Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae)
Abstract
Simple Summary
Abstract
1. Introduction
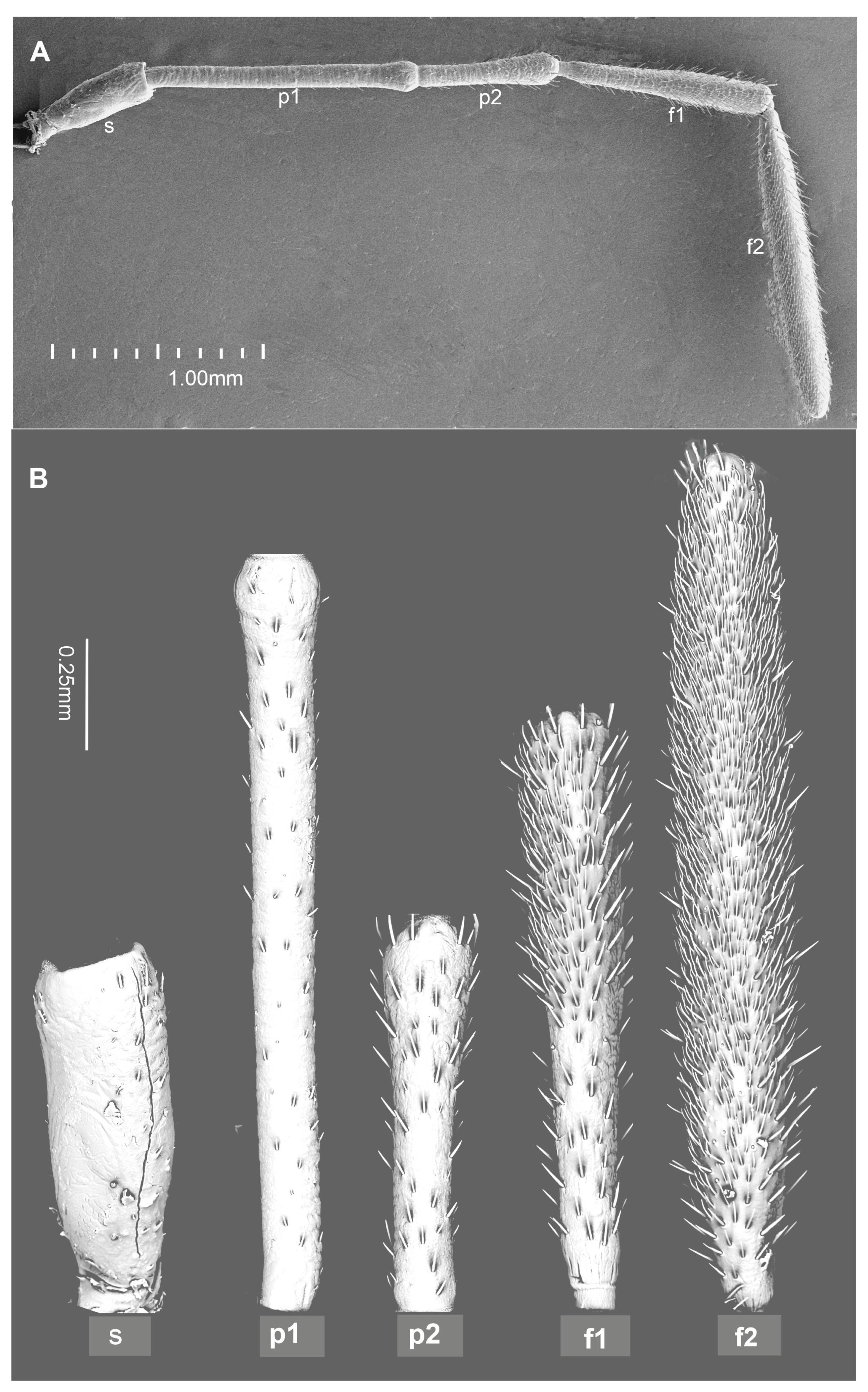
2. Materials and Methods
3. Results
3.1. Categories of the Sensilla
3.1.1. Olfactory Sensilla
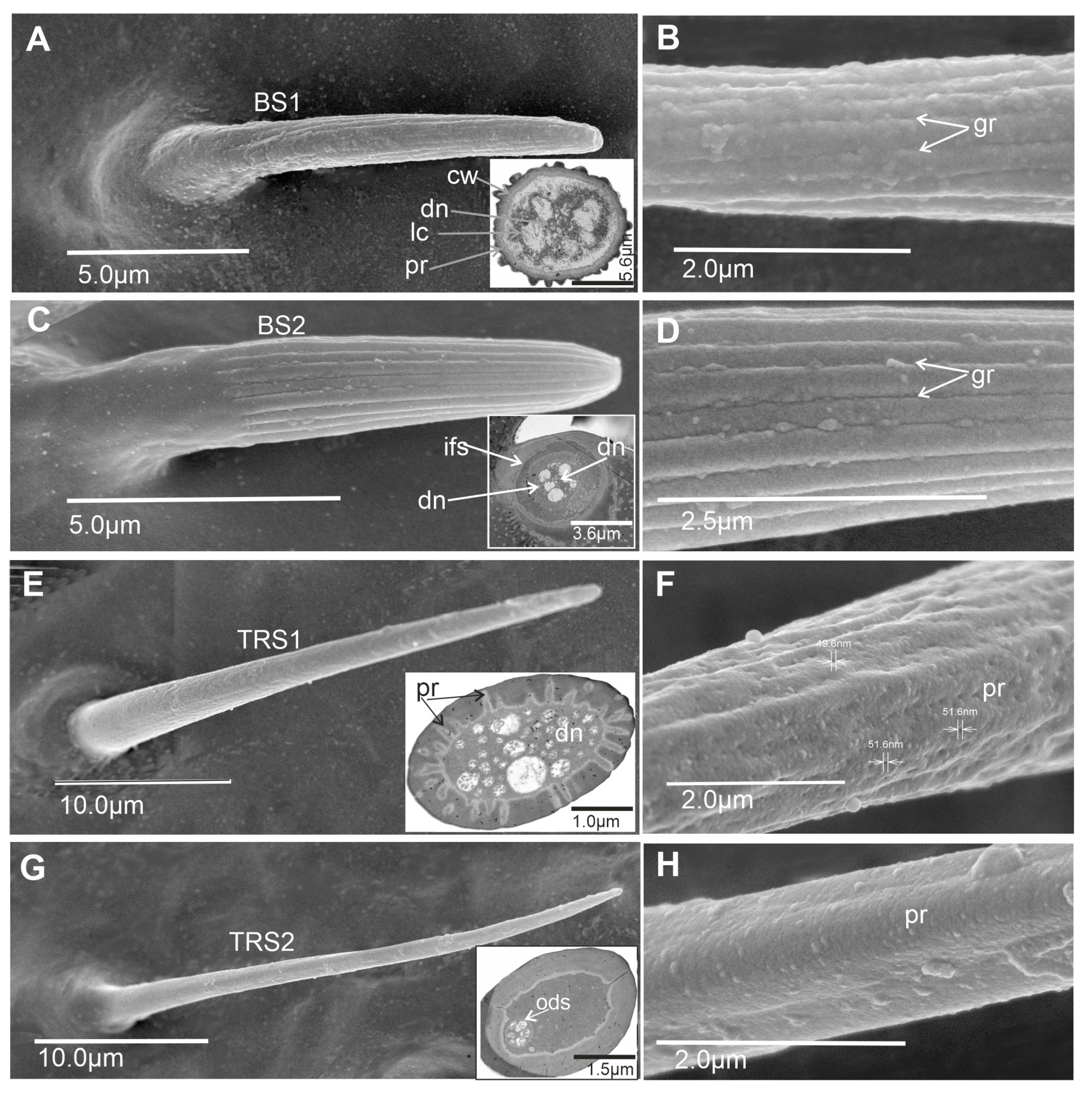
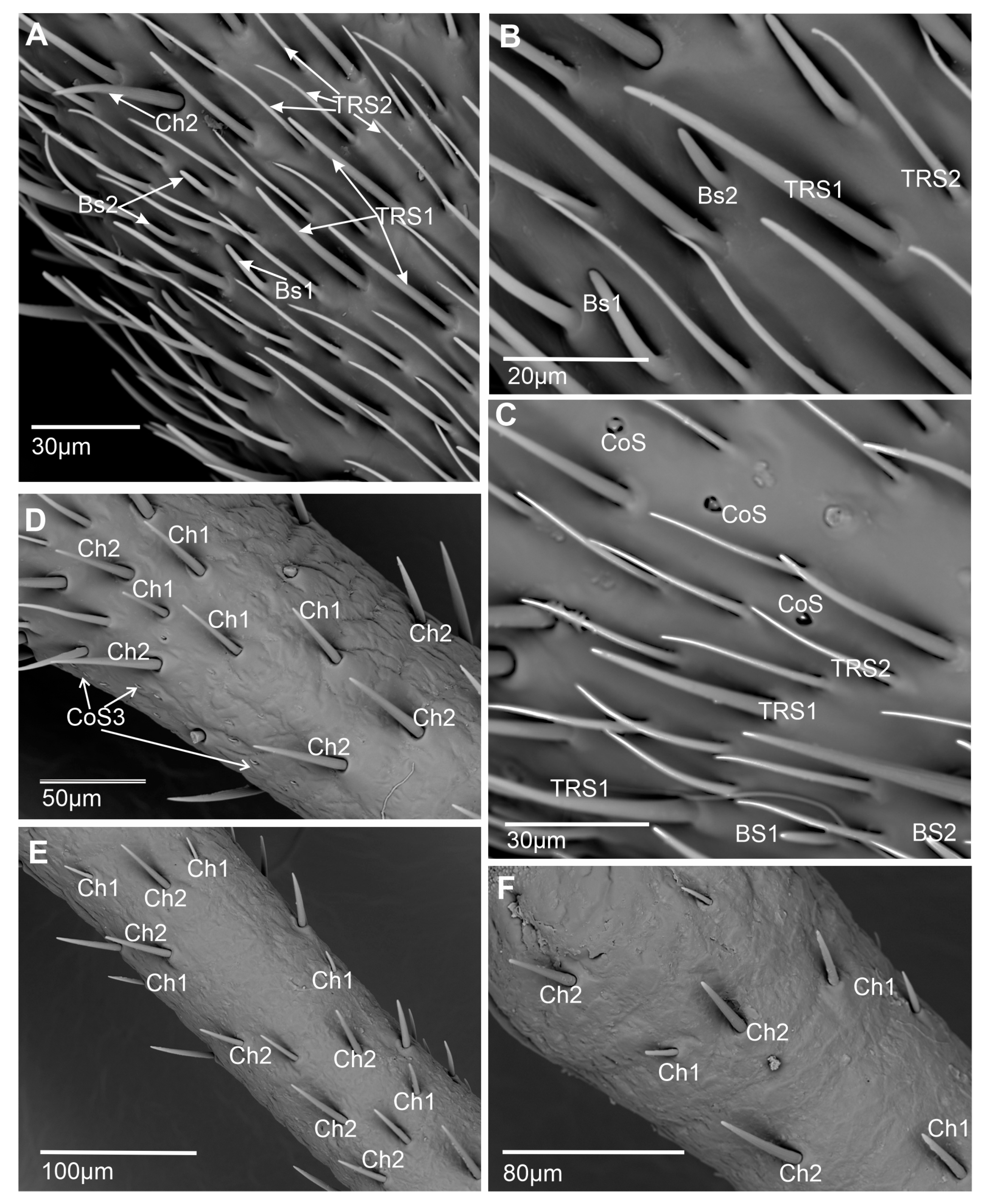
- Basiconic sensillum (BS1) is scattered on the first and second flagellomeres and frequently observed in the imaging area (Figure 5A–C). This cone-like sensillum is grooved and has a porous wall. The smooth proximal part is embedded in an inflexible socket (Figure 2A,B), and this sensillum is recognized as longer (L = 11.2–14.2 µm) than BS2 (Table 1). The sensillum’s