Resource Utilization of Residual Organic Sludge Generated from Bioenergy Facilities Using Hermetia illucens Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. H. illucens Larvae
2.2. Moisture Adjustment of BF-rOS and Preparation of BF-rOS/FW Mixture
2.3. BSFL Breeding
2.4. Analysis of Bioconversion Efficiency of BSFL
2.5. Analysis of Component Content of BSFL Frass
2.6. Analysis of Nutritional Compsition of BSFL
2.7. Analysis of Heavy Metals and Aflatoxins in BSFL
2.8. Evaluation of Cytotoxicity of BSFL
2.9. Statistical Analysis
3. Results
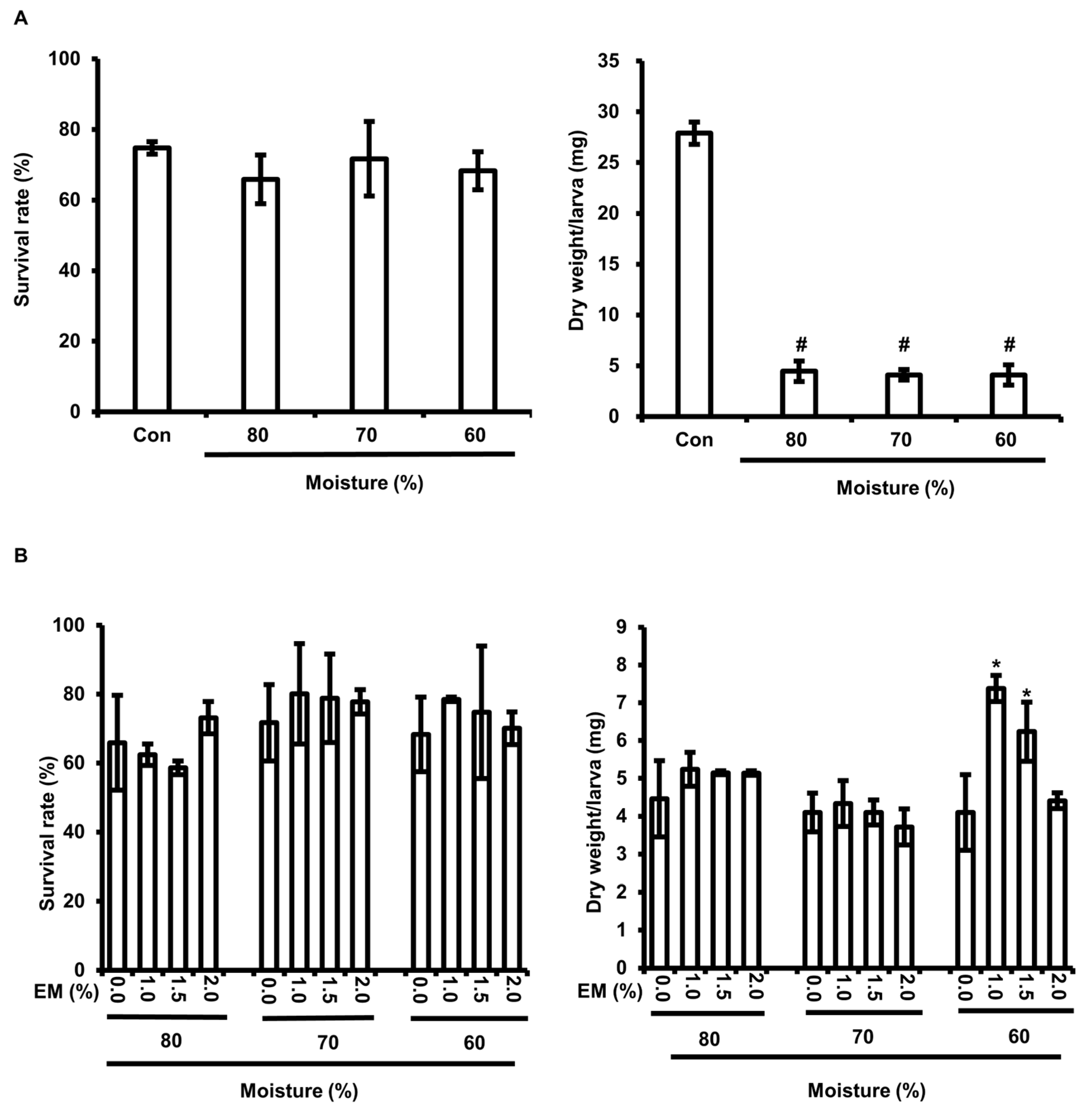
3.1. The Growth of BSFL When Fed BF-rOS
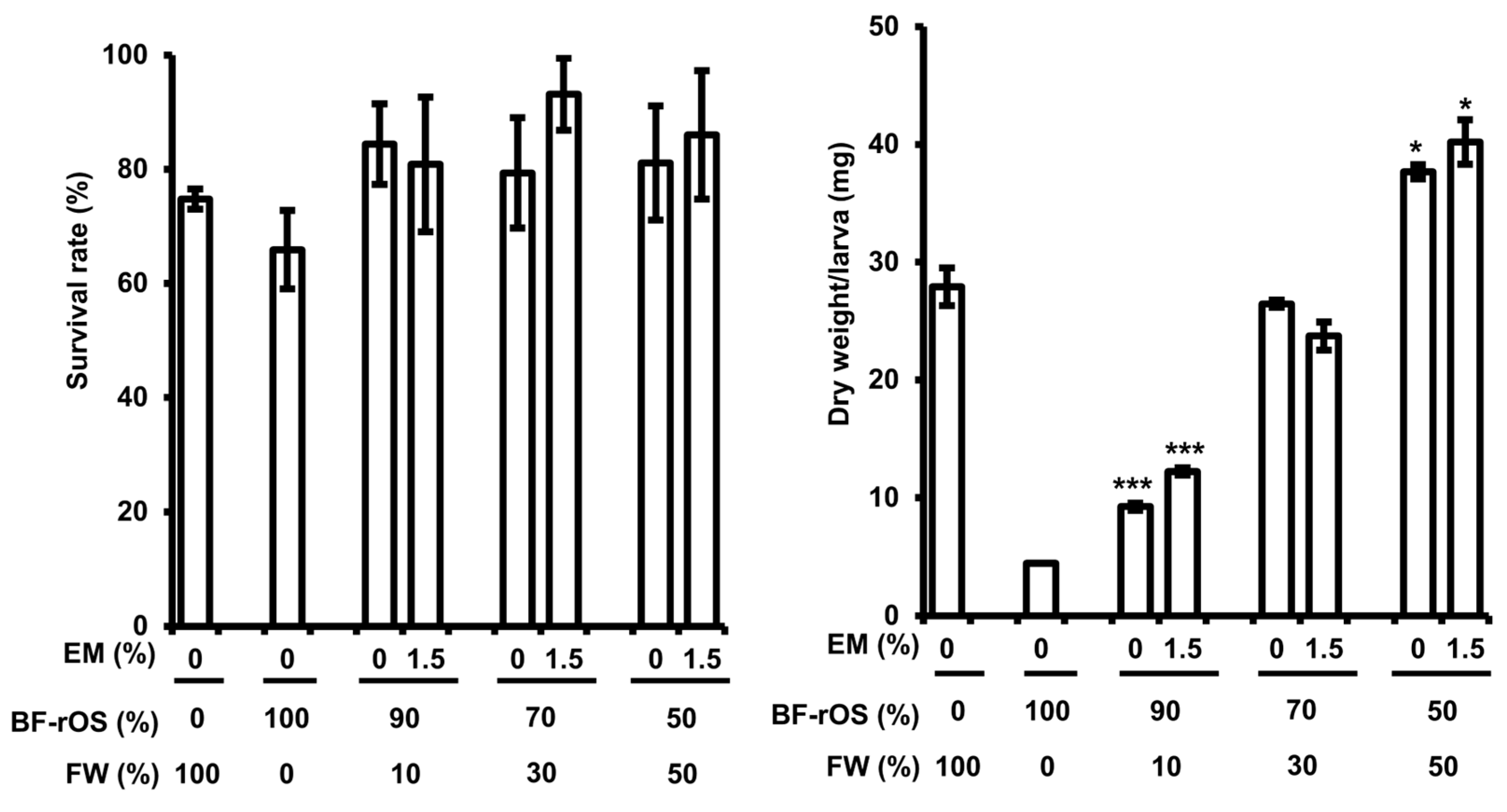
3.2. The Growth of BF-rOS When Fed BF-rOS/FW Mixture
3.3. Bioconversion Effciency of BF-rOS and BF-rOS/FW Mixtures by BSFL
3.4. Analysis of the Composition of BSFL Frass
3.5. Evaluation of BSFL Composition as Feed Ingredients
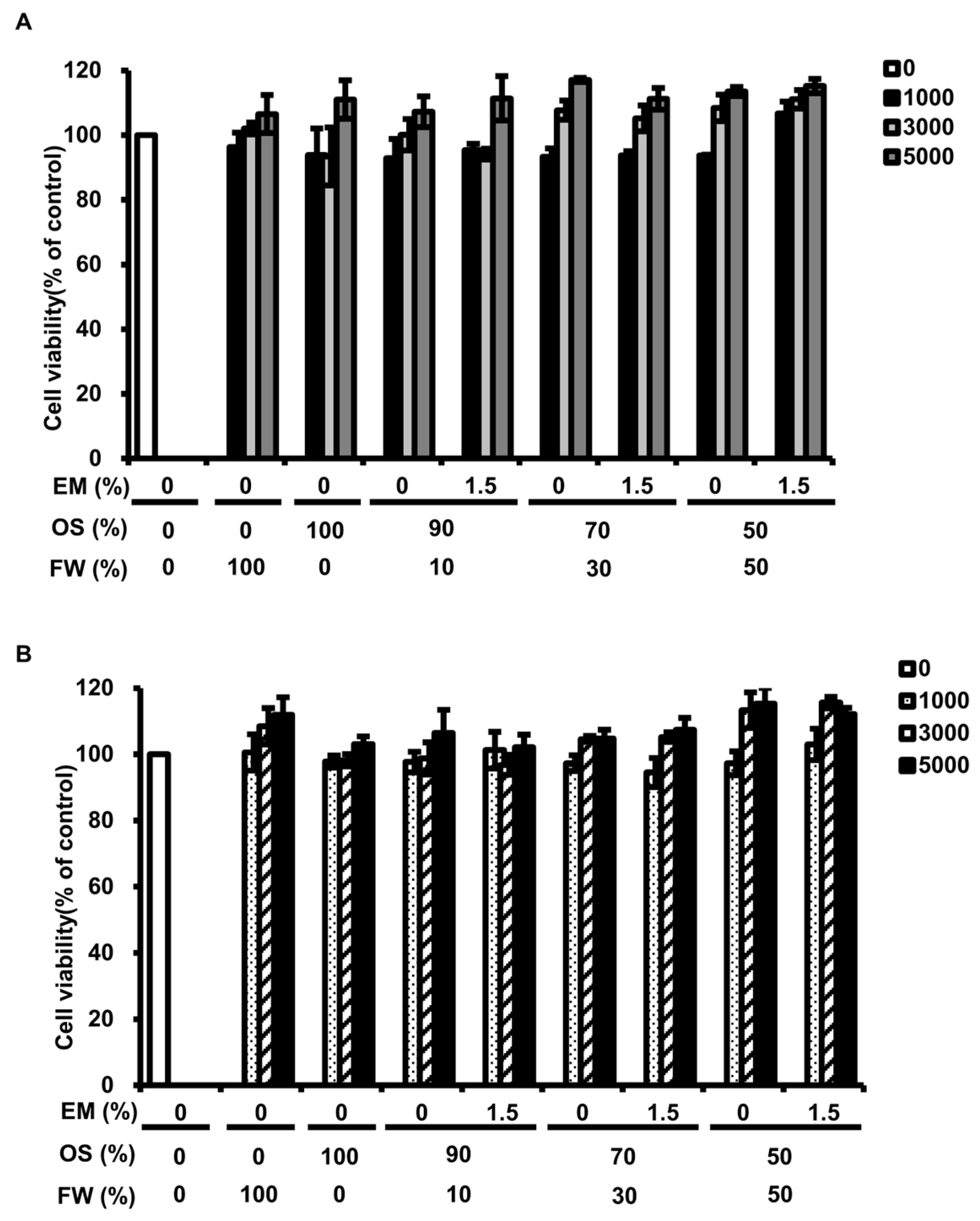
3.6. Evaluation of Cytotoxicity of BSFL
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Livestock and environment statistics: Manure and greenhouse gas emissions global, regional and country trends, 1990–2018. In FAOSTAT Analytical Briefs; FAO: Rome, Italy, 2020; p. 12. [Google Scholar]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, N.; Foley, J.A. Estimating historical changes in global land cover: Croplands from 1700 to 1992. Glob. Biogeochem. Cycles 1999, 13, 997–1027. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. A global assessment of the water footprint of farm animal products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, J.; Lin, J.-G.; Zhang, N.; Cao, W. Biogas energy generated from livestock manure in China: Current situation and future trends. J. Environ. Manag. 2021, 297, 113324. