The Impact of Wildflower Habitat on Insect Functional Group Abundance in Turfgrass Systems
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sampling
2.2. Identification
2.3. Data Analysis
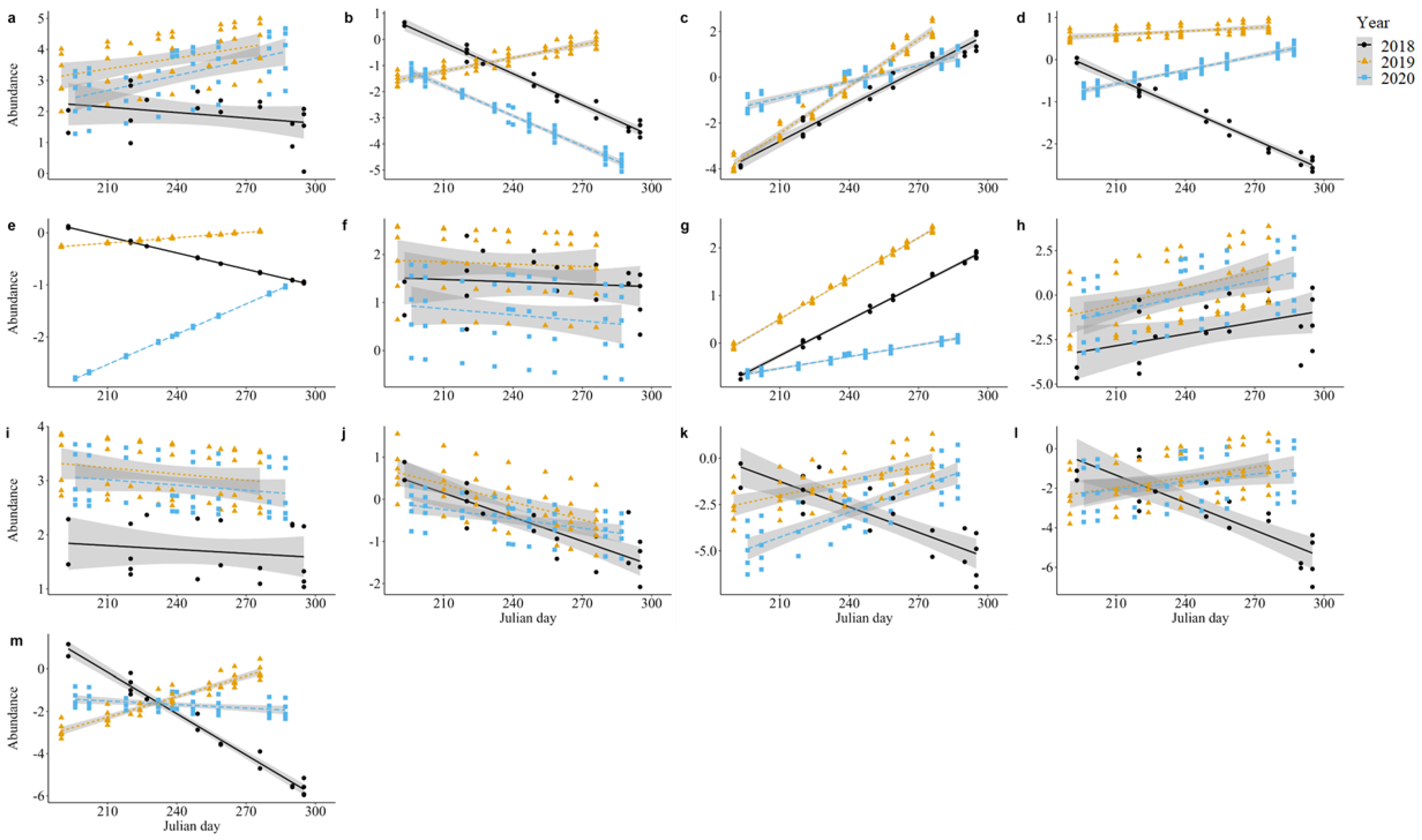
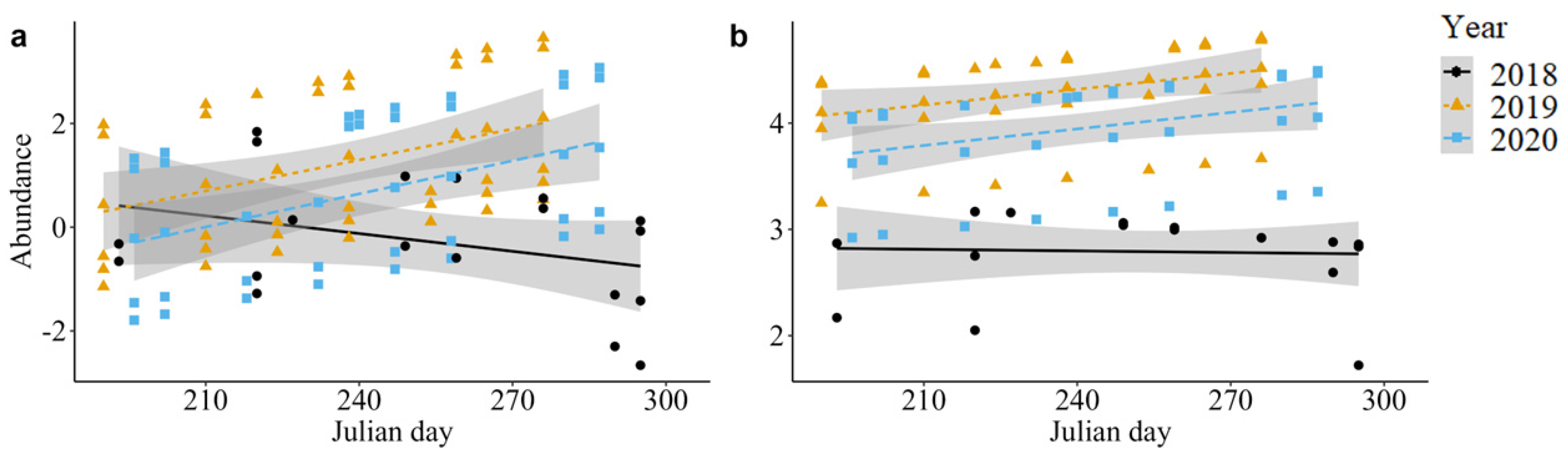
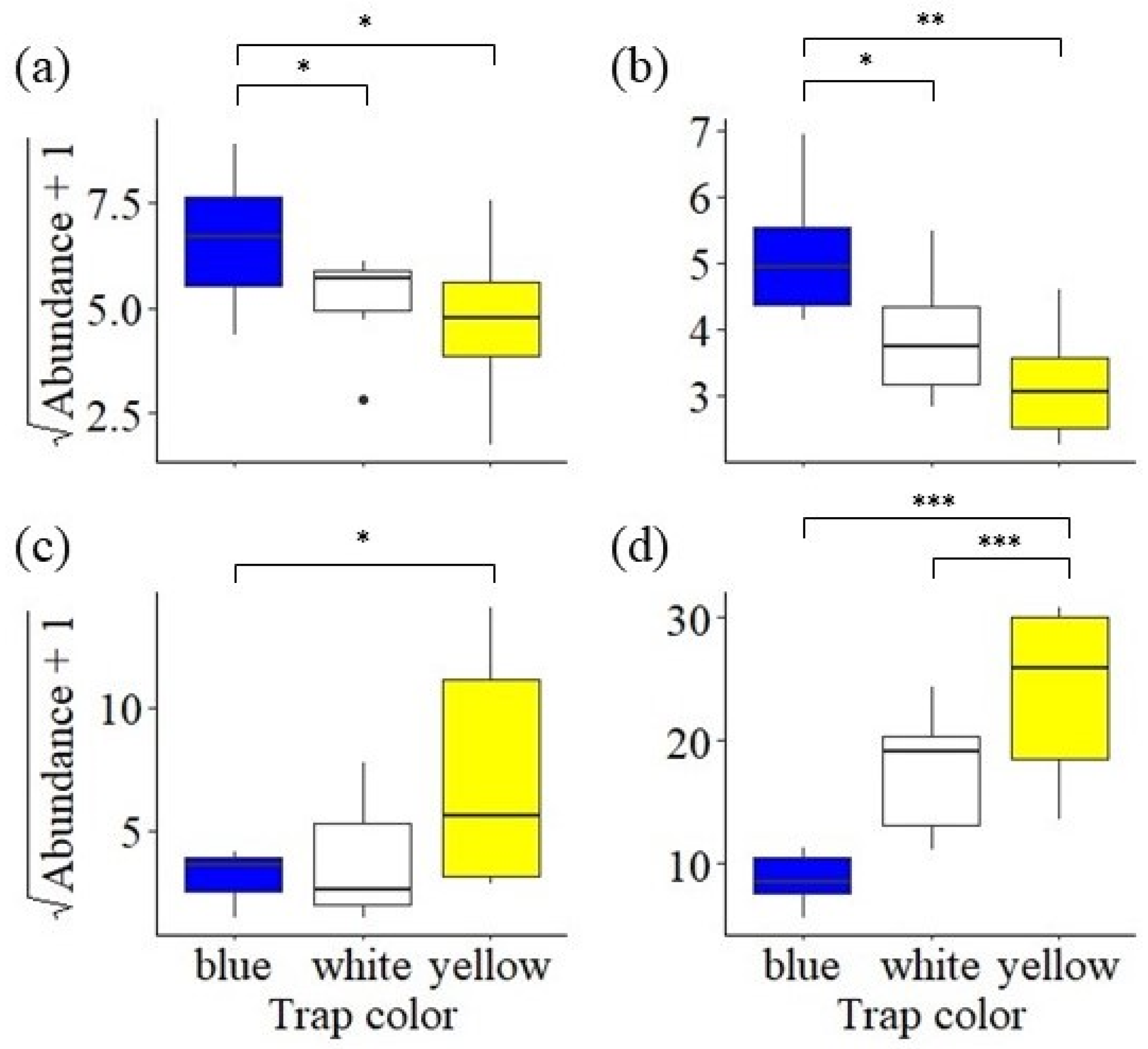
3. Results
3.1. Family Abundance
3.2. Guild Abundance
3.3. Trap Color Preference
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFrederick, Q.S.; LeBuhn, G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol. Conserv. 2006, 129, 372–381. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Fairbrass, A.J.; Falk, S.J.; Hale, J.D.; Matthews, T.J. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 2011, 6, e23459. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Potter, D.A.; Braman, S.K. Ecology and management of turfgrass insects. Annu. Rev. Entomol. 1991, 36, 383–406. [Google Scholar] [CrossRef]
- Reng-Moss, T.; Baxendale, F.; Riordan, T. Beneficial arthropods associated with buffalograss. J. Econ. Entomol. 1998, 91, 1167–1172. [Google Scholar] [CrossRef][Green Version]
- Norton, B.A.; Thomson, L.J.; Williams, N.S.; McDonnell, M.J. The effect of urban ground covers on arthropods: An experiment. Urban Ecosyst. 2014, 17, 77–99. [Google Scholar] [CrossRef]
- Francoeur, X.W.; Dagenais, D.; Paquette, A.; Dupras, J.; Messier, C. Complexifying the urban lawn improves heat mitigation and arthropod biodiversity. Urban For. Urban Green. 2021, 60, 127007. [Google Scholar] [CrossRef]