stem is wide, stiff, and rounded at the end. Additionally, the sensillum ultra-section indicated groups of dendrites in the lymph cavity (Lc) inside the sensillum, confirming its olfactory function.
- Basiconic sensillum (BS2) is distributed randomly in the first and second flagellomeres, with only several such sensillum observed (Figure 5A–C). The sensillum’s stem is wide, stiff, and rounded at the end, and it is embedded in inflexible sockets on the cuticle surface. BS2 is recognized as a short sensillum (L = 7.4–8.41 µm) with a grooved and multiporous wall (Figure 2C,D). These structures are present on the non-proximal area of the sensilla’s cuticle (Figure 2C). The ultra-section at the base of the sensillum indicated numerous dendrites (dn) (about 37) and documented its olfactory function.
- Trichoid sensillum (TRS1) has a round base and a long cylindrical cuticular multiporous shaft tapered apically into a sharp tip (Figure 2E,F). It is classified as a shorter sensillum (Table 1) than TRS2. Pores about 50 nm in diameter are densely distributed along the entire length of the sensillum. The base is embedded in an inflexible socket. The cross-section shows pores in the wall and numerous dendrites inside the lumen cavity of the sensillum, confirming its olfactory function. This sensillum is numerous on the first and second flagellomeres (Figure 5A–C).
