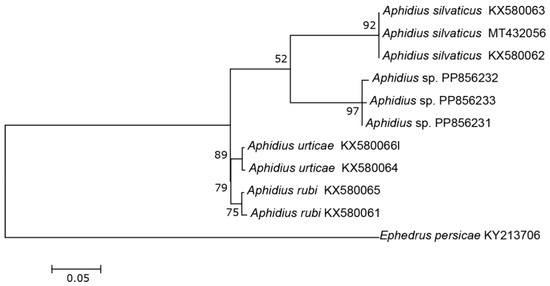
Figure 2.
The evolutionary history of the A. urticae sensu stricto group, inferred by using the Maximum Likelihood method based on the Tamura–Nei model.
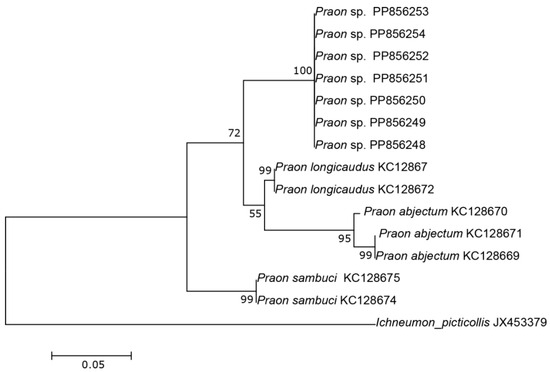
Figure 3.
The evolutionary history of the Praon abjectum species group, inferred by using the Maximum Likelihood method based on the Tamura–Nei model.
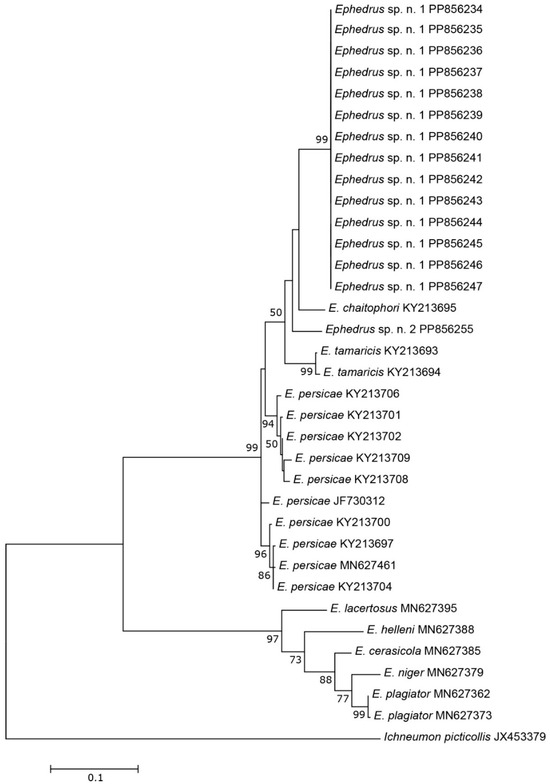
Figure 4.
The evolutionary history of subgenus Fovephedrus, inferred by using the Maximum Likelihood method based on the Tamura–Nei model.
The analysis of the morphological characters also revealed that all four species (Aphidius sp., Praon sp., Ephedrus sp. 1 and Ephedrus sp. 2) possess unique morphological traits or a combination of morphological characteristics that clearly distinguish them from other congeners.
Based on molecular and morphological evidence, we describe four species new to science, all collected from Norway: Aphidius norvegicus sp.n., Praon breviantennalis sp.n., Ephedrus borealis sp.n. and Ephedrus gardenforsi sp.n.
3.1. New Species Descriptions
3.1.1. Aphidius norvegicus sp.n. Tomanović & Kocić (Figure 5)
(GenBank Accession Numbers PP856231–PP856233)
Diagnosis. Morphologically, A. norvegicus sp.n. resembles species from Aphidius urticae s.str. group (Aphidius urticae Haliday, 1834, Aphidius rubi Starý, 1962, Aphidius silvaticus Starý, 1962) with respect to the costulated anterolateral area of the petiole, number of flagellomeres, proportion of length and width of flagellomere 1 and tergite 1. However, it can easily be discriminated by more elongated pterostigma (proportion of length and width of pterostigma 4.30–4.50 in A. norvegicus sp.n., while in the A. urticae s.str. group it is 3.40–4.10). The R1 vein (=metacarpus) in A. norvegicus sp.n. is shorter than pterostigma (the proportion between the pterostigma and R1 length is 1.20–1.50) while in A. urticae and A. silvaticus, pterostigma is subequal to the R1 vein. Additionally, A. norvegicus sp.n. has a length-to-width ratio of flagellomere 2 of 3.40–3.60, compared to 2.70–3.30 in A. rubi.
Description. Female. Head. Eyes oval, sparsely setose (Figure 5A). Malar index 0.16–0.18 times the longitudinal eye diameter. Clypeus oval, with 15–22 long setae (Figure 5A). Tentorial index (tentoriocular line/intertentorial line) 0.33–0.37. Antennae with 18–19 flagellomeres, with semierect setae which are subequal to half the segment diameter (Figure 5B). Flagellomere 1 (F1) and flagellomere 2 (Figure 5C) are 3.50–3.80 and 3.40–3.60 times as long as the median width, respectively. F1 is subequal to F2. F1 without and F2 with 1–4 longitudinal placodes (Figure 5C). Maxillary palps with four palpomeres, labial palps with three palpomeres. Mesosoma. Mesonotum with notaulices distinct only at the ascending part, slightly crenulated, with two rows of setae (Figure 5D). Propodeum areolated with narrow central pentagonal areola (Figure 5E). External and dentiparal areolae with 4–10 and 2–7 setae, respectively (Figure 5E). Fore wing. Pterostigma elongated, approx. 4.30–4.50 times as long as wide and approx. 1.20–1.50 times as long as the distal abscissa of R1 (=metacarpus) (Figure 5H); R1 vein 3.00 times longer than pterostigma width. Metasoma. Petiole 3.30–3.50 times as long as wide at the spiracle level (Figure 5F), with a prominent and adjective mediodorsal carina, with 7–9 costulae on its anterolateral area and about 10 setae in the lower dorsal part. The ovipositor sheath is almost straight in its dorsal margin (Figure 5G).
Colour. Body generally yellow to light brown. Head yellow to light brown, eyes dark brown. Scape yellow, pedicel light brown to brown, with a narrow yellow ring at the base of flagellomere 1. The remaining part of the antennae is brown. Legs yellow with dark apices. The remainder of the body is light brown.
Body length. 1.7–2.1 mm
Male. Unknown
Aphid host. Tribe Macrosiphini.
Distribution: Norway, Northern Ireland.
Etymology. The new species was named after the locus typicus of the type specimen.