- Lyman, G.T.; Throssell, C.S.; Johnson, M.E.; Stacey, G.A.; Brown, C.D. Golf course profile describes turfgrass, landscape, and environmental stewardship features. Appl. Turfgrass Sci. 2007, 4, 1–25. [Google Scholar] [CrossRef]
- Dobbs, E.K.; Potter, D.A. Forging natural links with golf courses for pollinator-related conservation, outreach, teaching, and research. Am. Entomol. 2015, 61, 116–123. [Google Scholar] [CrossRef][Green Version]
- Norton, B.A.; Bending, G.D.; Clark, R.; Corstanje, R.; Dunnett, N.; Evans, K.L.; Grafius, D.R.; Gravestock, E.; Grice, S.M.; Harris, J.A. Urban meadows as an alternative to short mown grassland: Effects of composition and height on biodiversity. Ecol. Appl. 2019, 29, e01946. [Google Scholar] [CrossRef]
- Frank, S.D.; Shrewsbury, P.M. Effect of conservation strips on the abundance and distribution of natural enemies and predation of Agrotis ipsilon (Lepidoptera: Noctuidae) on golf course fairways. Environ. Entomol. 2004, 33, 1662–1672. [Google Scholar] [CrossRef]
- Baldock, K.C. Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 2020, 38, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ganser, D.; Albrecht, M.; Knop, E. Wildflower strips enhance wild bee reproductive success. J. Appl. Ecol. 2021, 58, 486–495. [Google Scholar] [CrossRef]