- Trichoid sensillum (TRS2) has a round base and a long, thin, cylindrical cuticular multiporous shaft tapered apically into a sharp tip (Figure 2G,H). It is classified as a longer sensillum (Table 1) than TRS1. The base is embedded in an inflexible socket. The cross-section shows a few pores on the wall and several dendrites (at least five). This sensillum is numerous and distributed throughout the first and second flagellomeres (Figure 5A–C).
3.1.2. Thermo–Hygroreceptive Sensilla
- Coeloconic sensillum (CoS1) has only a few irregular cavities in several numbers (Figure 3A) in the middle and distal flagellum in both sexes. The short peg-like sensillum is embedded in an inflexible socket in a shallow, oval singular cavity (Figure 3B). The proximal part is wider than the distal, with a narrow, rounded end. The wall pores are invisible, so this sensillum is treated as non-porous. We could only identify a slightly invaginated pore (molting pore) at its apical tip. The cross-section below the cuticle surface shows the presence of the three dendrites; however, two dendrites (no. 1 and 2) are surrounded by an outer dendritic sheath (ods), similar to the third dendrite (no. 3) (Figure 3C). The composition of the three dendrites is visible in the cross-section at the base of the sensillum. Additionally, the microvilli (mr) are also observed. The number and arrangement of the three dendrites can suggest a thermo–hygroreceptive function of the sensillum.
- Coeloconic sensillum (CoS2) is present in several numbers in the middle and distal flagellums (Figure 3D). The short peg-like sensillum is probably embedded in an inflexible socket in a shallow cavity of two chambers (Figure 3D). The proximal part is perhaps broader than the distal, with a blunt end and slight protrusion. The surface of the peg is smooth and shows no indications of wall pores except the slightly invaginated molting pore at the apical tip (Figure 3E). The ultra-section at the base of the peg shows the presence of the three dendrites; however, two dendrites (no. 1 and 2) (Figure 3E) are surrounded separately by a dendritic sheath from the third dendrite (no. 3). Dendrite no. 3 probably terminates at the base of the sensillum, while the other two dendrites probably extend into the lumen of the peg to its distal end. The single dendrite at the base is likely responsible for thermoreception, while the other two dendrites are responsible for hygroreception.
- Coeloconic sensillum (CoS3) is a cone-like sensillum with a profoundly grooved, porous wall. The base of the sensillum is probably embedded in an inflexible socket in a deeper and narrower cavity than CoS1 and CoS2 (Figure 3G). The stem slightly protrudes from the cavity. Numerous sensilla of this type were observed on the lateral side of the basiflagellum (Figure 3F). The ultrastructure of the peg shows the presence of multiple dendrites (Figure 3H,I), suggesting their olfactory function.
3.1.3. Mechanoreceptive Sensilla
- Chaetic sensillum (Ch) belongs to the group of mechanosensilla, primarily distinguished by their length (Ch1 = 14.3–20.5; Ch2 = 28.4–36.4; Ch3 = 50.2–60.0; Ch4 = 66.5–73.0) (Figure 4A) (Table 1). These sensilla are stout bristles connected to the cuticular surface by a socket equipped with a flexible external membrane (mb), allowing possible deformations of the sensilla (Figure 4B). Mechanoreceptive chaetic sensilla are straight in shape, broad at the basal part, and narrow at the distal part. The external surface of the stem is grooved, but the pattern differs from the wall grooves of the chemosensilla. These sensilla are positioned on the antennal surface at a larger angle (about 45°) than basiconic and trichoid sensilla, making them visible as they stick out (Figure 1B and Figure 4A).
- Campaniform sensillum (CaS) is a dome and oval-shaped structure with a single pore in the middle (Figure 4F) and embedded in sockets with a flexible membrane (sm) (Figure 4G). The campaniform sensillum has an ultrastructure similar to the chaetic sensillum. Dendrites in the distal outer segment are encased by a dendrite sheath (ods) and form a tubular body (tb) (cytoskeletal complex structure) consisting of multiple tiny, tightly packed microtubules (Figure 4G). The tormogen (to) cell forms the large lymph cavity and numerous microvilli (mr), and the tubular body (tb) attaches to the center of the cap and terminates at its base. Several sensilla (2–4) are located in different places in each antennomere, functioning as proprioceptors responding to strains in the exoskeleton.
