Influence of Distance, Environmental Factors, and Native Vegetation on Honeybee (Apis mellifera) Foraging in Arid Shrublands and Grasslands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
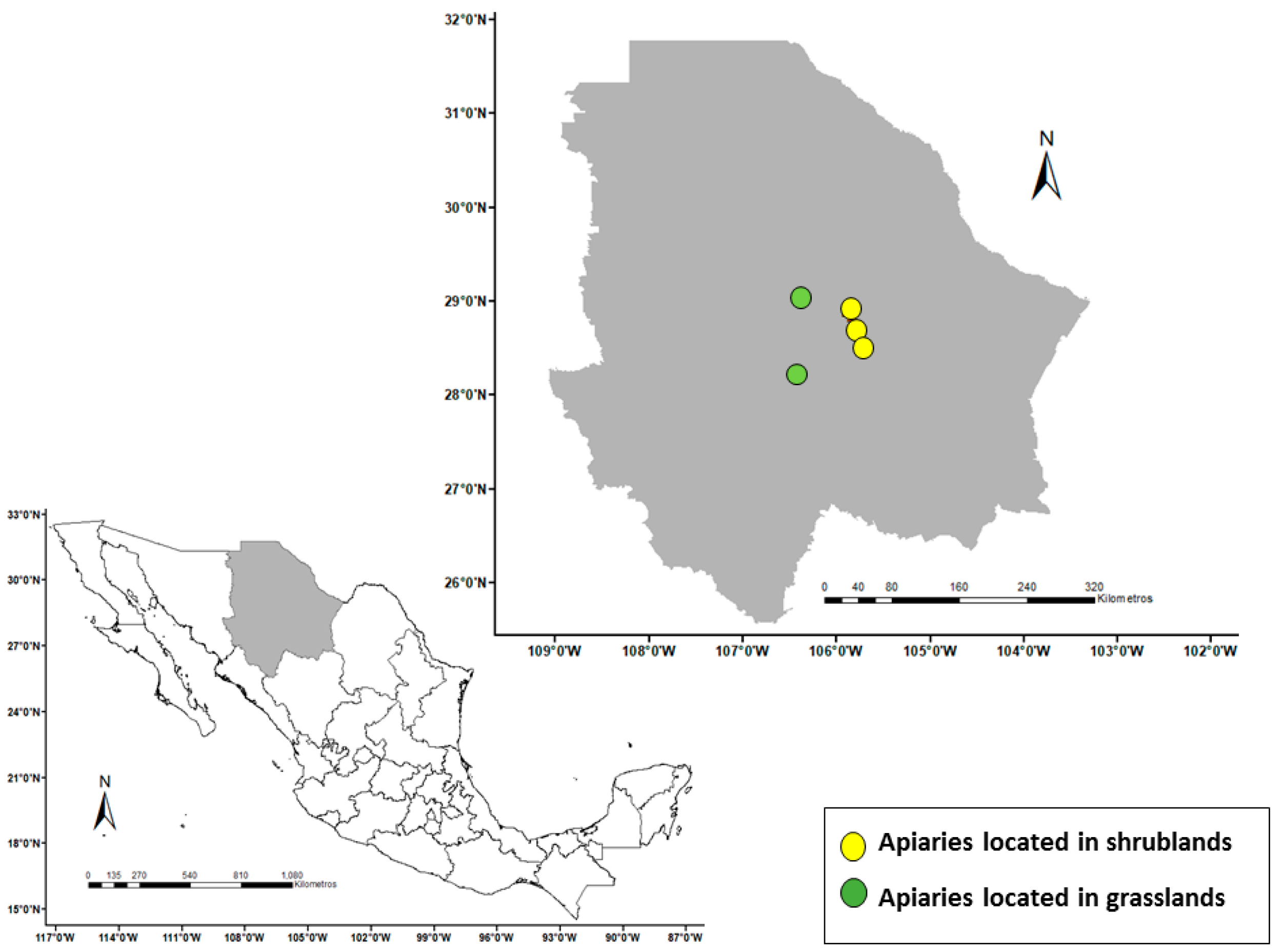
2.1. General Geospatial Analysis of the Apiary Sites
2.2. Vegetation Characterization
Floral Production
2.3. Foraging and Vegetation