Material. Norway: holotype ♀, Rogaland, Suldal, Vårvik (59.54282N, 6.64587E), 27.06.2021–03.08. 2021, leg. Eva Songe Paulsen and Rune Roalkvam, collected by malaise trap; paratypes: 1♀, Rogaland, Sandnes, Svanholmen (58.88880N, 5.70937E), 01.06.2022–15.06.2022, malaise trap, leg. Alf Tore Mjøs. United Kingdom, Northern Ireland: 1♀, Moneyreagh (54.532919N, 5.849970E), 2022, leg. Martin Wohlfarter (collaborative collection efforts of Koppert B.V. (Martin Wohlfarter) and Koppert UK Ltd. (Jasper Hubert and David Davidson), in the organic vegetable farm with the diverse adjacent garden, sampled aphid mummy from the Rosa sp. Holotype and paratypes slide mounted and deposited in the collection of the Institute of Zoology, Faculty of Biology, University of Belgrade, Serbia.
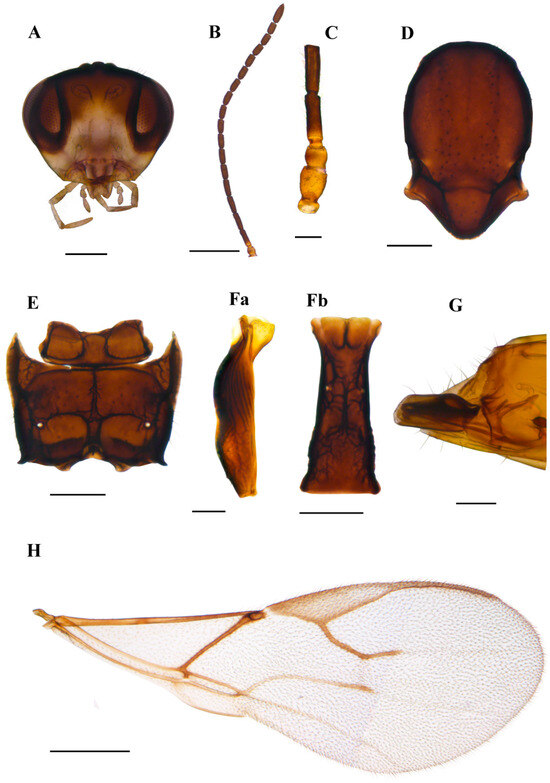
Figure 5.
Aphidius norvegicus sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (Fa) petiole—lateral aspect (Fb) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
Figure 5.
Aphidius norvegicus sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (Fa) petiole—lateral aspect (Fb) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
3.1.2. Praon breviantennalis sp.n. Kocić & Tomanović (Figure 6)
(GenBank Accession Numbers PP856248–PP856254)
Diagnosis. Praon breviantennalis sp.n. can easily be discriminated from other Praon species by short antennae (13–14 antennomeres) and a very long R1 vein, which is equal in length to the pterostigma.
Description. Female. Head. Malar space equal to 0.10–0.15 of longitudinal eye diameter. Clypeus oval, with 10–16 long setae. Tentorial index 0.20–0.28 (Figure 6A). Maxillary palps with four palpomeres, labial palps with three palpomeres. Antennae with 13–14 antennomeres, moderately thickened, with semierect setae, which are subequal to or shorter that half of the segment diameter (Figure 6B). F1 and F2 are moderately elongated, 3.50–4.00 and 2.70–3.20 times as long as wide, respectively (Figure 6C). F1 is 1.30–1.40 times longer than F2. F1 without, and F2 without or with 1 (2) longitudinal placodes. Mesosoma. Mesonotum (Figure 6D) with central lobe covered with dense setae. Lateral lobes of mesonotum with large glabrous areas. Notaulices deep and distinct throughout. Propodeum (Figure 6E) smooth, densely pubescent with small central glabrous areas. Forewing. Pterostigma 3.40–3.70 times as long as wide (Figure 6H) and equal to distal abscissa of R1 (=metacarpus). The first half of the m–cu vein is usually sclerotized and coloured; the remaining part is colourless; Rs+M vein colourless (Figure 6H). Metasoma. Petiole elongated, 1.10–1.40 times as long as wide (Figure 6F) at the level of the spiracles. The distance between spiracles and the apex is less than the width at the level of spiracles (proportion between width at the level of spiracles and distance between spiracles and apex of petiole 1.75–2.10). Petiole with 2–4 long setae along the petiole sides. Ovipositor sheath (Figure 6G) with a slightly concave dorsal margin. Apex round, with two conical spines on its upper and lower edge.
Colour. Head brown, with mouthparts yellow to light brown. Scape and pedicel brown, annellus yellow, 1/3–2/3 of flagellomere 1 yellow or light brown. The remaining parts of the antennae are brown. Mesosoma and metasoma are light brown to brown. Legs yellow with dark apices. The petiole is yellow to light brown.
Body length. 1.4–1.7 mm
Male. Unknown
Aphid host: Unknown.
Distribution: Norway.
Etymology. The new species was named after the unique character among the congeners, short antennae.
Material. Holotype: 1♀ Norway, Rogaland, Sandnes, Svanholmen (58.88878N, 5.71121E), 15.08.2021–31.08.2021, leg. Alf Tore Mjǿs, malaise trap paratypes: 1♀, same as holotype; 2♀ same as previous, 16.07.2021–31.07.2021, 2♀ same as previous, 31.08.2021–15.09.2021; 1♀ Rogaland, Sola, Roynebergsletta plen (58.90041N, 5.68971E), 15.06.2021–29.06.2021, leg. Alf Tore Mjǿs, malaise trap; 2♀ same as previous 15.09.2021–01.10.2021, 1♀ same as previous 29.06.2021–16.08.2021. Holotype and eight paratypes slide mounted, one female stored in ethanol. Material is deposited in the collection of the Institute of Zoology, Faculty of Biology, University of Belgrade, Serbia.
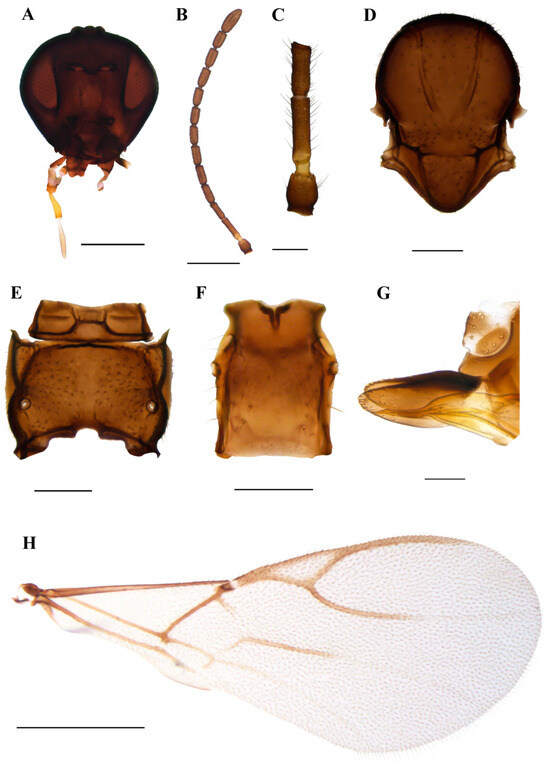
Figure 6.
