Expression and Functional Analysis of the Smo Protein in Apis mellifera
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Drug Feeding
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Smo Gene Amplification
2.5. Expression of Smo
2.6. Localization of Smo Genes in the Antennae
2.7. EAG Recording
2.8. Behavioral Tests
2.9. Statistical Analysis
3. Results
3.1. Identification of Smo Genes
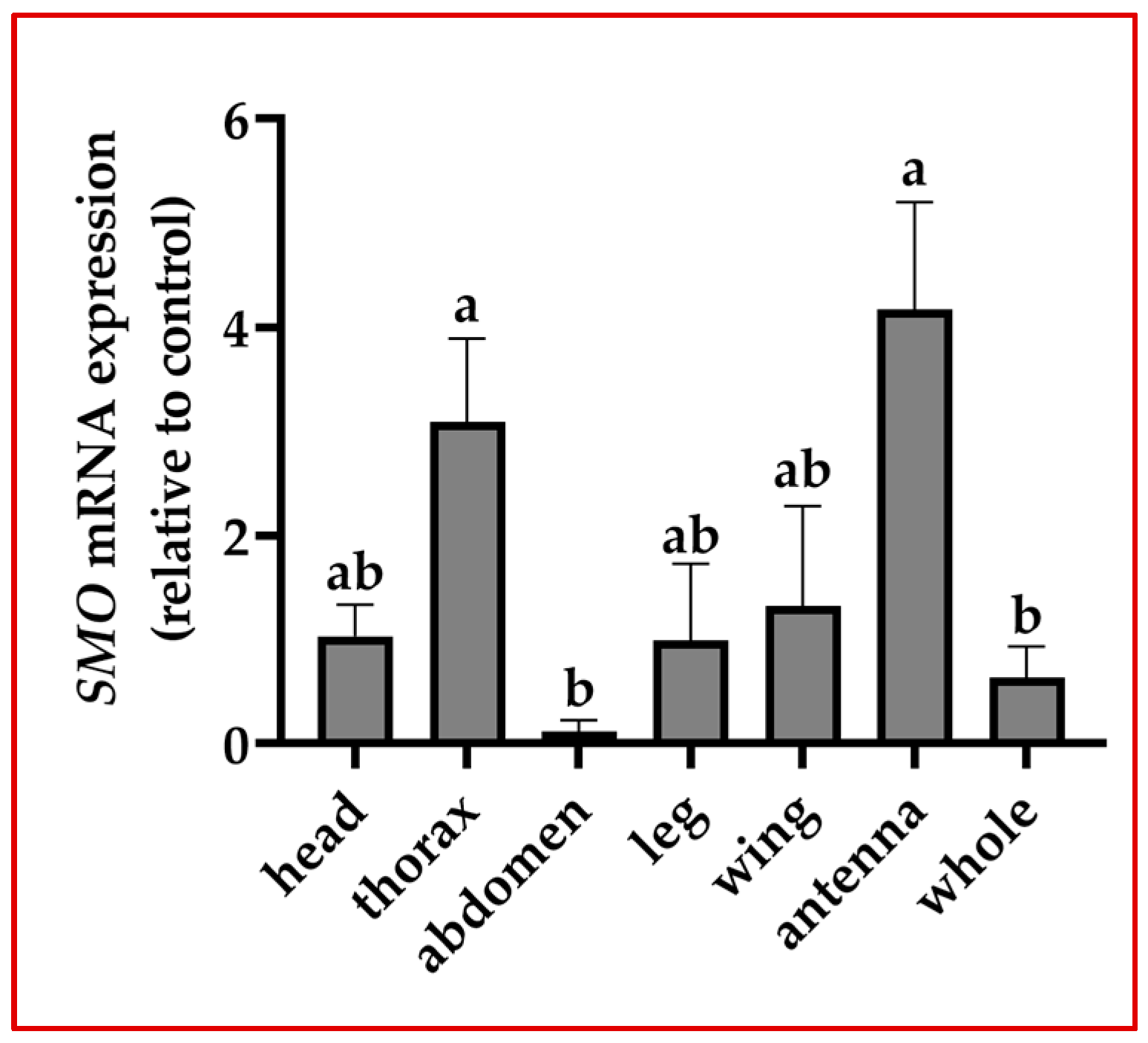
3.2. Analysis of Differential Tissue Expression of Smo
3.3. Location of Smo
3.4. Effects of Cyclopamine and Purmorphamine on Smo Gene Expression
3.4.1. Cyclopamine
3.4.2. Purmorphamine
3.4.3. Regulation of OR by Smo
3.5. EAG Recording
3.6. Y-Tube Olfactometer Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Embalabala, R.J.; Brockman, A.A.; Jurewicz, A.R.; Kong, J.A.; Ryan, K.; Guinto, C.D.; Álvarez-Buylla, A.; Chiang, C.; Ihrie, R.A. GLI3 is required for OLIG2+ progeny production in adult dorsal neural stem cells. Cells 2022, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Kageyama, R. Dual activation of Shh and Notch signaling induces dramatic enlargement of neocortical surface area. Neurosci. Res. 2021, 176, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Xu, M.; Yang, J.; Ma, X. The role of Hedgehog and Notch signaling pathway in cancer. Mol. Biomed. 2022, 3, 44. [Google Scholar] [CrossRef]
- Liu, A. Proteostasis in the Hedgehog signaling pathway. Semin. Cell Dev. Biol. 2019, 93, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, X.; Ni, L.; Liu, C.; Zheng, Y.; You, H.; Li, M.; Xiu, C.; Zhang, L.; Gong, T.; et al. Hedgehog signaling controls bone homeostasis by regulating osteogenic/adipogenic fate of skeletal stem/progenitor cells in mice. J. Bone Mineral Res. 2022, 37, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Shen, Y.Q.; Yang, X.; Li, X.H.; Xu, S.H.; Zhao, L.B.; Li, R.; Xiong, X.; Bai, S.J.; Wu, Q.Y.; et al. MicroRNA transcriptomics analysis identifies dysregulated hedgehog signaling pathway in a mouse model of acute intracerebral hemorrhage exposed to hyperglycemia. J. Stroke Cerebrovasc. Dis. 2022, 31, 106281. [Google Scholar] [CrossRef]
- Wang, J.; Ware, K.; Bedolla, A.; Allgire, E.; Turcato, F.C.; Weed, M.; Sah, R.; Luo, Y. Disruption of Sonic Hedgehog signaling accelerates age-related neurogenesis decline and abolishes stroke-induced neurogenesis and leads to increased anxiety behavior in stroke mice. Transl. Stroke Res. 2022, 13, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Pegah, A.; Tayebinia, H.; Khazaei, S.; Feizi, F.; Mazaheri, S.; Ghiasian, M.; Khazaei, M. The overexpression of sonic hedgehog associates with collateral development and amelioration of oxidative stress in stroke patients. J. Stroke Cerebrovasc. Dis. 2022, 31, 106408. [Google Scholar] [CrossRef]