2.4. Foraging and Environmental Parameters
2.5. Statistical Analysis
3. Results and Discussion
3.1. Geospatial Characteristics of the Apiary Sites
3.1.1. Shrubland Sites
3.1.2. Grassland Sites
3.2. Botanical Composition
3.2.1. Shrublands
3.2.2. Grasslands
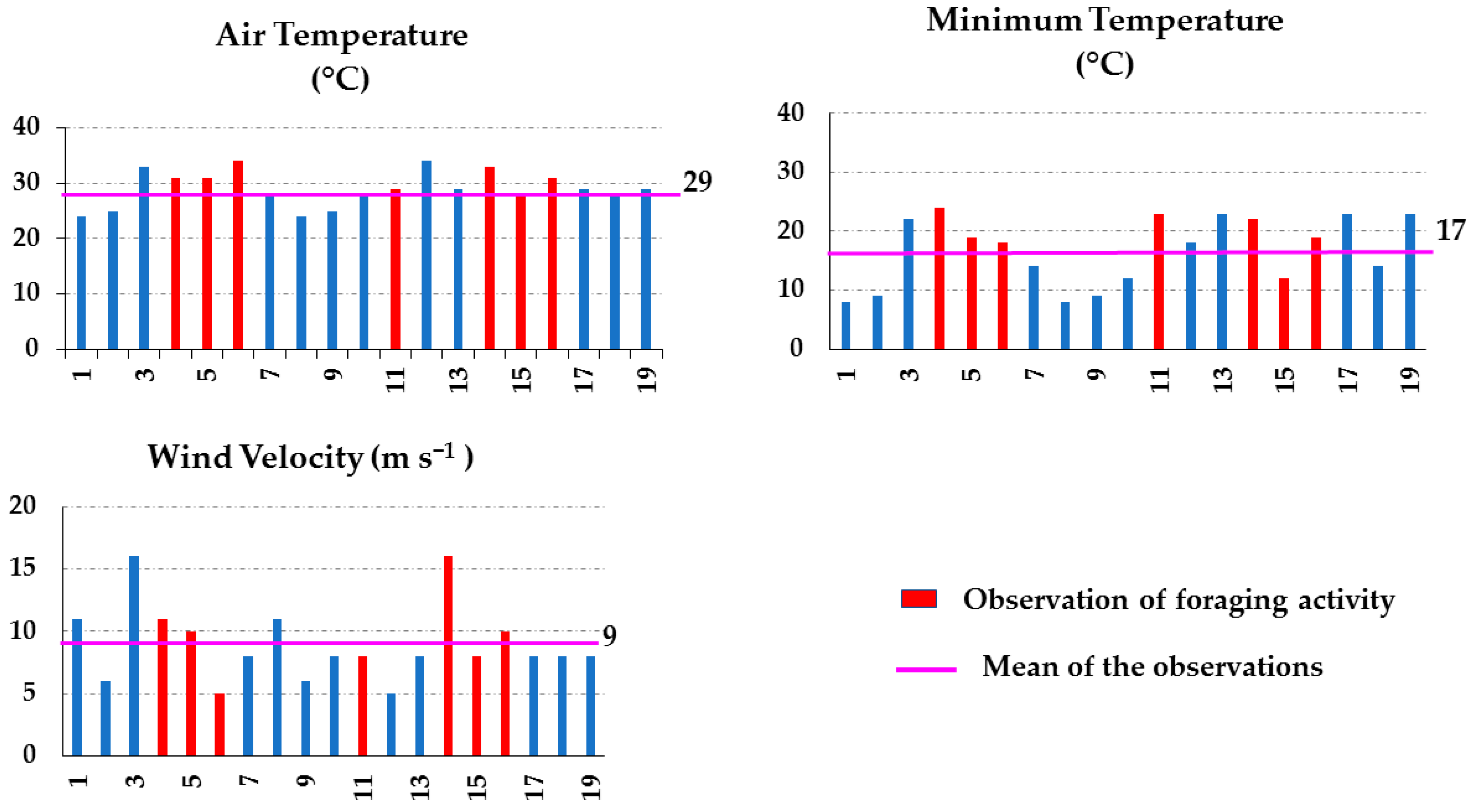
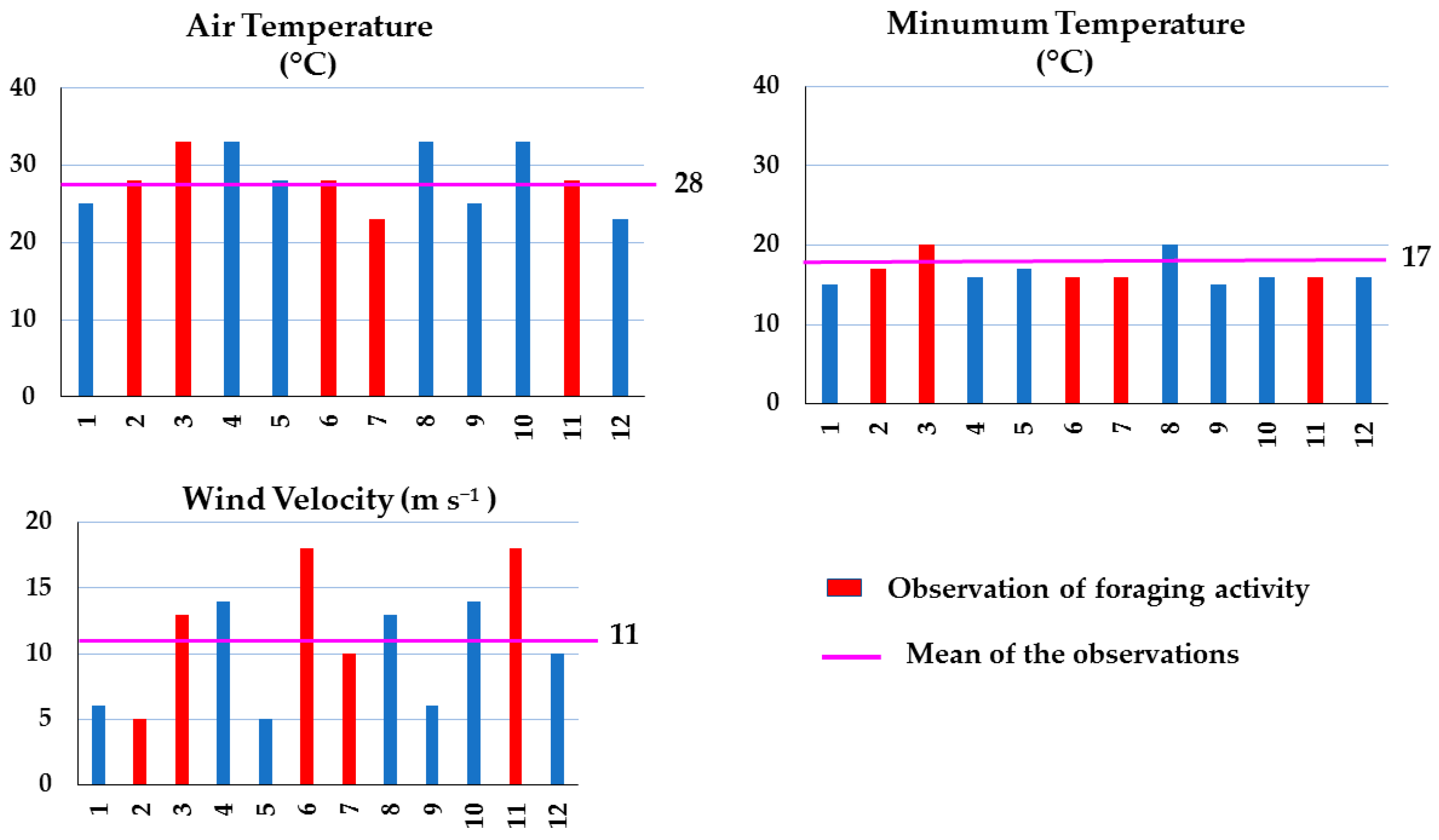
3.3. Foraging and Environmental Parameters