Praon breviantennalis sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
Figure 6.
Praon breviantennalis sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
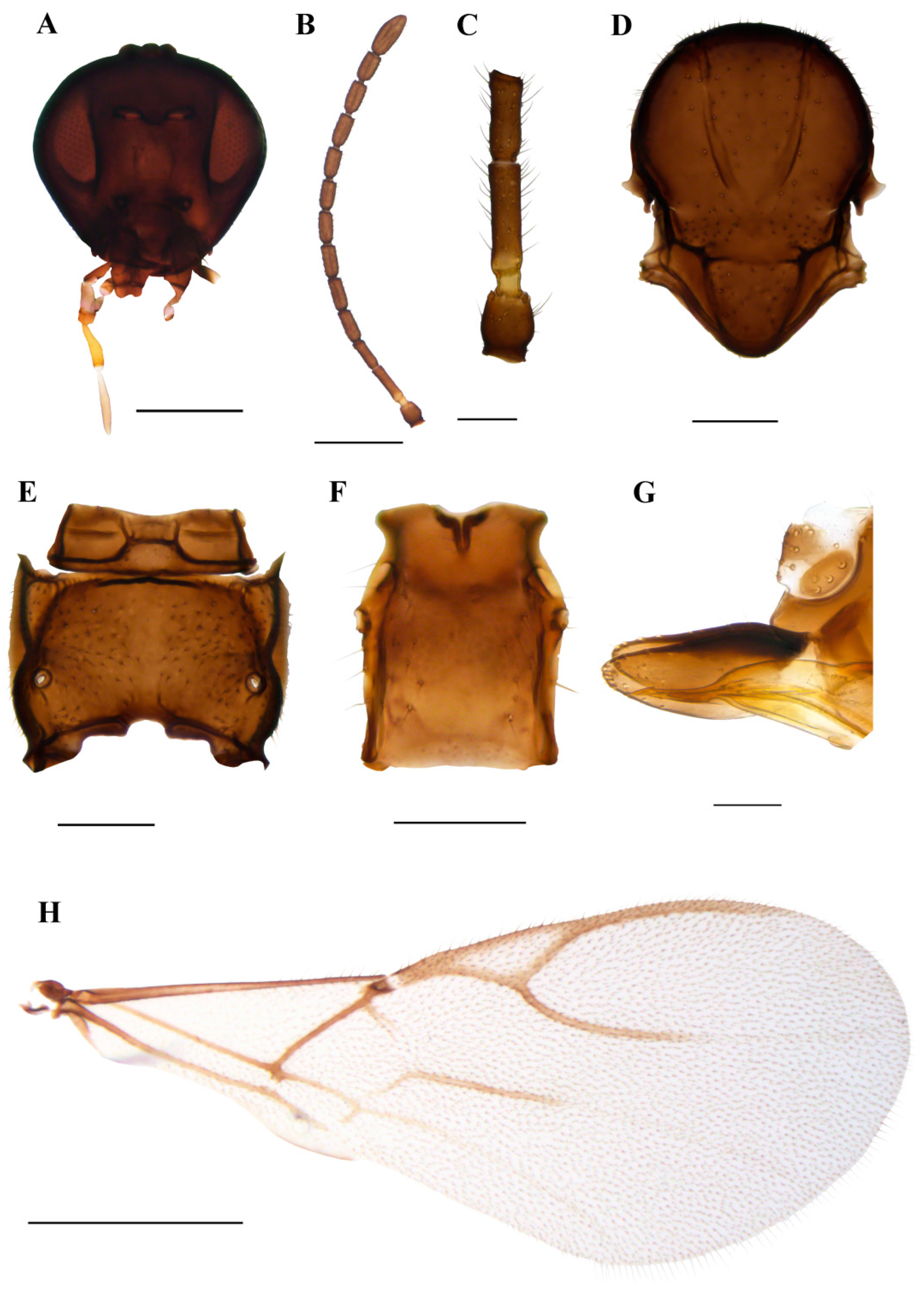
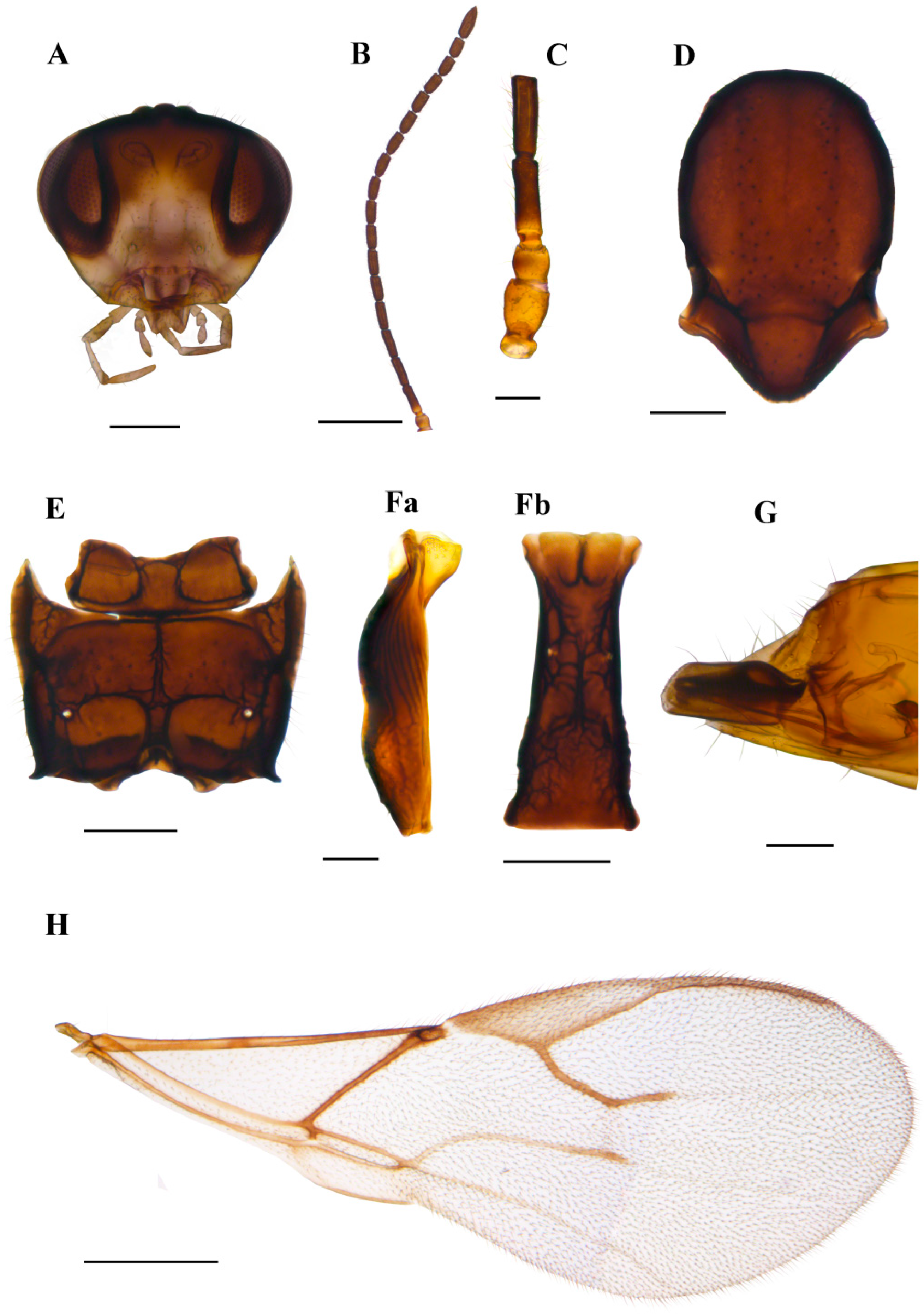
3.1.3. Ephedrus gardenforsi sp. n. Kocić & Tomanović (Figure 7)
(GenBank Accession Number PP856255)
Diagnosis. The new species belongs to the Fovephedrus subgenus (former Ephedrus persicae species group), based on the short fore wing 3RSa vein. It differs from the nominative species E. persicae by the longer F1, which is lightly coloured (F1 l/w is 4.8 in E. gardenforsi vs 3.8–4.2 in E. persicae). It differs from Ephedrus lonicerae Tomanović, Kavallieratos & Starý, 2009 with respect to the length and colouration of F1 as well as the number of foveal pits on mesoscutum (in E. lonicerae F1 is 5.0–5.3 times as long as wide, F1 and half of F2 are yellow, the mesoscutum has two foveal pits, while in E. gardenforsi sp.n., F1 is about 4.8 times as long as wide, F1 is yellowish at the first part and the mesoscutum has one foveal pit). It differs from Ephedrus tamaricis Tomanović & Petrović, 2016 with respect to the colouration of F1 (yellowish at the first part), a shorter pterostigma (pterostigma 4.8–5.0 times as long as wide) and a more elongated petiole (1.5 times as long as wide), while in E. tamaricis, F1 has only a basal yellow ring with the remainder of the flagellomere being brown. The pterostigma length/width ratio is 5.0–5.22, and the petiole is 1.2–1.4 times as long as wide at the spiracle level. The new species is most similar to E. chaitophori; however, in this species, F8 and F9 are visibly separated, thus not forming a club as in E. gardenforsi (Figure 7B). The ovipositor sheath in E. gardenforsi lacks a subapical constriction, which is present in E. chaitophori.
Description. Female. Head. Eyes medium-sized, without noticeable setae (Figure 7A). Clypeus is slightly convex, bearing sparse long setae. Tentorial index 0.5, tentorial pits wide and deep. Malar space equal to 0.35 of longitudinal eye diameter. Mandibles bidentate. Maxillary palps with four palpomeres, labial palps with two palpomeres, both covered with sparse long setae. Antennae with 11 antennomeres with long setae