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Si, B.; Tan, X.; Xu, J.; Xu, W.; Zhou, L.; Chen, J.; Zhang, Y.; Zhou, X. Valorization of livestock manure for bioenergy production: A perspective on the fates and conversion of antibiotics. Resour. Conserv. Recycl. 2022, 183, 106352. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef] [PubMed]
- Walling, E.; Vaneeckhaute, C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manag. 2020, 276, 111211. [Google Scholar] [CrossRef] [PubMed]
- Kaparaju, P.; Rintala, J. Mitigation of greenhouse gas emissions by adopting anaerobic digestion technology on dairy, sow and pig farms in Finland. Renew. Energy 2011, 36, 31–41. [Google Scholar] [CrossRef]
- Gonzalez-Salazar, M.A.; Venturini, M.; Poganietz, W.-R.; Finkenrath, M.; Leal, M.R.L. Combining an accelerated deployment of bioenergy and land use strategies: Review and insights for a post-conflict scenario in Colombia. Renew. Sustain. Energy Rev. 2017, 73, 159–177. [Google Scholar] [CrossRef]
- Abbas, Y.; Jamil, F.; Rafiq, S.; Ghauri, M.; Khurram, M.S.; Aslam, M.; Bokhari, A.; Faisal, A.; Rashid, U.; Yun, S. Valorization of solid waste biomass by inoculation for the enhanced yield of biogas. Clean Technol. Environ. Policy 2020, 22, 513–522. [Google Scholar] [CrossRef]
- Park, W.-K.; Park, N.-B.; Shin, J.-D.; Hong, S.-G.; Kwon, S.-I.; Kang, K.-K. Study on characteristics of biogas production and liquid fertilizer with anaerobic co digestion of livestock manure and food waste. Korean J. Soil Sci. Fertil. 2011, 44, 895–902. [Google Scholar] [CrossRef]