3.3.1. Shrublands
3.3.2. Grasslands
3.4. Foraging and Apiary Distance
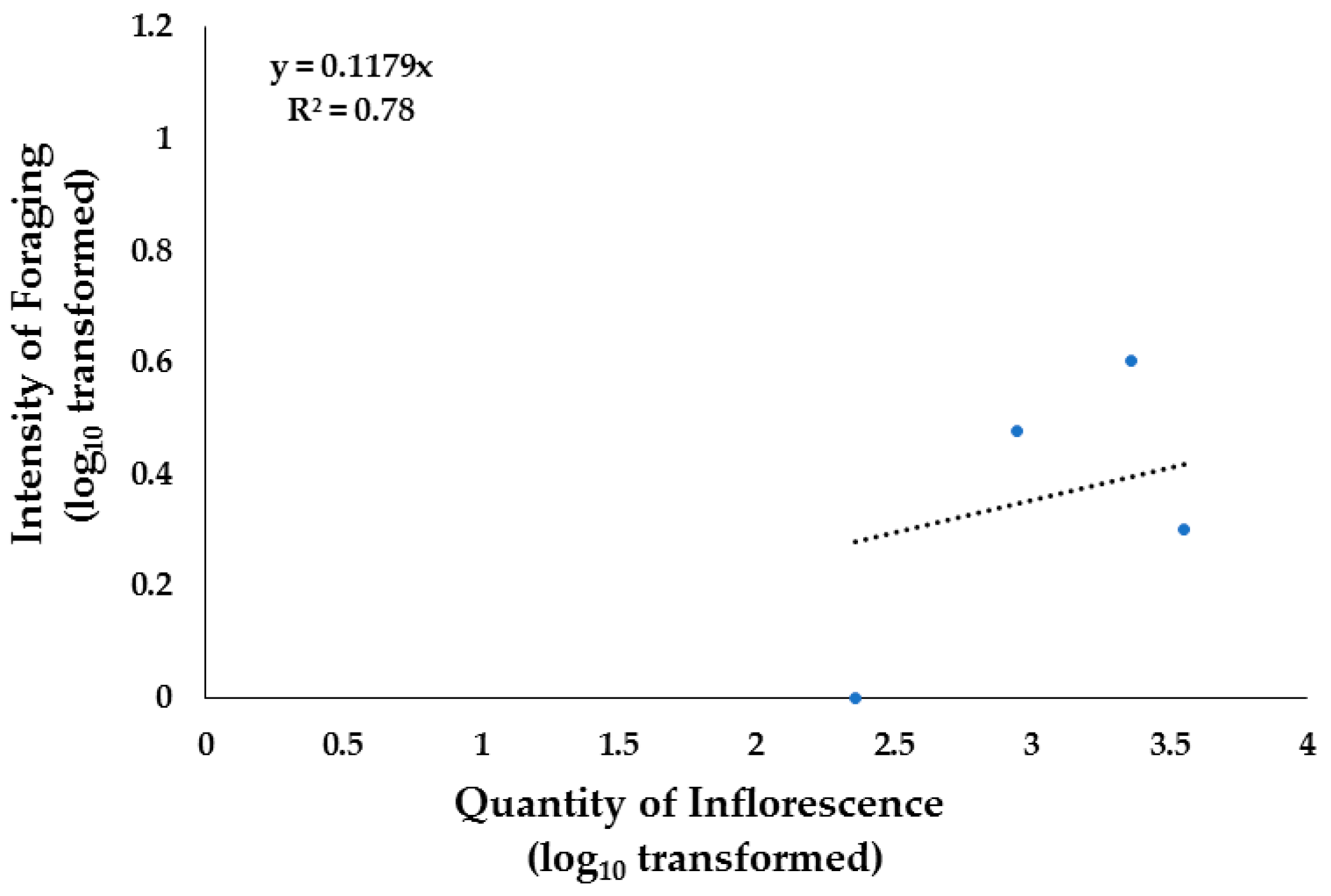
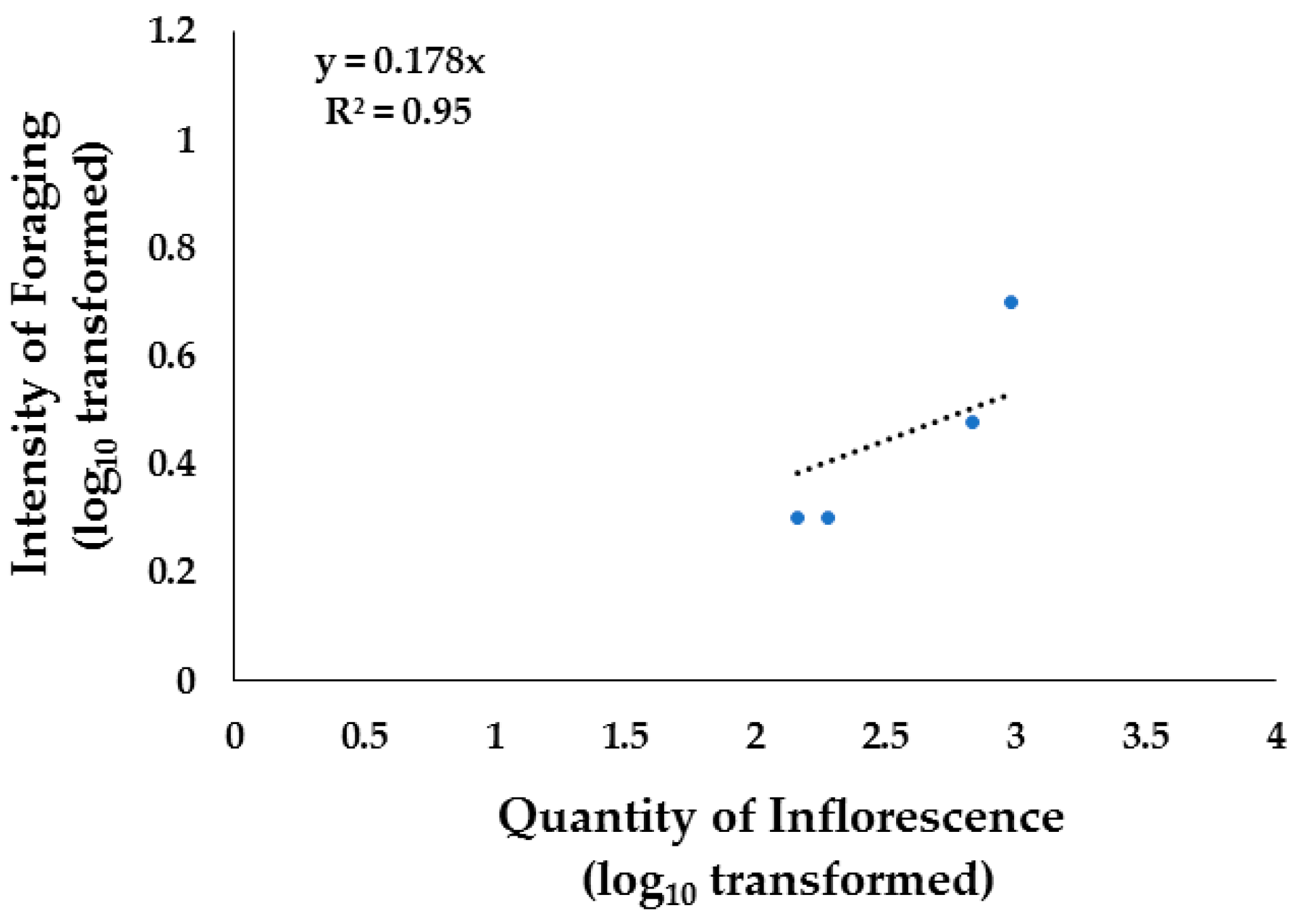
3.5. Foraging and Vegetation
3.5.1. Shrublands
3.5.2. Grasslands
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinlan, G.M.; Sponsler, D.; Gaines-Day, H.R.; McMinn-Sauder, H.B.; Otto, C.R.; Smart, A.H.; Grozinger, C.M. Grassy–herbaceous land moderates regional climate effects on honey bee colonies in the Northcentral US. Environ. Res. Lett. 2022, 17, 064036. [Google Scholar] [CrossRef]
- Selby, C. Pollinators under Pressure. Newsl. Nativ. Plant Soc. N. M. 2015, 40, 6. Available online: https://www.npsnm.org/wp-content/uploads/2012/02/NPSNM_2015_July_web.pdf (accessed on 2 March 2023).