that are subequal to segment diameter. The last two apical segments (F8 and F9) are not well separated, giving the impression of a club (Figure 7B). The first flagellar segment (F1) is 1.44 times as long as the second flagellar segment (F2) (Figure 7C). F1 is elongated, 4.8 times as long as wide, bearing 1–2 longitudinal placodes (=rhinariae). The second flagellar segment (F2), with 2–3 longitudinal placodes, is 2.9 times as long as wide (Figure 7C). Mesosoma. Mesoscutum with notaulices present only at the ascending part (Figure 7D). The mesoscuteal fovea is present, yet small and feebly visible. Distinctive setae are present on the surface of the mesoscutum, following the direction of notaulices and evenly distributed around the foveal pit. Propodeum areolated, with 4–5 setae present on external areolae and smooth dentiparal areolae (Figure 7E). Fore wing pterostigma 4.8–5.0 times as long as wide. 3RSa/2RS and 3RSb/3Rsa vein ratios 0.76–0.78 and 3.0–3.3, respectively. 3RSa vein 1.2 times longer than r-m (Figure 7H). Metasoma. Petiole short, 1.5 times as long as wide, bearing rugosities along the dorsal surface and with 5 long setae on lateral sides (Figure 7F). The ovipositor sheath is elongated, 3.1 times as long as wide, with 4 and 3 setae along the dorsal and ventral side, respectively (Figure 7G).
Colour. Head brown, mouthparts light brown. Scape and pedicel brown, anellus yellow, F1 yellowish in the first part, gradually getting brown towards the distal part. The remainder of the flagellar segments are brown. The mesonotum, propodeum, petiole, abdomen and ovipositor sheath are brown. Legs yellowish. Fore wings hyaline.
Body length. 1.5 mm.
Male: unknown
Aphid host: unknown
Distribution: Norway.
Etymology: The new species is named in honour of the Swedish entomologist Ulf Gärdenfors, who has greatly contributed to the knowledge of the subfamily Aphidiinae, especially the genus Ephedrus.
Material: Holotype: 1♀, Norway, Sola, Forus, Røynebergsletta, (58.90017N, 5.68956E), 29.06.2021–16.07.2021, collected using a malaise trap, leg. Alf Tore Mjøs. Holotype slide mounted and deposited in the collection of the Institute of Zoology, Faculty of Biology, University of Belgrade.
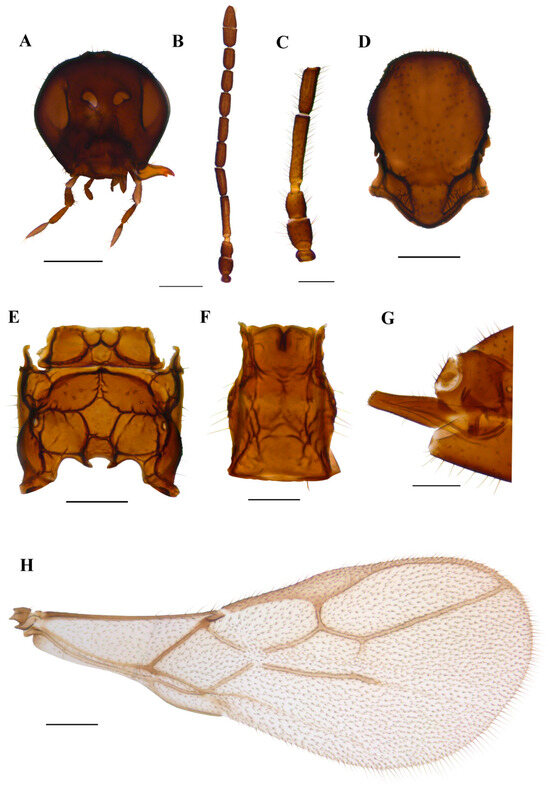
Figure 7.