- Peg sensillum (PeS) is the conical-shaped stiff sensillum with a non-porous wall but a flexible socket, categorized as the proprioceptors that occur in the proximal section of the pedicel and are directed to control the position of the scapus (Figure 4H).
4. Discussion
4.1. Morphology and Ultrastructure of Basiconic and Trichoid Sensilla
4.2. Morphology and Ultrastructure of Coeloconic Sensilla
4.3. Mechanoreception (Exteroceptors and Proprioceptors)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panizzi, A.R.; Grazia, J. Introduction to True Bugs (Heteroptera) of the Neotropics. In True Bugs (Heteroptera) of the Neotropics; Entomology in Focus, 2; Panizzi, A.R., Grazia, J., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2015; pp. 3–14. [Google Scholar] [CrossRef]
- Panizzi, A.R. Stink Bugs (Hemiptera: Pentatomidae), Emphasizing Economic Importance. In Encyclopedia of Entomology, 1st ed.; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 2120–2122. [Google Scholar]
- Schuh, R.T.; Slater, J.A. Classification and Natural History. In True Bugs of the World (Hemiptera: Heteroptera); Cornell University Press: Ithaca, NY, USA, 1995; p. 338. [Google Scholar]
- Rider, D.A. Pentatomidae. In Catalogue of the Heteroptera of the Palaearctic Region; Aukema, B., Rieger, C., Eds.; Netherlands Entomological Society: Amsterdam, The Netherlands, 2006; Volume 5, pp. 233–402. [Google Scholar]
- Lupoli, R. Graphosoma lineatum (L., 1758) et G. italicum (O.F. Müller, 1766), deux espèces valides et distinctes, probablement issues de la transgression zancléenne méditerranéenne (Hemiptera Pentatomidae). L’Entomologiste 2017, 73, 19–33. [Google Scholar]
- Krall, B.S.; Bartelt, R.J.; Lewis, C.J.; Whitman, D.W. Chemical defense in the stink bug Cosmopepla bimaculata. J. Chem. Ecol. 1999, 25, 2477–2494. [Google Scholar] [CrossRef]
- Sillén-Tullberg, B.; Leimar, O. The evolution of gregariousness in distasteful insects as a defense against predators. Am. Nat. 1988, 132, 723–734. [Google Scholar] [CrossRef]
- Ryan, M.F. Insect Chemoreception Fundamental and Applied; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002; pp. 113–125. [Google Scholar]
- Gonzaga-Segura, J.; Valdéz-Carrasco, J.; Castrejón-Gómez, V.R. Sense organs on the antennal fagellum of Leptoglossus zonatus (Heteroptera: Coreidae). Ann. Entomol. Soc. Am. 2013, 106, 510–517. [Google Scholar] [CrossRef]
- Taszakowski, A.; Masłowski, A.; Daane Kent, M.; Brożek, J. Closer view of antennal sensory organs of two Leptoglossus species (Insecta, Hemiptera, Coreidae). Sci. Rep. 2023, 13, 617. [Google Scholar] [CrossRef]
- Chapman, R.F. Mechanoreception. Chemoreception. In The Insects, Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 610–652. [Google Scholar]
- Steinbrecht, R.A. On the question of nervous syncytia: Lack of axon fusion in two insect sensory nerves. J. Cell Sci. 1969, 4, 39–53. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticle mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.Y., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 1–69. [Google Scholar]
- Keil, T.A. Morphology and development of the peripheral olfactory organs. In Insect Olfaction; Hansson, B.S., Ed.; Springer: New York, NY, USA, 1999; pp. 5–47. [Google Scholar]
- Vosshall, L.B. Olfaction in Drosophila. Curr. Opin. Neurobiol. 2000, 10, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Diongue, A.; Yang, J.T.; Lai, P.Y. Biomorphometric characteristics of different types of sensilla detected on the antenna of Helicoverpa armigera by scanning electron microscopy. J. Asia-Pac. Entomol. 2013, 16, 23–28. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. The fine structure of olfactory sensilla in the silk moth (Insecta, Lepidoptera). Receptor processes and stimulus conduction apparatus. Appar. Z. Zellforsch. Mikrosk. Anat. 1973, 139, 533–565. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Functional morphology of pheromone-sensitive sensilla. In Pheromone Biochemistry; Prestwich, G.D., Blomquist, G.J., Eds.; Academic Press: New York, NY, USA, 1987; pp. 353–384. [Google Scholar]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Suwannapong, G.; Benbow, M.E. The Biology of Insect Odors: Sources and Olfaction, chapter V. In The Biology of Odors; Weiss, L.E., Atwood, J.M., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 166–175. [Google Scholar]