- Peña-Chora, G.; Toledo-Hernández, E.; Sotelo-Leyva, C.; Damian-Blanco, P.; Villanueva-Flores, A.G.; Alvarez-Fitz, P.; Palemón-Alberto, F.; Ortega-Acosta, S.A. Presence and distribution of pests and diseases of Apis mellifera (Hymenoptera: Apidae) in Mexico: A review. Eur. Zool. J. 2023, 90, 224–236. [Google Scholar] [CrossRef]
- Guezen, J.M.; Forrest, J.R. Seasonality of floral resources in relation to bee activity in agroecosystems. Ecol. Evol. 2021, 11, 3130–3147. [Google Scholar] [CrossRef] [PubMed]
- Baena-Díaz, F.; Chévez, E.; de la Merced, F.R.; Porter-Bolland, L. Apis mellifera en México: Producción de miel, flora melífera y aspectos de polinización. Rev. Mex. Cienc. Pecu. 2022, 13, 525–548. [Google Scholar] [CrossRef]
- Balvino-Olvera, F.J.; Lobo, J.A.; Aguilar-Aguilar, M.J.; Ruiz-Guzmán, G.; González-Rodríguez, A.; Ruiz-Mercado, I.; Ghilardi, A.; Arizmendi, M.C.; Quesada, M. Long-term spatiotemporal patterns in the number of colonies and honey production in Mexico. Sci. Rep. 2023, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cuellar, A.K.; De la Mora, A.; Contreras-Escareño, F.; Morfin, N.; Tapia-González, J.M.; Macías-Macías, J.O.; Petukhova, T.; Correa-Benítez, A.; Guzman-Novoa, E. Genotype, but not climate, affects the resistance of honey bees (Apis mellifera) to viral Infections and to the Mite Varroa destructor. Vet. Sci. 2022, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Arechavaleta-Velasco, M.E.; García-Figueroa, C.; Alvarado-Avila, L.Y.; Ramírez-Ramírez, F.J.; Alcalá-Escamilla, K.I. Resultados e impacto de la investigación en genética y mejoramiento genético de las abejas melíferas desarrollada por el INIFAP en México. Rev. Mex. Cienc. Pecu. 2021, 12, 224–242. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Polo, R.O.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Guarna, M.M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Zavala-Beltrán, J.I.; Santiago, M.A.L.; Alcalá, R.V.; Batalla, B.M.M. Analysis of beekeeping profitability by strata in Aguascalientes, Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 453–468. [Google Scholar] [CrossRef]
- Gallardo-López, F.; Castellanos-Potenciano, B.P.; Díaz-Padilla, G.; Pérez-Vásquez, A.; Landeros-Sánchez, C.; Sol-Sánchez, Á. Cognitive dissonance in the face of climate change in beekeepers: A case study in Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 238–255. [Google Scholar] [CrossRef]
- Córdova-Rodríguez, A.; Aragón-Moreno, A.A.; Islebe, G.A.; Torrescano-Valle, N. Botanical characterization of Apis mellifera honeys in areas under different degrees of disturbance in the southern Yucatan peninsula, México. Palynology 2023, 47, 2215290. [Google Scholar] [CrossRef]
- Decourtye, A.; Mader, E.; Desneux, N. Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 2010, 41, 264–277. [Google Scholar] [CrossRef]
- Bloom, E.H.; Graham, K.K.; Haan, N.L.; Heck, A.R.; Gut, L.J.; Landis, D.A.; Milbrath, M.O.; Quinlan, G.M.; Wilson, J.K.; Zhang, Y.; et al. Responding to the US national pollinator plan: A case study in Michigan. Front. Ecol. Environ. 2022, 20, 84–92. [Google Scholar] [CrossRef]
- Grüter, C.; Hayes, L. Sociality is a key driver of foraging ranges in bees. Curr. Biol. 2022, 32, 5390–5397. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P. What’s killing American honey bees. PLoS Biol. 2007, 5, e168. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Pettis, S.J. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Owayss, A.A.; Ibrahim, Y.Y.; Basuny, N.K. A review of impacts of temperature and relative humidity on various activities of honey bees. Insect. Soc. 2017, 64, 455–463. [Google Scholar] [CrossRef]