Ephedrus gardenforsi sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
Figure 7.
Ephedrus gardenforsi sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (D) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
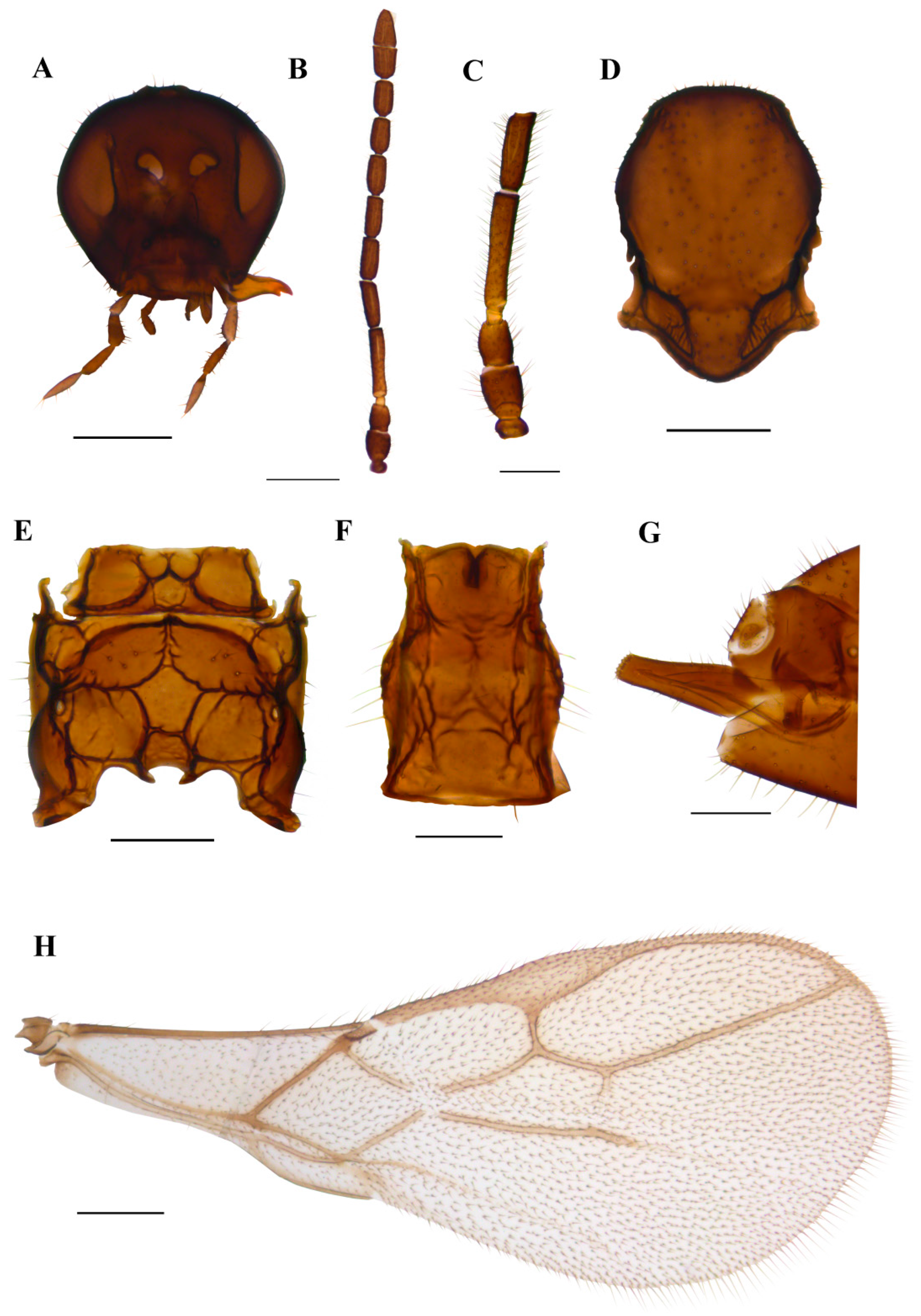
3.1.4. Ephedrus borealis sp.n. Kocić & Tomanović (Figure 8)
(GenBank Accession Numbers PP856234–PP856247)
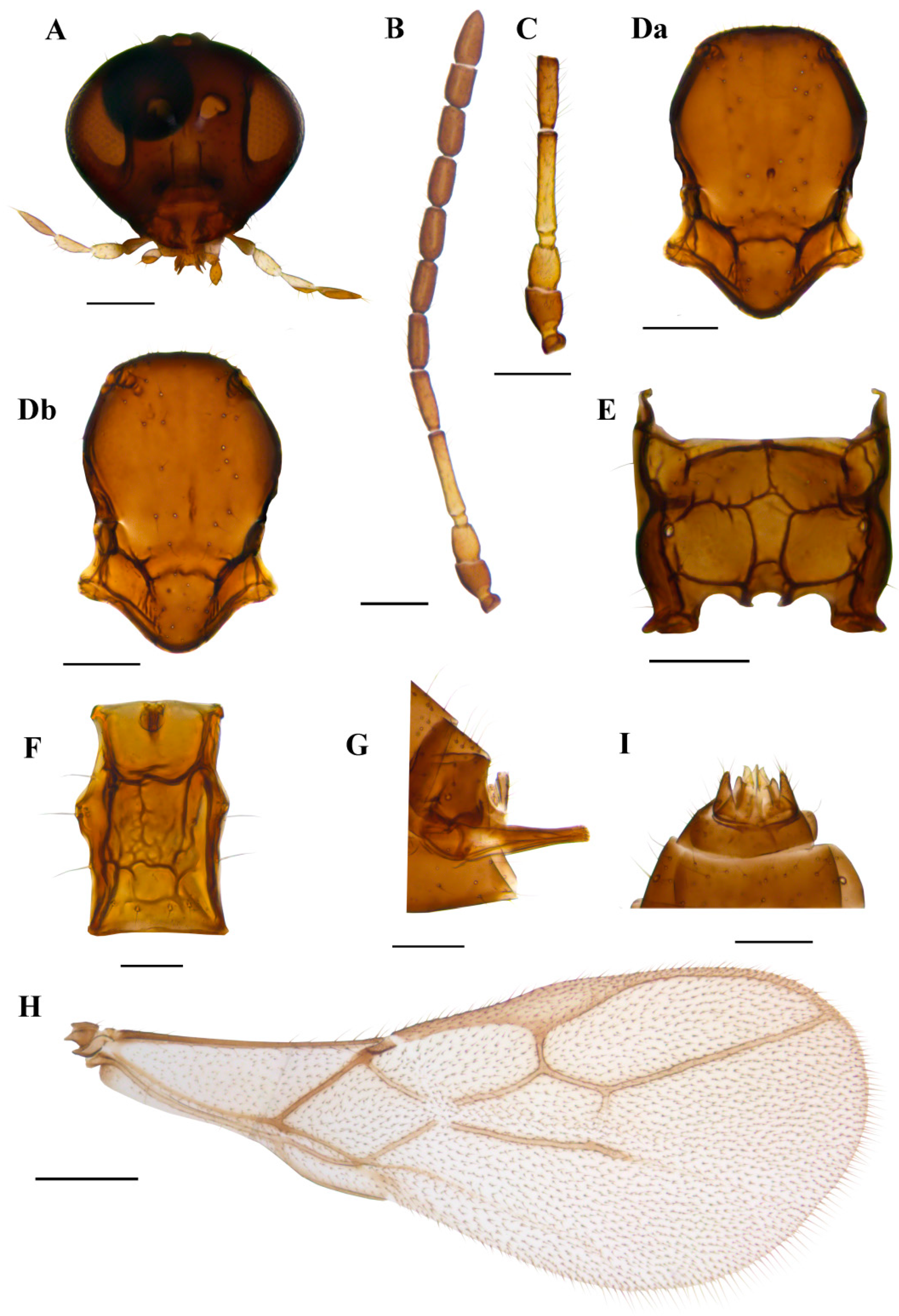
Diagnosis. The new species belongs to the subgenus Fovephedrus (Ephedrus persicae species group) due to the ratio of 3RSa/2RS. It differs from all other species of the subgenus with respect to a very long first flagellar segment F1 (5.7–6.3), elongated petiole (1.9–2.3) and ovipositor sheath, a narrow central areola of propodeum and a densely pubescent body.