- Dhungana, B.; Lohani, S.P.; Marsolek, M. Anaerobic co-digestion of food waste with livestock manure at ambient temperature: A biogas based circular economy and sustainable development goals. Sustainability 2022, 14, 3307. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Pastor, B.; Velasquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic waste substrates induce important shifts in gut microbiota of black soldier fly (Hermetia illucens L.): Coexistence of conserved, variable, and potential pathogenic microbes. Front. Microbiol. 2021, 12, 635881. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B. Assessing the microbiota of black soldier fly larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Evaluation of antimicrobial activity in the extract of defatted Hermetia illucens fed organic waste feed containing fermented effective microorganisms. Animals 2022, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.-Y.; Park, K.Y.; Chung, H. Use of black soldier fly larvae for food waste treatment and energy production in Asian countries: A review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using Black Soldier Fly larvae: A review. J. Environ. Manag. 2019, 251, 109569. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Duan, Y.; Zhang, Z. Effects of black soldier fly larvae (Diptera: Stratiomyidae) on food waste and sewage sludge composting. J. Environ. Manag. 2020, 256, 109967. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Gutiérrez, F.R.; Nguyen, D.H.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment-prospects and constraints. Proc. WasteSafe 2011, 2, 13–15. [Google Scholar]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Beskin, K.V.; Holcomb, C.D.; Cammack, J.A.; Crippen, T.L.; Knap, A.H.; Sweet, S.T.; Tomberlin, J.K. Larval digestion of different manure types by the black soldier fly (Diptera: Stratiomyidae) impacts associated volatile emissions. Waste Manag. 2018, 74, 213–220. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Jung, S.; Jung, J.-M.; Tsang, Y.F.; Bhatnagar, A.; Chen, W.