- Dobbs, E.K.; Potter, D.A. Naturalized habitat on golf courses: Source or sink for natural enemies and conservation biological control? Urban Ecosyst. 2016, 19, 899–914. [Google Scholar] [CrossRef]
- Dale, A.G.; Perry, R.L.; Cope, G.C.; Benda, N. Floral abundance and richness drive beneficial arthropod conservation and biological control on golf courses. Urban Ecosyst. 2020, 23, 55–66. [Google Scholar]
- Braman, S.K.; Pendley, A.F.; Corley, W. Influence of commercially available wildflower mixes on beneficial arthropod abundance and predation in turfgrass. Environ. Entomol. 2002, 31, 564–572. [Google Scholar] [CrossRef]
- Frank, T.; Kehrli, P.; Germann, C. Density and nutritional condition of carabid beetles in wildflower areas of different age. Agric. Ecosyst. Environ. 2007, 120, 377–383. [Google Scholar] [CrossRef]
- Billeisen, T.L.; Kilpatrick, L.D.; Seth-Carley, D.; Brandenburg, R.L. Presence of pollinator-friendly habitat on pollinator communities in managed turfgrass systems. Int. Turfgrass Soc. Res. J. 2022, 14, 295–303. [Google Scholar] [CrossRef]
- Albrecht, M.; Knecht, A.; Riesen, M.; Rutz, T.; Ganser, D. Time since establishment drives bee and hoverfly diversity, abundance of crop-pollinating bees and aphidophagous hoverflies in perennial wildflower strips. Basic Appl. Ecol. 2021, 57, 102–114. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Succession of bee communities on fallows. Ecography 2001, 24, 83–93. [Google Scholar] [CrossRef]