- Hartenstein, V. Development of Insect Sensilla. In Comprehensive Molecular Insect Science; Lawrence, I., Gilbert, L.I., Gill, K.S., Eds.; Elsevier Pergamon: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany; London, UK; New York, NY, USA; Oxford, UK; Paris, France; San Diego, CA, USA; San Francisco, CA, USA; Singapore; Sydney, Australia; Tokyo, Japan, 2005; pp. 384–386. [Google Scholar]
- Keil, T.A. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Keil, T.A.; Steinbrecht, R.A. Mechanosensitive and olfactory sensilla of insects. In Insect Ultrastructure, 2nd ed.; King, R.C., Akai, H., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 477–516. [Google Scholar]
- McIver, S.B. Mechanoreception. In Comprehensive Insect Physiology Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 71–132. [Google Scholar]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo- and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 6, 69–139. [Google Scholar]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Rani, U.P.; Madhavendra, S.S. External morphology of antennal and rostral sensilla in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Brézot, P.; Tauban, D.; Renou, M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensillum types and numerical growth during the post-embryonic development. Int. J. Insect Morphol. Embryol. 1996, 25, 427–441. [Google Scholar] [CrossRef]
- Sinitsina, E.E.; Krutov, V.V. Antennal and labial sense organs in a bug Podizus maculiventris (Hemiptera, Pentatomidae). Zool. Zhurnal. 1996, 75, 1172–1173. [Google Scholar]
- Silva, C.C.A.; de Capdeville, G.; Moraes, M.C.B.; Falcão, R.; Solino, L.F.; Laumann, R.A.; Silva, J.P.; Borges, M. Morphology, distribution and abundance of antennal sensilla in three stink bug species (Hemiptera: Pentatomidae). Micron 2010, 41, 289–300. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.J.; Liu, C.Y.; Meng, L.P.; Zhou, Y.L. Fine structure and distribution of antennal sensilla of stink bug Arma chinensis (Heteroptera: Pentatomidae). Entomol. Fenn. 2014, 25, 186–198. [Google Scholar] [CrossRef]
- Ahmad, A.; Parveen, S.; Brożek, J.; Dey, D. Antennal sensilla of phytophagous and predatory pentatomids (Hemiptera: Pentatomidae): A comparative study of four genera. Zool. Anz. 2016, 261, 48–55. [Google Scholar] [CrossRef]
- Li, X.; Tian, L.; Li, H.; Cai, W. Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae). Biology 2021, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Giovannini, I.; Anfora, G.; Rossi Stacconi, M.V.; Malek, R.; Maistrello, L.; Guidetti, R.; Romani, R. A closer look at the antennae of the invasive Halyomorpha halys: Fine structure of the sensilla. Bull. Insectol. 2019, 72, 187–199. [Google Scholar]
- Romani, R.; Stacconi, M.V.R. Mapping and ultrastructure of antennal chemosensilla of the wheat bug Eurygaster maura. Insect Sci. 2009, 16, 193–203. [Google Scholar] [CrossRef]
- Shields, V.D. High resolution ultrastructural investigation of insect sensory organs using feld emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Steinbrecht, R.A. Pore structures in insect olfactory sensilla: A review of data and concepts. Int. J. Insect Morphol. Embryol. 1997, 26, 229–245. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.C.; Roh, H.S.; Kim, J.; Oh, H.W.; Kim, J.A.; Park, C.G. Morphology and distribution of antennal sensilla of the bean bug Riptortus pedestris (Hemiptera: Alydidae). Microsc. Res. Tech. 2016, 79, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; McBridec, C.S. Evolution of olfactory circuits in insects. J. Comp. Physiol. A 2020, 206, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, K.E. Responses of Insect Olfactory Neurons to Single; Pheromone Molecules, chapter 1. In Olfactory Concepts of Insect Control—Alternative to Insecticides; Picimbon, J.-F., Ed.; Springer Nature: Cham, Switzerland, 2019; Volume 2, p. 29. [Google Scholar]
- Menini, A. The Neurobiology of Olfaction; CRC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hansson, B.S. Insect Olfaction; Springer: Berlin, Germany, 2013. [Google Scholar]