-H.; Lin, K.-Y.A.; Kwon, E.E. Biodiesel production from black soldier fly larvae derived from food waste by non-catalytic transesterification. Energy 2022, 238, 121700. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Optimization of feed components to improve Hermetia illucens growth and development of oil extractor to produce biodiesel. Animals 2021, 11, 2573. [Google Scholar] [CrossRef]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Higa, T.; Wididana, G. The concept and theories of effective microorganisms. In Proceedings of the First International Conference on Kyusei Nature Farming, Khon Kaen, Thailand, 17–21 October 1989; US Department of Agriculture: Washington, DC, USA, 1991; pp. 118–124. [Google Scholar]
- Van Keulen, J.; Young, B. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Kofroňová, J.; Melliti, A.; Vurm, R. Biogas Digestate and Sewage Sludge as Suitable Feeds for Black Soldier Fly (Hermetia illucens) Larvae. Toxics 2024, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Beniers, J.; Graham, R. Effect of protein and carbohydrate feed concentrations on the growth and composition of black soldier fly (Hermetia illucens) larvae. J. Insects Food Feed 2019, 5, 193–199. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
| Feed | EM (%) | Initial Weight of Feed (g) | Relative Residual Reduction (%) |
|---|---|---|---|
| BF-rOS (80%) 1 | 0.0 | 1000 | 0.00 ± 1.45 |
| 1.0 | 1000 | −10.30 ± 0.80 * | |
| 1.5 | 1000 | −9.62 ± 4.18 * | |
| 2.0 | 1000 | −5.76 ± 1.28 | |
| BF-rOS (70%) | 0.0 | 1000 | −74.08 ± 4.18 *** |
| 1.0 | 1000 | −77.49 ± 1.90 *** | |
| 1.5 | 1000 | −74.53 ± 2.91 *** | |
| 2.0 | 1000 | −101.77 ± 2.91 *** | |
| BF-rOS (60%) | 0.0 | 1000 | −157.38 ± 4.44 *** |
| 1.0 | 1000 | −149.66 ± 2.54 *** | |
| 1.5 | 1000 | −153.74 ± 3.18 *** | |