- Campbell, J.W.; Milne, M.; Dinh, B.T.; Daniels, J.C.; Ellis, J.D. Spider (Araneae) abundance and species richness comparison between native wildflower plantings and fallow controls in intensively managed agricultural areas. Arthropod-Plant Interact. 2020, 14, 263–274. [Google Scholar] [CrossRef]
- Frank, T.; Aeschbacher, S.; Zaller, J.G. Habitat age affects beetle diversity in wildflower areas. Agric. Ecosyst. Environ. 2012, 152, 21–26. [Google Scholar] [CrossRef]
- Noordijk, J.; Musters, C.; van Dijk, J.; de Snoo, G.R. Invertebrates in field margins: Taxonomic group diversity and functional group abundance in relation to age. Biodivers. Conserv. 2010, 19, 3255–3268. [Google Scholar] [CrossRef]
- Denys, C.; Tscharntke, T. Plant-insect communities and predator-prey ratios in field margin strips, adjacent crop fields, and fallows. Oecologia 2002, 130, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S. Insects: Their Natural History and Diversity: With a Photographic Guide to Insects of Eastern North America; Firefly Books: Richmond Hill, ON, USA, 2006. [Google Scholar]
- Discover Life. Available online: https://www.discoverlife.org/ (accessed on 4 February 2023).
- BugGuide.Net. Available online: https://bugguide.net/node/view/15740 (accessed on 4 February 2023).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R. emmeans: Estimated M arginal Means, Aka Least-Squares Means, R Package Version 1.8.0. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 June 2024).