Description. Female. Head. Eyes medium sized, head with scattered long setae along the surface. Clypeus convex, bearing 10 long setae. Tentorial index 0.4–0.5, tentorial pits wide. Malar space equal to 0.19–0.22 of longitudinal eye diameter. Maxillary palps with four palpomeres, labial palps with two palpomeres, both covered with multiple long setae (Figure 8A). Antennae with 11 antennomeres with long setae that are slightly longer than the segment diameter (Figure 8B). The last two apical segments (F8 and F9) are well separated. The first flagellar segment (F1) is 1.37–1.55 times as long as the second flagellar segment (F2) (Figure 8C). F1 is long, 5.7–6.3 times as long as wide, without longitudinal placodes (=rhinariae). The second flagellar segment (F2) usually has one longitudinal placode, sometimes has none, and is 3.0–3.5 times as long as wide. Mesosoma. A mesoscutum with notaulices is present only at the ascending part, with two longitudinal rows of setae. In most specimens, one mesoscuteal fovea is clearly visible (Figure 8Da). In several, two foveal pits are visible, irregular, elongated and shallow (Figure 8Db). Propodeum with narrow central areola, with 3–5 setae present on external and 0–1 on dentiparal areolae (Figure 8E). Fore wing pterostigma 5.5–6.0 times as long as wide. 3RSa/2RS and 3RSb/3Rsa vein ratios 0.6–0.75 and 3.2–3.5, respectively. 3RSa vein 1.1–1.2 times longer than r-m (Figure 8H). Metasoma. Petiole 1.9–2.3 times as long as wide, bearing rugosities along the dorsal surface and with 3 long setae on lateral sides (Figure 8F). The ovipositor sheath is moderately elongated, with 2 and 1 setae along the dorsal and ventral sides, respectively (Figure 8G).
Colour. Head brown, mouthparts yellowish. Scape brown to light brown, pedicel and annelus yellow, 2/3 or complete F1 yellow. Sometimes F2 has a yellow or light brown basal ring, grading into darker brown at the distal part. The remainder of the flagellar segments are brown. The mesonotum, propodeum, petiole and abdomen are yellowish brown to brown. The ovipositor sheath is brown. Legs yellow. Fore wings hyaline.
Body length. 1.5–1.7 mm.
Male. Eyes smaller than in females. The first flagellar segment is 1.15–1.20 times as long as the second flagellar segment, bearing 0–1 and 1–2 longitudinal placodes, respectively. F1 and F2 are 4.3–4.4 and 2.7–2.8 times as long as wide. The mesonotum has notaulices only at the ascending part, with two longitudinal rows of setae. The mesoscuteal foveal pit is present, deep and narrow, or the mesoscutum has two connected, shallow and irregular pits. The propodeum is as in females, with narrow central pentagonal areola and 2 and 1 setae present on the external and dentiparal areolae, respectively. Fore wing pterostigma is 5.5 times as long as wide, 3RSa/2RS veins ratio 0.6–0.65. Petiole is 1.7–1.8 times as long as wide at the spiracle level. Male genitalia as in Figure 6I. The colour is slightly darker than in females. Scape and pedicel are brown, annelus is light brown or yellow. F1 is yellow or light brown in the first 2/3 of the segment, and the remaining flagellar segments are brown.
Aphid host. Unknown.
Distribution: Norway.
Etymology: Name of the new species derived from its known distribution, borealis meaning northern in Latin.
Material: Holotype ♀ Norway, Rogaland, Hå, Brusand (58.53837N, 5.74777E), 31.08.2020–17.09.2020, malaise trap, leg. Alf Tore Mjǿs paratypes: 2♂, same as holotype; 1♀1♂ Rogaland, Time, Linemyra (58.71556N, 5.63867E), 31.08.2021–30.09.2021, malaise trap, leg. Alf Tore Mjøs, 1♀1♂ same as previous, 01.08.2021–30.08.2021, 2♀4♂ same as previous, 29.06.2021–20.07.2021, 3♀ same as previous, 21.07.2021–31.08.2021, 2♀2♂ same as previous, 01.06.2021–29.06.2021.
Holotype and seven female and six male paratypes slide mounted, two female and four male paratypes stored in ethanol. Material is deposited in the collection of the Institute of Zoology, Faculty of Biology, University of Belgrade, Serbia.
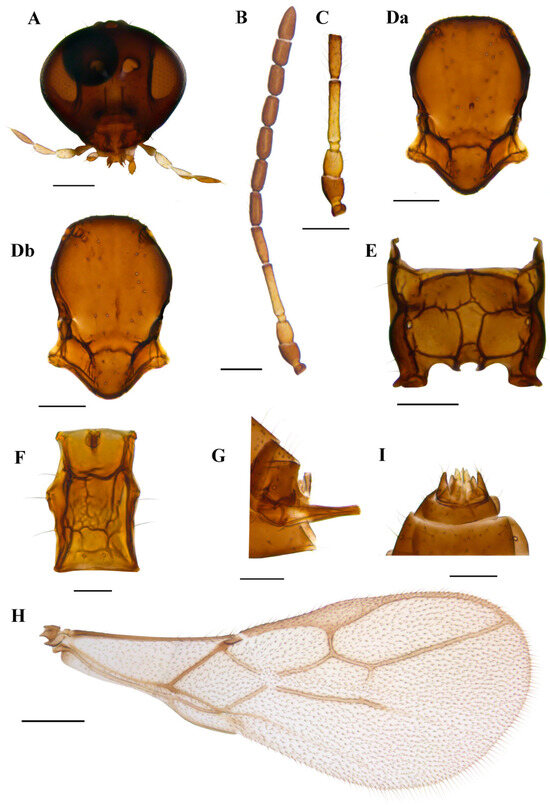
Figure 8.