- Guarino, L.; Jarvis, A.; Hijmans, R.J.; Maxted, N. 36 Geographic Information Systems. In Managing Plant Genetic Diversity; Engels, J.M.M., Rao, V.R., Brown, A.H.D., Jackson, M.T., Eds.; International Plant Genetic Resources Institute and and CIAT: Cali, Colombia, 2002; pp. 387–404. [Google Scholar] [CrossRef][Green Version]
- De Smith, M.J.; Goodchild, M.F.; Longley, P. Geospatial Analysis: A Comprehensive Guide to Principles, Techniques and Software Tools; Troubador Publishing Ltd.: Leicester, UK, 2007; pp. 33–39. [Google Scholar]
- Krigas, N.; Papadimitriou, K.; Mazaris, A.D. GIS and ex situ plant conservation. Environ. Sci. Geogr. Biol. 2012, I, 153–174. [Google Scholar] [CrossRef]
- Mercado-Rojas, A.L. Ubicación de Zonas Aptas para el Cultivo de Abejas Mediante Percepción Remota. Bachelor’s Thesis, Universidad Autónoma del Estado de México, Toluca, Estado de México, México, 2020. [Google Scholar]
- ESRI. ArcGIS Version 10.3. Environmental Systems Research Institute. 2014. Available online: www.arcgis.com (accessed on 11 June 2023).
- INEGI. Modelos Digitales de Elevación de Alta Resolución LiDAR, con Resolución de 5m. Superficie. ASCII. E14D29C4. Available online: https://www.inegi.org.mx/app/biblioteca/ficha.html?upc=702825796341#:~:text=Un%20Modelo%20Digital%20de%20Elevaci%C3%B3n,a%20trav%C3%A9s%20de%20la%20luz (accessed on 2 March 2022).
- Badger, J.; Bauwens, I.; Casso, P.; Davis, N.; Hahmann, A.; Hansen, S.B.K.; Hansen, B.O.; Heathfield, D.; Knight, O.J.; Lacave, O.; et al. Global Wind Atlas 3.0. World Bank Group. 2019. Available online: https://globalwindatlas.info/en (accessed on 2 May 2022).
- González-Suárez, M.; Mora-Olivo, A.; Villanueva-Gutiérrez, R.; Lara-Villalón, M.; Vanoye-Eligio, V.; Guerra-Pérez, A. Diversidad de la flora de interés apícola en el estado de Tamaulipas, México. Rev. Mex. Cienc. Pecu. 2020, 11, 914–932. [Google Scholar] [CrossRef]
- Melgoza, A.; Fierro, L.C. Manual de Métodos de Muestreos de Vegetación; Serie Técnico Científica; Departamento de Manejo de Pastizales, INIP-SARH: Chihuahua, México, 1980; Volume 1, pp. 46–79. [Google Scholar]
- Herrick, J.E.; Van Zee, J.W.; McCord, S.E.; Courtright, E.M.; Karl, J.W.; Burkett, L.M. Monitoring Manual for Grasslands, Shrublands, and Savanna Ecosystems. Vol. 1: Core Methods, 2nd ed.; USDA-ARS Jornada Experimental Range: Las Cruces, NM, USA, 2016. [Google Scholar]
- Hughes, H.G.; Varner, L.W.; Blankenship, L.H. Estimating Shrub Production from Plant Dimensions. J. Range Manag. 1987, 4, 367–369. [Google Scholar] [CrossRef]
- Guallpa-Calva, M.A.; Guilcapi-Pacheco, E.D.; Espinoza-Espinoza, A.E. Estimación de la flora melífera para la productividad apícola de la estación experimental Tunshi en el sector de Licto, Riobamba. Dom. Cienc. 2020, 6, 181–202. [Google Scholar] [CrossRef]
- Danner, N.; Molitor, A.M.; Schiele, S.; Härtel, S.; Steffan-Dewenter, I. Season and landscape composition affect pollen foraging distances and habitat use of honey bees. Ecol. Appl. 2016, 26, 1920–1929. [Google Scholar] [CrossRef]
- Hagler, J.R.; Mueller, S.; Teuber, L.R.; Machtley, S.A.; Van Deynze, A. Foraging range of honey bees, Apis mellifera, in alfalfa seed production fields. J. Insect Sci. 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Bänsch, S.; Tscharntke, T.; Ratnieks, F.L.; Härtel, S.; Westphal, C. Foraging of honey bees in agricultural landscapes with changing patterns of flower resources. Agric. Ecosyst. Environ. 2020, 291, 106792. [Google Scholar] [CrossRef]
- Weather Spark. Datos Historicos Meteorologicos. Available online: https://es.weatherspark.com/h/r/3253/Datos-hist%C3%B3ricos-meteorol%C3%B3gicos-en-Valent%C3%ADn-G%C3%B3mez-Far%C3%ADas-M%C3%A9xico (accessed on 2 February 2023).