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- Frank, T.; Künzle, I. Effect of early succession in wildflower areas on bug assemblages (Insecta: Heteroptera). Eur. J. Entomol. 2006, 103, 61–70. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Myers, M.C.; Hoksch, B.J.; Mason, J.T. Butterfly response to floral resources during early establishment at a heterogeneous prairie biomass production site in Iowa, USA. J. Insect Conserv. 2012, 16, 457–472. [Google Scholar] [CrossRef]
- Stein, D.S.; Debinski, D.M.; Pleasants, J.M.; Toth, A.L. Evaluating native bee communities and nutrition in managed grasslands. Environ. Entomol. 2020, 49, 717–725. [Google Scholar] [CrossRef]
- Eccard, J.A. Can rolling composite wildflower blocks increase biodiversity in agricultural landscapes better than wildflowers strips? J. Appl. Ecol. 2022, 59, 1172–1177. [Google Scholar] [CrossRef]
- Tscharntke, T.; Karp, D.S.; Chaplin-Kramer, R.; Batáry, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—Five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. USA 2013, 110, 5534–5539. [Google Scholar] [CrossRef]
- Tashiro, H.; Mitchell, W.C. Biology of the fiery skipper, Hylephila phyleus (Lepidoptera: Hesperiidae), a turfgrass pest in Hawaii. Proc. Hawaiian Entomol. Soc. 1985, 25, 131–138. [Google Scholar]
- Ainslie, G.G. The corn leaf-tier, Lerema accius S. & A. Fla. Entomol. 1922, 6, 1–14. [Google Scholar]
- Majewska, A.A.; Altizer, S. Planting gardens to support insect pollinators. Conserv. Biol. 2020, 34, 15–25. [Google Scholar] [CrossRef]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullman, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.R.; Isaacs, R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl. Ecol. 2014, 15, 701–711. [Google Scholar] [CrossRef]
- Ogilvie, J.E.; Griffin, S.R.; Gezon, Z.J.; Inouye, B.D.; Underwood, N.; Inouye, D.W.; Irwin, R.E. Interannual bumble bee abundance is driven by indirect climate effects on floral resource phenology. Ecol. Lett. 2017, 20, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, A.L.; Youngsteadt, E.; Frank, S.D. Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst. 2018, 21, 419–428. [Google Scholar] [CrossRef]
- Boyer, K.J.; Fragoso, F.P.; Dieterich Mabin, M.E.; Brunet, J. Netting and pan traps fail to identify the pollinator guild of an agricultural crop. Sci. Rep. 2020, 10, 13819. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, L.; Berglund, H.-L.; Jonason, D.; Milberg, P. Color pan traps often catch less when there are more flowers around. Ecol. Evol. 2020, 11, 3830–3840. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.W.; Hanula, J.L. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested systems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Geroff, R.K.; Gibbs, J.; McCravy, K.W. Assessing bee (Hymenoptera: Apoidea) diversity of an Illinois restored tallgrass prairie: Methodology and conservation considerations. J. Insect Conserv. 2014, 18, 951–964. [Google Scholar] [CrossRef]
- Gollan, J.R.; Ashcroft, M.B.; Batley, M. Comparison of yellow and white pan traps in surveys of bee fauna in New South Wales, Australia (Hymenoptera: Apoidea: Anthophila). Aust. J. Entomol. 2010, 50, 174–178. [Google Scholar] [CrossRef]
- Ramírez-Freire, L.; Alaní-Flores, G.J.; Ayala-Barajas, R.; Quiroz-Martínez, H.; Velazco-Macías, C.G. Bees of the genus Agapostemon (Hymenoptera: Halictidae) of the state of Nuevo León, Mexico. Rev. Mex. Biodivers. 2020, 83, 63–72. [Google Scholar]