Ephedrus borealis sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (Da) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing; paratype female (Db) mesonotum—dorsal aspect; paratype male (I) aedeagus. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
Figure 8.
Ephedrus borealis sp. n. holotype female (A) head (B) antenna (C) scape, pedicel, first and second flagellomere (Da) mesonotum—dorsal aspect (E) propodeum—dorsal aspect (F) petiole—dorsal aspect (G) ovipositor sheath—lateral aspect (H) fore wing; paratype female (Db) mesonotum—dorsal aspect; paratype male (I) aedeagus. Scale bars: 200 µm (A,D,E); 500 µm (B,H); 100 µm (C,F,G).
- Key for Identification of Female Parasitoids Belonging to Subgenus Fovephedrus (Ephedrus persicae Species Group)
- 1
- Number of antennomeres 12, central areola of propodeum not clearly defined......................................………………E. antennalis Tomanović
- –
- Number of antennomeres 11, central areola of propodeum clearly defined………………………………………………………………………...2
- 2
- F1 very long, 5.7–6.3 times as long as wide, narrow central propodeal areola, petiole elongated, 1.9–2.3 times as long as wide at spiracle level (Figure 8) …………………………………………………………………………………………......……….E. borealis sp.n. Kocić & Tomanović
- –
- F1 3.8–5.3 times as long as wide, wide central areola of propodeum, petiole subquadrate, 1.1–1.6 times as long as wide at spiracle level…………………………………………………………………………………………………………………………………………………………....3
- 3
- Mesoscutum with two foveal pits, F1 elongated, 5.0–5.3 times as long as wide.........……….....E. lonicerae Tomanović, Kavallieratos & Starý
- –
- Mesoscutum with one foveal pit, F1 3.8–4.9 times as long as wide …………………………………………………………………………………...4
- 4
- F1 uniformly brown, 3.8–4.2 times as long as wide………………………………………………………………………………..E. persicae Froggatt
- –
- F1 with the yellow ring at the base, or 1/2 to 2/3 yellow, 4.5–4.9 times as long as wide………………………………………………………….....5
- 5
- F1 with narrow yellow ring at the base, F1 subequal to F2 (1.2–1.3)…….…………………..…………………E. tamaricis Tomanović & Petrović
- –
- Approx. 1/2 to 2/3 of F1 yellow, F1 1.3–1.5 times longer than F2…………………………................………………………………………………...6
- 6
- Flagellomeres F8 and F9 well separated, ovipositor sheath elongated with subapical constriction……………….....E. chaitophori Gärdenfors
- –
- F8 and F9 not well separated, forming a club, ovipositor sheath without subapical constriction (Figure 7)..…………………………………………………………………………………………………......................E. gardenforsi sp.n. Kocić & Tomanović
4. Discussion
Only sporadic faunistic research articles on Braconidae have reported data on the species found in Norway [13,14,15,16]. The preliminary results of our study revealed four species new to science, indicating that the current number of recorded species in Norway is significantly lower than the actual diversity. All specimens, except for one female reared from an aphid mummy, were collected using malaise traps. Although this type of sampling results in the loss of data on tritrophic interactions (plant–aphid–parasitoid), the diversity of the sampled material often greatly surpasses the number of species collected by rearing.
The genus Aphidius, with more than 130 described species [17] distributed worldwide, is one of the largest members of the subfamily Aphidiinae. Several species, including Aphidius colemani Viereck, A. ervi Haliday, and A. matricariae Haliday are used in biological control programs for pest aphids and are commercially distributed across the world. Only 11 species of this genus have been reported from Norway [4]. A newly identified species, Aphidius norvegicus sp. n., morphologically belongs to the A. urticae sensu stricto group, which includes A. urticae, A. rubi and A. silvaticus [18,19]. Phylogenetic reconstruction of the barcoding region was in congruence with morphological analysis, revealing that the new species is genetically distinct from other species of the group, exhibiting significantly high evolutionary divergence (Figure 2). Although the tritrophic associations of the material collected from Norway are currently unknown, an additional female was reared from an aphid mummy of the tribe Macrosiphini found on Rosa sp. Three additional sequences of the same species are available in the BOLD database (GMNWJ1781-14 GMNWK3447-14, GMNWK2015-14), all originating from Norway.
Currently, there are 33 recorded species of the genus Praon in Europe, with only seven reported from Norway [4]. The host range of these species varies from strict specialists to generalists like Praon volucre (Haliday, 1833), which parasitizes a broad range of aphid hosts. The species within this genus exhibit variability in the number of antennal segments, ranging from the lowest documented in Praon necans Mackauer, 1959 (15–16), Praon abjectum Haliday, 1833 (15–16) Praon rosaecola Starý, 1961 and Praon staryi Kavallieratos & Lykouressis, 2000 (usually 16–17 in both species) to a maximum of 23 in Praon longicorne Marshall, 1896. The newly described P. breviantennalis sp.n. possesses a unique character among its congeners: very short antennae consisting of only 13–14 antennomeres. Based on morphological analysis results, this species belongs to the Praon abjectum species group [18,20]. In the phylogenetic tree, sequences belonging to P. breviantennalis sp. n. formed a separate clade and confirmed that this species is genetically distinct from other taxa (Figure 3).
The genus Ephedrus, considered basal within the subfamily Aphidiinae and containing several important biological control agent species, has been relatively well–studied, particularly in Europe. Out of more than 40 described species, only four have been detected in Norway, all belonging to the subgenus Ephedrus Haliday [4]. The first comprehensive review of the Palearctic species of the genus was provided by Gärdenfors [21], where E. persicae species group was established with two members, E. persicae and the newly described E. chaitophori Gärdenfors. Over the past decades, additional new species belonging to the E. persicae group have been described—E. lonicerae Tomanović, Kavallieratos & Starý [22], E. tamaricis Tomanović & Petrović [23], and E. antennalis Tomanović [24]. Kocić et al. [25] proposed a new subgeneric classification and raised the species from the E. persicae species group to the subgenus Fovephedrus Chen, 1986. Based on morphological and molecular analyses, two new species (E. borealis sp.n. and E. gardenforsi sp.n.) both belong to Fovephedrus (Ephedrus persicae species group) and are the first reports of this subgenus in Norway. Ephedrus borealis sp. n. exhibits clear morphological characters that easily distinguish it from other taxa, such as a long first flagellar segment, a narrow pentagonal areola of propodeum (unusual for the genus), and a densely setose body. Although its aphid host is unknown, it is possible that this species parasitizes underground aphids or other hosts that produce a large amount of honeydew, with the dense setae across its body surface serving as an adaptation to the special conditions it encounters while searching for a host. Another new member of the genus, Ephedrus gardenforsi sp. n., has so far been collected only once. This species morphologically resembles E. chaitophori, and it is possible that it has been misidentified in previous samples. Therefore, a careful re-examination of the E. chaitophori material should be conducted. Prior to this study, only five species of the subgenus were known (E. persicae, E. tamaricis, E. chaitophori, E. antennalis and E. lonicerae). The results of our molecular analysis position both E. borealis and E. gardenforsi close to E. chaitophori, a specialized parasitoid of aphids found on poplars (Populus).