| 2.0 | 1000 | −144.21 ± 6.40 *** | |
| FW | 0.0 | 1000 | 50.75 ± 2.59 *** |
| BF-rOS:FW (90:10) 2 | 0.0 | 1000 | −1.45 ± 9.94 |
| 1.5 | 1000 | 9.44 ± 0.95 | |
| BF-rOS:FW (70:30) | 0.0 | 1000 | 20.79 ± 3.54 * |
| 1.5 | 1000 | 20.79 ± 6.08 * | |
| BF-rOS:FW (50:50) | 0.0 | 1000 | 36.22 ± 1.59 ** |
| 1.5 | 1000 | 41.22 ± 0.32 ** |
| Composition | BF-rOS | BSFL Frass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed [Mixture Ratio of BF-rOS and FW (EM)] | |||||||||
| 100/0 (0.0) | 0/100 (0.0) | 90/10 (0.0) | 90/10 (1.5) | 70/30 (0.0) | 70/30 (1.5) | 50/50 (0.0) | 50/50 (1.5) | ||
| OM (%) | 69.63 | 74.55 | 71.72 | 75.82 | 72.71 | 73.98 | 75.55 | 72.67 | 72.47 |
| As (mg/kg) | ND | ND | 0.55 | 0.16 | 0.27 | 0.31 | 0.38 | ND | 0.37 |
| Cd (mg/kg) | 0.0063 | 0.0336 | ND | 0.034 | 0.064 | 0.035 | 0.062 | 0.077 | 0.11 |
| Ag (mg/kg) | 0.21 | ND | ND | 0.21 | 0.13 | ND | ND | ND | ND |
| Pb (mg/Kg) | 0.48 | 0.49 | 1.42 | 0.90 | 0.76 | 0.73 | 0.16 | 0.74 | 0.60 |
| Cr (mg/kg) | 7.67 | 6.26 | 7.10 | 6.10 | 6.72 | 6.44 | 5.88 | 7.35 | 7.36 |
| Cu (mg/kg) | 191.10 | 169.00 | 28.70 | 156.60 | 167.30 | 152.70 | 156.60 | 141.80 | 141.90 |
| Ni (mg/kg) | 7.49 | 6.02 | 1.86 | 5.56 | 5.64 | 5.56 | 6.49 | 5.39 | 5.59 |
| Zn (mg/kg) | 376.04 | 344.20 | 46.20 | 308.10 | 336.70 | 285.10 | 303.00 | 284.70 | 259.20 |
| NaCl (%) | 1.03 | 0.92 | 5.33 | 1.20 | 1.21 | 1.76 | 1.75 | 1.77 | 1.88 |
| MO (%) | 19.60 | 14.16 | 19.47 | 13.19 | 14.70 | 16.00 | 14.10 | 15.62 | 16.75 |
| C/N (%) | 33.80 | 38.42 | 35.68 | 42.60 | 35.82 | 42.27 | 41.74 | 36.52 | 38.34 |
| HCl (%) | 1.88 | 1.75 | 0.90 | 1.56 | 1.02 | 1.69 | 1.68 | 0.98 | 1.24 |
| CAP (mg/kg) | ND | ND | 0.031 | ND | 0.010 | 0.020 | 0.020 | 0.080 | 0.080 |
| Nutritional Composition (%) | Feed [Mixture Ratio of BF-rOS and FW (EM)] | |||||||