- Hallem, E.A.; Ho, M.; Carlson, J.R. The molecular basis of odor coding in the Drosophila antenna. Cell 2004, 117, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375. [Google Scholar] [CrossRef]
- Backus, E.S. Sensory system and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Structure and function of insect olfactory sensilla. In Ciba Foundation Symposium 200—Olfaction in Mosquito Host Interactions; Bock, G.R., Cardew, G., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 158–183. [Google Scholar] [CrossRef]
- Altner, H. Insect sensillum specificity and structure: An approach to a new typology. In Olfaction and Taste; Le Magnen, J., Macleod, P., Eds.; Information Retrieval: London, UK, 1977; Volume 6, pp. 295–303. [Google Scholar]
- Isidoro, N.; Romani, R.; Bin, F. Antennal multiporous sensilla: Their gustatory features for host recognition in female parasitic wasps (Insecta, Hymenoptera: Platygastroidea). Microsc. Res. Tech. 2001, 55, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Yersen, L.W.; Ball, H.J. Antennal hygroreceptors of the milkweed bug, Oncopeltus fasciatus (Dallas) (Hemiptera, Lygaeidae). Ann. Entomol. Soc. Am. 1959, 52, 279–284. [Google Scholar]
- Raman, K. Behavioural attributes of Oxycarenus laetus Kirby towards different malvaceous seeds. Phytophaga 1988, 2, 57–71. [Google Scholar]
- Ventura, M.U.; Panizzi, A.R. Morphology of olfactory sensilla and its role in host plant recognition by Neomegalotomus parvus (Westwood) (Heteroptera: Alydidae). Braz. Arch. Biol. Technol. 2005, 48, 589–597. [Google Scholar] [CrossRef]
- Xiao, Y.; Fadamiro, H.Y. Host preference and development of Leptoglossus zonatus (Hemiptera: Coreidae) on satsuma mandarin. J. Econ. Entomol. 2009, 102, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Nowińska, A.; Brożek, J. Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 2017, 136, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Sass, H.; Altner, I. Relationship between structure and function of antenna1 chemo-, hygro-, and thermoreceptive sensilla in Periplaneta americana. Cell Tissue Res. 1977, 176, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, E.; Johansson, K.U.I.; Elofsson, R. The aesthetasc concept: Structural variations of putative olfactory receptor cell complexes in Crustacea. Microsc. Res. Tech. 1992, 22, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Tichy, H. Hygro- and thermoreceptive triad in antennal sensillum of the stick insect, Carausius morosus. J. Comp. Physiol. 1979, 132, 149–152. [Google Scholar] [CrossRef]
- Altner, H.; Routil, C.; Loftus, R. The structure of bimodal chemoreceptive, thermoreceptive, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981, 215, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Yokohari, F. The coelocapitular sensillum, an antennal hygro- and thermoreceptive sensillum of the honey bee, Apis mellifera L. Cell Tissue Res. 1983, 233, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo-and hygroreceptors. Annu. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Altner, H.; Tichy, H.; Altner, I. Lamellated outer dendritic segments of a sensory cell within a poreless thermoreceptive and hygroreceptive sensillum of insect Carausius morosus. Cell Tissue Res. 1978, 191, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.S.; Kleineidam, C.J.; Leitinger, G.; Römer, H. Ultrastructure and electrophysiology of thermosensitive sensilla coeloconica in a tropical katydid of the genus Mecopoda (Orthoptera, Tettigoniidae). Arthropod Struct. Dev. 2018, 47, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Selzer, R.; Altner, H. Lamellated outer dendritic segments of a chemoreceptor within wall-pore sensilla in the labial palp pit organ of the butterfly, Pieris rapae L (Insecta, Lepidoptera). Cell Tissue Res. 1985, 240, 333–342. [Google Scholar] [CrossRef]
- Ruchty, M.; Romani, R.; Kuebler, L.S.; Ruschioni, S.; Roces, F.; Isidoro, N.; Kleineidam, C.J. The thermo-sensitive sensilla coeloconica of leaf-cutting ants (Atta vollenweideri) Arthropod Struct. Dev. 2009, 38, 195–205. [Google Scholar]
- Hansson, B.S.; Ochieng, S.A.; Grosmaitre, X.; Anton, S.; Njagi, P.G.N. Physiological responses and central nervous projections of antennal olfactory receptor neurons in the adult desert locust, Schistocerca gregaria (Orthoptera: Acrididae). J. Comp. Physiol. A 1996, 179, 157–167. [Google Scholar] [CrossRef]