Tritrophic interactions are crucial for studying Aphidiinae, as they provide key insights into their biology, host specificity, and distribution, which align with those of their aphid hosts. In addition to continuing the exploration of the Norwegian Aphidiinae fauna through malaise trapping, future studies should focus on rearing from aphid hosts to reveal the tritrophic interactions of the newly described species.
Author Contributions
K.K. and Ž.T. conceived and designed the experiments. A.T.M. and E.S.P. provided specimens that were used for morphological and molecular analyses. K.K., J.Č., N.P. and A.P. carried out laboratory work and data analysis. Ž.T. and K.K. provided species descriptions. K.K., A.P. and Ž.T. wrote the original draft of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant numbers 451-03-65/2024-03/200178 and 451-03-66/2024-03/200178) and the Serbian Academy of Sciences and Arts (grant no. F131).
Data Availability Statement
The new species sequences analysed in this study are deposited in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers PP856231–PP856255.
Acknowledgments
We would like to thank Equinor and Forus Næringspark for interesting research opportunities and financial support for this study. Bess Jahres Stiftelse provided funding for DNA barcoding a large number of Aphidiinae from the “MUST Malaise” project. We would like to express our gratitude to Martin Wohlfarter (Koppert B.V.) and Jasper Hubert and David Davidson (Koppert UK Ltd.) for providing additional sample of A. norvegicus sp.n.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Petrović, A. Sizing the knowledge gap in taxonomy: The last dozen years of Aphidiinae research. Insects 2022, 13, 170. [Google Scholar] [CrossRef]
- The Norwegian Biodiversity Information Centre (NBIC). 2024. Available online: https://www2.artsdatabanken.no/ (accessed on 23 May 2024).
- SLU Swedish Species Information Centre, Swedish Taxonomical Database. Available online: https://namnochslaktskap.artfakta.se/ (accessed on 23 May 2024).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.J.; Wharton, R.A. Morphology and terminology. In Manual of the New World Genera of the Family Braconidae; Wharton, R.A., Marsh, P.M., Sharkey, M.J., Eds.; International Society of Hymenopterists: Washington, DC, USA, 1997. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A. MEGA 6: Molecular evolutionary genetics analysis 864 version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M.A. Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Hofsvang, T.; Hagvar, E.B. Primary parasitoids (Hym., Aphidiidae) and hyperparasitoids on aphids from Norway. Fauna Nor. Ser. B 1983, 30, 60–62. [Google Scholar]
- Riedel, M.; Hansen, L.O.; Berg, O. Braconidae (Hymenoptera) of Norway, Part 1. Nor. J. Entomol. 2002, 49, 97–108. [Google Scholar]
- Westrum, K.; Klingen, I.; Hofsvang, T.; Hågvar, E.B. Checklist of primary parasitoids and hyperparasitoids (Hymenoptera, Apocrita) on aphids (Hemiptera, Aphididae) from Norway. Nor. J. Entomol. 2010, 57, 142–153. [Google Scholar]
- Riedel, M.; Hansen, L.O. Braconidae (Hymenoptera) of Norway, Part II. Nor. J. Entomol. 2014, 61, 147–159. [Google Scholar]
- Yu, D.; van Achterberg, C.; Horstmann, K. Taxapad 2016. Ichneumonoidea 2015 (Biological and Taxonomical Information), Taxapad Interactive Catalogue Database on Flashdrive (Nepean). 2016. Available online: www.taxapad.com (accessed on 6 February 2024).
- Tomanović, Ž.; Žikić, V.; Petrović, A. Fauna of parasitoid wasps (Hymenoptera, Braconidae, Aphidiinae) of Serbia; Monographs, Book 15; Serbian Academy of Sciences and Arts: Beograd, Serbia, 2021; 262p. (In Serbian) [Google Scholar]
- Tomanović, Ž.; Žikić, V.; Petrović, A. Aphidius Nees (Hymenoptera, Braconidae, Aphidiinae) in Serbia: Key to species identification including parasitoid–aphid host list. Acta Entomol. Serbica 2023, 28, 61–76. [Google Scholar]
- Kavallieratos, N.G.; Tomanović, Ž.; Starý, P.; Athanassiou, C.G.; Fasseas, C.; Petrović, O.; Ljubiša, Ž. Stanisavljević, Maria Anagnou Veroniki, Praon Haliday (Hymenoptera: Braconidae: Aphidiinae) of Southeastern Europe: Key, host range and phylogenetic relationships. Zool. Anz. 2005, 243, 181–209. [Google Scholar] [CrossRef]
- Gärdenfors, U. Taxonomic and biological revision of Paleartcic Ephedrus Haliday (Hymenoptera, Braconidae, Aphidiinae). Entomol. Scand. Suppl. 1986, 27, 1–95. [Google Scholar]
- Žikić, V.; Tomanović, Ž.; Ivanović, A.; Kavallieratos, N.G.; Starý, P.; Stanisavljević, L.j.; Rakhshani, E. Morphological characterization of Ephedrus persicae biotypes (Hymenoptera: Braconidae: Aphidiinae) in the Palaearctic. Ann. Entomol. Soc. Am. 2009, 102, 1–11. [Google Scholar] [CrossRef]
- Petrović, A.; Kocić, K.; Kos, K.; Plećaš, M.; Žikić, V.; Kavallieratos, N.G.; Tomanović, Ž. High genetic diversity and a new cryptic species within the Ephedrus persicae species group (Hymenoptera: Braconidae: Aphidiinae). Biologia 2016, 71, 1386–1394. [Google Scholar] [CrossRef]
- Tomanović, Ž.; Petrović, A.; Kocić, K.; Čkrkić, J.; Žikić, V. Two new morphologically interesting species of the genus Ephedrus Haliday (Hymenoptera, Braconidae, Aphidiinae). J. Hymenopt. Res. 2020, 77, 167–174. [Google Scholar] [CrossRef]
- Kocić, K.; Petrović, A.; Čkrkić, J.; Mitrović, M.; Tomanović, Ž. Phylogenetic relationships and subgeneric classification of European Ephedrus species (Hymenoptera, Braconidae, Aphidiinae). ZooKeys 2019, 878, 1–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).