- Moreira, E.F.; Santos, R.L.d.; Penna, U.L.; Angel-Coca, C.; Freitas de Oliveira, F.; Viana, B.F. Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Steudel, B.; Kessler, M. Sampling Hymenoptera along a precipitation gradient in tropical forests: The effectiveness of different coloured pan tramps. Entomol. Exp. Appl. 2010, 137, 262–268. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wratten, S.D.; Sedcole, J.R. The role of odour and visual cues in then pan-trap catching of hoverflies (Diptera: Syrphidae). Ann. Appl. Biol. 2006, 148, 173–178. [Google Scholar] [CrossRef]
- Campbell, A.J.; Biesmeijer, J.C.; Varma, V.; Wäckers, F.L. Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic Appl. Ecol. 2012, 13, 363–370. [Google Scholar] [CrossRef]
- Saarikivi, J.; Idström, L.; Venn, S.; Niemelä, J.; Kotze, D.J. Carabid beetle assemblages associated with urban golf courses in the greater Helsinki area. Eur. J. Entomol. 2010, 107, 553–561. [Google Scholar] [CrossRef]
- Braman, K.S.; Pendley, R.F. Relative and seasonal abundance of beneficial arthropods in centipede grass as influenced by management practices. J. Econ. Entomol. 1993, 86, 494–504. [Google Scholar] [CrossRef]
- Gelernter, W.D.; Stowell, L.J.; Johnson, M.E.; Brown, C.D. Documenting trends in land-use characteristics and environmental stewardship programs on US golf courses. Crop Forage Turfgrass Manag. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Terando, A.J.; Costanza, J.; Belyea, C.; Dunn, R.R.; McKerrow, A.; Collazo, J.A. The southern megalopolis: Using the past to predict the future of urban sprawl in the Southeast US. PLoS ONE 2014, 9, e102261. [Google Scholar] [CrossRef]
| Grouping | Year | Julian Day | Year * Julian Day | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Χ2 Statistic | df | p-Value | Χ2 Statistic | df | p-Value | Χ2 Statistic | df | p-Value | |
| Family | Apidae | 0.44 | 2 | 0.80 | 6.24 | 1 | 0.01 * | 0.71 | 2 | 0.70 |
| Calliphoridae | 6.63 | 2 | 0.04 * | 0.00 | 1 | 0.95 | 1.55 | 2 | 0.46 | |
| Chloropidae | 4.68 | 2 | 0.10 | 1.92 | 1 | 0.17 | 4.59 | 2 | 0.10 | |
| Cicadellidae | 11.26 | 2 | <0.01 ** | 0.14 | 1 | 0.71 | 3.08 | 2 | 0.21 | |
| Crabronidae | 0.38 | 2 | 0.83 | 1.30 | 1 | 0.25 | 3.86 | 2 | 0.14 | |
| Dolichopodidae | 6.18 | 2 | <0.05 * | 2.73 | 1 | 0.10 | 10.41 | 2 | <0.01 ** | |
| Formicidae | 8.40 | 2 | 0.02 * | 3.76 | 1 | 0.05 | 9.41 | 2 | <0.01 ** | |
| Halictidae | 16.43 | 2 | <0.001 *** | 2.59 | 1 | 0.11 | 1.22 | 2 | 0.54 | |
| Hesperiidae | 33.61 | 2 | <0.001 *** | 20.68 | 1 | <0.001 *** | 4.52 | 2 | 0.10 | |
| Ichneumonidae | 29.68 | 2 | <0.001 *** | 25.93 | 1 | <0.001 *** | 1.06 | 2 | 0.69 | |
| Miridae | 5.65 | 2 | 0.06 | 2.41 | 1 | 0.12 | 5.87 | 2 | 0.05 | |
| Sarcophagidae | 23.93 | 2 | <0.001 *** | 1.26 | 1 | 0.26 | 0.02 | 2 | 0.99 | |
| Syrphidae | 7.35 | 2 | 0.03 * | 40.98 | 1 | <0.001 *** | 11.15 | 2 | <0.01 ** | |
| Guild | Wasps | 8.62 | 2 | 0.01 * | 4.11 | 1 | 0.04 | 12.22 | 2 | <0.01 ** |
| Predatory flies | 59.48 | 2 | <0.001 *** | 1.74 | 1 | 0.19 | 3.00 | 2 | 0.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamon, L.E.; Kilpatrick, L.D.; Billeisen, T.L. The Impact of Wildflower Habitat on Insect Functional Group Abundance in Turfgrass Systems. Insects 2024, 15, 520. https://doi.org/10.3390/insects15070520
Hamon LE, Kilpatrick LD, Billeisen TL. The Impact of Wildflower Habitat on Insect Functional Group Abundance in Turfgrass Systems. Insects. 2024; 15(7):520. https://doi.org/10.3390/insects15070520
Chicago/Turabian StyleHamon, Laura E., Lauren D. Kilpatrick, and Terri L. Billeisen. 2024. "The Impact of Wildflower Habitat on Insect Functional Group Abundance in Turfgrass Systems" Insects 15, no. 7: 520. https://doi.org/10.3390/insects15070520
APA StyleHamon, L. E., Kilpatrick, L. D., & Billeisen, T. L. (2024). The Impact of Wildflower Habitat on Insect Functional Group Abundance in Turfgrass Systems. Insects, 15(7), 520. https://doi.org/10.3390/insects15070520