- Osborne, C.P.; Salomaa, A.; Kluyver, T.A.; Visser, V.; Kellogg, E.A.; Morrone, O.; Vorontsova, M.S.; Clayton, W.D.; Simpson, D.A. A global database of C4 photosynthesis in grasses. New Phytolog. 2014, 204, 441–446. [Google Scholar] [CrossRef]
- Westhoff, P.; Gowik, U. Evolution of C4 photosynthesis—Looking for the master switch. Plant Physiol. 2010, 154, 598–601. [Google Scholar] [CrossRef]
- Nippert, J.B.; Fay, P.A.; Knapp, A.K. Photosynthetic traits in C3 and C4 grassland species in mesocosm and field environments. Environ. Exp. Bot. 2007, 60, 412–420. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Al-Ghamdi, A.A.; Mohamed, A.A. Tolerance of two honey bee races to various temperature and relative humidity gradients. Environ. Exp. Bot. 2012, 10, 133–138. [Google Scholar]
- Clarke, D.; Robert, D. Predictive modelling of honey bee foraging activity using local weather conditions. Apidologie 2018, 49, 386–396. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Tadesse, A.; Belay, T. Relating climatic factors to foraging behavior of honeybees (Apis mellifera) during blooming period of Guizotia abyssinica (LF). Livest. Res. Rural Dev. 2014, 26, 2–7. [Google Scholar]
- Vicens, N.; Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Tan, K.; Yang, S.; Wang, Z.; Radloff, S.E.; Oldroyd, B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie 2012, 43, 618–623. [Google Scholar] [CrossRef]
- Joshi, N.C.; Joshi, P.C. Foraging behaviour of Apis spp. on apple lowers in a subtropical environment. N. Y. Sci. J. 2010, 3, 71–76. [Google Scholar]
- Blažytė-Čereškienė, L.; Vaitkevičienė, G.; Venskutonytė, S.; Būda, V. Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirb. Agric. 2010, 97, 61–70. [Google Scholar]
- Burrill, R.M.; Dietz, A. The response of honey bees to variations in solar radiation and temperature. Apidologie 1981, 12, 319–328. [Google Scholar] [CrossRef]
- Gobierno de Mexico. Proyecciones de Cambio Climático y Descarga de Fichas Climáticas por Estado. Available online: https://atlasvulnerabilidad.inecc.gob.mx/page/Proyecciones/P_08.html (accessed on 15 September 2023).
- Luber, G.; McGeehin, M. Climate change and extreme heat events. Am. J. Prev. Med. 2008, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Switanek, M.; Crailsheim, K.; Truhetz, H.; Brodschneider, R. Modelling seasonal effects of temperature and precipitation on honey bee winter mortality in a temperate climate. Sci. Total Environ. 2017, 579, 1581–1587. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 2003, 270, 569–575. [Google Scholar] [CrossRef]
- Beekman, M.; Sumpter, D.J.T.; Seraphides, N.; Ratnieks, F.L.W. Comparing foraging behavior of small and large honey-bee colonies by decoding waggle dances made by foragers. Funct. Ecol. 2004, 18, 829–835. [Google Scholar] [CrossRef]
- Otto, C.R.V.; Bailey, L.L.; Smart, A.H. Patch utilization and flower visitations by wild bees in a honey bee-dominated, grassland landscape. Ecol. Evol. 2021, 11, 14888–14904. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.M.; Milbrath, M.O.; Otto, C.R.; Isaacs, R. Honey bee (Apis mellifera) colonies benefit from grassland/pasture while bumble bee (Bombus impatiens) colonies in the same landscapes benefit from non-corn/soybean cropland. PLoS ONE 2021, 16, e0257701. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Márquez, M. Memoria y aprendizaje en la escogencia floral de las abejas. Acta Biol. Colomb. 2009, 14, 125–136. [Google Scholar]
- Mattu, V.K.; Raj, H.; Thakur, M.L. Foraging behavior of honeybees on apple crop and its variation with altitude in Shimla hills of western Himalaya. Int. J. Sci. Nat. 2012, 3, 296–301. [Google Scholar]