|---|---|---|---|---|---|---|---|---|
| 100/0 (0.0) | 0/100 (0.0) | 90/10 (0.0) | 90/10 (1.5) | 70/30 (0.0) | 70/30 (1.5) | 50/50 (0.0) | 50/50 (1.5) | |
| Crude protein | 49.89 | 38.62 | 47.3 | 47.4 | 44.98 | 44.3 | 43.16 | 44.50 |
| Crued fat | 1.34 | 30.56 | 8.60 | 7.97 | 16.97 | 15.26 | 23.61 | 21.18 |
| Crude fiber | 9.71 | 7.88 | 9.67 | 9.61 | 8.69 | 8.53 | 7.97 | 8.46 |
| Crude ash | 18.93 | 8.38 | 15.37 | 15.99 | 12.73 | 12.39 | 11.74 | 12.39 |
| Moisture | 5.00 | 6.01 | 5.15 | 4.58 | 4.17 | 6.75 | 3.44 | 1.81 |
| Heavy Metal (mg/kg) | Feed [Composition Ratio of BF-rOS and FW (EM) in Each Feed] | |||||||
|---|---|---|---|---|---|---|---|---|
| 100/0 (0.0) | 0/100 (0.0) | 90/10 (0.0) | 90/10 (1.5) | 70/30 (0.0) | 70/30 (1.5) | 50/50 (0.0) | 50/50 (1.5) | |
| As | ND | ND | ND | ND | ND | ND | ND | ND |
| Cd | 0.22 | 0.42 | 0.18 | 0.15 | 0.21 | 0.24 | 0.20 | 0.26 |
| Pb | 1.19 | 1.33 | 1.25 | 0.94 | 0.80 | 0.74 | 0.82 | 1.00 |
| Hg | 0.055 | 0.100 | 0.057 | 0.043 | 0.050 | 0.050 | 0.047 | 0.058 |
| Cr | 3.88 | 1.99 | 3.46 | 3.26 | 3.03 | 2.79 | 2.41 | 2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Resource Utilization of Residual Organic Sludge Generated from Bioenergy Facilities Using Hermetia illucens Larvae. Insects 2024, 15, 541. https://doi.org/10.3390/insects15070541
Lee K-S, Yun E-Y, Goo T-W. Resource Utilization of Residual Organic Sludge Generated from Bioenergy Facilities Using Hermetia illucens Larvae. Insects. 2024; 15(7):541. https://doi.org/10.3390/insects15070541
Chicago/Turabian StyleLee, Kyu-Shik, Eun-Young Yun, and Tae-Won Goo. 2024. "Resource Utilization of Residual Organic Sludge Generated from Bioenergy Facilities Using Hermetia illucens Larvae" Insects 15, no. 7: 541. https://doi.org/10.3390/insects15070541