- Shanbhag, S.R.; Singh, K.; Singh, R.N. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Res. 1995, 282, 237–249. [Google Scholar] [CrossRef] [PubMed]
- French, A.S. Transduction mechanisms of mechanosensilla. Annu. Rev. Entomol. 1988, 33, 39–58. [Google Scholar] [CrossRef]
- Guo, J.S.; Wang, X.Q.; Li, D.T.; Song, D.D.; Zhang, C.X. Three-dimensional architecture of a mechanoreceptor in the brown planthopper, Nilaparvata lugens, revealed by FIB-SEM. Cell Tissue Res. 2020, 379, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, H.; Zhang, Y.; Zhang, X. Morphology and distribution of antennal, maxillary palp and labial palp sensilla of the adult bruchid beetles, Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Entomol. Res. 2018, 48, 466–479. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, N.; Brożek, J.; Dai, W. Antennal morphology and sexual dimorphism of antennal sensilla in Callitettix versicolor (Fabricius) (Hemiptera: Cercopidae). Insects 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Chakilam, S.; Brożek, J.; Chajec, Ł.; Poprawa, I.; Gaidys, R. Ultra-morphology and mechanical function of the trichoideum sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha). Insects 2022, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brożek, J.; Dai, W. Functional morphology and sexual dimorphism of antennae of the pear lace bug Stephanitis nashi (Hemiptera: Tingidae). Zool. Anz. 2020, 286, 11–19. [Google Scholar] [CrossRef]
- Catalá, S. Antennal sensilla of Triatominae (Hemiptera, Reduviidae): A comparative study of five genera. Int. J. Insect Morphol. Embryol. 1997, 26, 67–73. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 138, 307–319. [Google Scholar] [CrossRef]
- Sun, L.; Gao, Y.; He, J.; Cui, L.; Meissner, J.; Verbavatz, J.M.; Li, B.; Feng, X.; Liang, X. Ultrastructural organization of NompC in the mechanoreceptive organelle of Drosophila campaniform mechanoreceptors. Proc. Natl. Acad. Sci. USA 2019, 16, 7343–7352. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L. The fine structure of campaniform sensilla on the halteres of Drosophila melanogaster. J. Morphol. 1969, 128, 443–463. [Google Scholar] [CrossRef]
- Pringle, J.W.S. Proprioception in Insects: II. The Action of the Campaniform Sensilla on the Legs. J. Exp. Biol. 1938, 15, 114–131. [Google Scholar] [CrossRef]


| Type of Sensillum | Subtypes of Sensilla | Range of Length (µm) | Wall | Tip | Socket | Distribution |
|---|---|---|---|---|---|---|
| Basiconic sensilla | BS1 BS2 | 11.2–14.2 7.4–8.4 | Groves and porous Deeply grooves and porous | Rounded | Inflexible | Middle and distiflagellum |
| Trichoid sensilla | TRS1 TRS2 | 27.0–29.1 30.0–33.2 | Porous Porous | Acute | Inflexible | Middle and distiflagellum |
| Coeloconic sensilla | CoS1 CoS2 CoS3 | - - - | No porous No porous Groves and porous | Blunted | Inflexible | Middle and distiflagellum |
| Chaetic sensilla | Ch1 Ch2 Ch3 Ch4 | 14.3–20.5 28.4–36.4 50.2–60.0 66.5–73.0 | Groves and no porous | Sharp | Flexible | On each antennomeres, but Ch3 and Ch4 are more numerous on the two last antennomeres |
| Campaniform sensillum | CaS | Molting pore | Cupola | Flexible | Several on each antennomeres | |
| Peg sensillum | PeS | No porous | Rounded | Flexible | Proximal edge of the pedicel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brożek, J.; Poprawa, I.; Wegierek, P.; Stroiński, A. Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae). Insects 2024, 15, 528. https://doi.org/10.3390/insects15070528
Brożek J, Poprawa I, Wegierek P, Stroiński A. Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae). Insects. 2024; 15(7):528. https://doi.org/10.3390/insects15070528
Chicago/Turabian StyleBrożek, Jolanta, Izabela Poprawa, Piotr Wegierek, and Adam Stroiński. 2024. "Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae)" Insects 15, no. 7: 528. https://doi.org/10.3390/insects15070528
APA StyleBrożek, J., Poprawa, I., Wegierek, P., & Stroiński, A. (2024). Functional Morphology and Ultrastructure of the Peripheral Antennal Sensillar System of Graphosoma italicum (Müller, 1766) (Insecta: Hemiptera: Pentatomidae). Insects, 15(7), 528. https://doi.org/10.3390/insects15070528