| Scientific Name | Botanical Composition (%) Apiary Number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Aloyisia gratissima | 3.5 | 3.6 | |
| Celtis pallida | 4.7 | ||
| Condalia ericoides | 3.0 | ||
| Echinocereus stramineus | 0.6 | ||
| Flourensia cernua | 16.8 | 19.0 | 20.7 |
| Fouquieria splendens | 0.9 | ||
| Jatropha dioica | 4.8 | ||
| Koeberlinia spinosa | 1.8 | 2.7 | |
| Larrea tridentata | 15.3 | 17.3 | 14.4 |
| Mimosa aculeaticarpa | 1.2 | 6.0 | |
| Neltuma glandulosa | 50.1 | 45.8 | 48.0 |
| Vachellia constrica | 1.5 | 1.8 | 1.2 |
| Vachellia biaciculata | 1.8 | 0.9 | |
| Valchellia wrightii | 5.0 | 1.1 | |
| Others | 1.9 | 2.9 | 1.7 |
| Scientific Name | Botanical Composition % Apiary Number | |
|---|---|---|
| 4 | 5 | |
| Amaranthus palmeri | 2.1 | |
| Aristida adscensionis | 9.3 | |
| Aristida divaricata | 5.9 | |
| Baccharis salicifolia | 2.5 | |
| Bothriochloa barbinodis | 0.9 | |
| Bouteloua curtipendula | 11.0 | 1.3 |
| Bouteloua gracilis | 16.0 | 39.1 |
| Cenchrus incertus | 0.8 | |
| Chloris virgata | 1.5 | 2.5 |
| Crotalaria pumila | 12.8 | |
| Drymaria arenarioides | 2.2 | |
| Enneapogon desvauxii | 1.9 | |
| Eragrostis cilianensis | 0.9 | |
| Eragrostis intermedia | 9.2 | |
| Eragrostis lehmanniana | 0.9 | |
| Hilaria mutica | 3.0 | 0.7 |
| Melinis repens | 2.7 | |
| Mimosa aculeaticarpa | 6.8 | 38.8 |
| Neltuma glandulosa | 0.7 | |
| Quercus emory | 3.5 | |
| Setaria macrostachya | 1.4 | |
| Solanum rostratum | 1.8 | |
| Tribulus terrestres | 2.1 | 3.5 |
| Others | 10.1 | 4.1 |
| Environmental Parameter | Regression Equation | R2 | Probability Value |
|---|---|---|---|
| Air temperature (°C) | y = −0.11x + 3.5 | 0.87 | 0.0001 |
| Minimum temperature (°C) | y = −0.15x + 3.33 | 0.80 | 0.0001 |
| Wind velocity (ms −1) | y = −0.008x + 1.1 | 0.68 | 0.0003 |
| Relative humidity (%) | y = −0.03x + 0.96 | 0.41 | 0.002 |
| Cloudiness | y = 0.17x + 0.25 | 0.09 | 0.29 |
| Atmospheric pressure (mbar) | y = −0.0006x + 0.81 | 0.006 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baez-Gonzalez, A.D.; Royo-Marquez, M.H.; Perez-Quintana, C.A.; Hernández-Bernal, A.I.; Melgoza-Castillo, A.; Titulaer, M.; Vega-Mares, J.H. Influence of Distance, Environmental Factors, and Native Vegetation on Honeybee (Apis mellifera) Foraging in Arid Shrublands and Grasslands. Insects 2024, 15, 543. https://doi.org/10.3390/insects15070543
Baez-Gonzalez AD, Royo-Marquez MH, Perez-Quintana CA, Hernández-Bernal AI, Melgoza-Castillo A, Titulaer M, Vega-Mares JH. Influence of Distance, Environmental Factors, and Native Vegetation on Honeybee (Apis mellifera) Foraging in Arid Shrublands and Grasslands. Insects. 2024; 15(7):543. https://doi.org/10.3390/insects15070543
Chicago/Turabian StyleBaez-Gonzalez, Alma Delia, Mario Humberto Royo-Marquez, Carlos Alejandro Perez-Quintana, Adrián Isaac Hernández-Bernal, Alicia Melgoza-Castillo, Mieke Titulaer, and Jose Humberto Vega-Mares. 2024. "Influence of Distance, Environmental Factors, and Native Vegetation on Honeybee (Apis mellifera) Foraging in Arid Shrublands and Grasslands" Insects 15, no. 7: 543. https://doi.org/10.3390/insects15070543
APA StyleBaez-Gonzalez, A. D., Royo-Marquez, M. H., Perez-Quintana, C. A., Hernández-Bernal, A. I., Melgoza-Castillo, A., Titulaer, M., & Vega-Mares, J. H. (2024). Influence of Distance, Environmental Factors, and Native Vegetation on Honeybee (Apis mellifera) Foraging in Arid Shrublands and Grasslands. Insects, 15(7), 543. https://doi.org/10.3390/insects15070543