- Palla, A.R.; Hilgendorf, K.I.; Yang, A.V.; Kerr, J.P.; Hinken, A.C.; Demeter, J.; Kraft, P.; Mooney, N.A.; Yucel, N.; Burns, D.M.; et al. Primary cilia on muscle stem cells are critical to maintain regenerative capacity and are lost during aging. Nat. Commun. 2022, 13, 1439. [Google Scholar] [CrossRef]
- Uehara, K.; Koyanagi-Aoi, M.; Koide, T.; Itoh, T.; Aoi, T. Epithelial-derived factors induce muscularis mucosa of human induced pluripotent stem cell-derived gastric organoids. Stem Cell Rep. 2022, 17, 820–834. [Google Scholar] [CrossRef]
- Otsuka, A.; Levesque, M.P.; Dummer, R.; Kabashima, K. Hedgehog signaling in basal cell carcinoma. J. Dermatol. Sci. 2015, 78, 95–100. [Google Scholar] [CrossRef]
- Sheng, M.; Lin, Y.; Xu, D.; Tian, Y.; Zhan, Y.; Li, C.; Farmer, D.G.; Kupiec-Weglinski, J.W.; Ke, B. CD47-mediated Hedgehog/SMO/GLI1 signaling promotes mesenchymal stem cell immunomodulation in mouse liver inflammation. Hepatology 2021, 74, 1560–1577. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Zhang, Y.J.; Abbas, M.N.; Hao, X.W.; Zhao, Y.Z.; Liang, H.H.; Cui, H.J.; Yang, L.Q. Hedgehog promotes cell proliferation in the midgut of silkworm, Bombyx mori. Insect Sci. 2020, 27, 697–707. [Google Scholar] [CrossRef]
- Athar, M.; Li, C.; Kim, A.L.; Spiegelman, V.S.; Bickers, D.R. Sonic hedgehog signaling in basal cell nevus syndrome. Cancer Res. 2014, 74, 4967–4975. [Google Scholar] [CrossRef]
- Sommer, A.; Lemmon, M.A. Smoothening out the patches. Science 2018, 362, 26–27. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Akhshi, T.; Shannon, R.; Trimble, W.S. The complex web of canonical and non-canonical Hedgehog signaling. BioEssays 2022, 44, e2100183. [Google Scholar] [CrossRef]
- Bortolotti, L.; Costa, C. Chemical communication in the honey bee society. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Paoli, M.; Galizia, G.C. Olfactory coding in honeybees. Cell Tissue Res. 2021, 383, 35–58. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Retzke, T.; Das Chakraborty, S.; Fabian, B.; Hansson, B.S.; Knaden, M.; Sachse, S. Odor mixtures of opposing valence unveil inter-glomerular crosstalk in the Drosophila antennal lobe. Nat. Commun. 2019, 10, 1201. [Google Scholar] [CrossRef]
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Ellis, K.; Álvarez-Ocaña, R.; Arguello, J.R.; Hansson, B.S.; Jefferis, G.S.; Caron, S.J.; et al. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef]
- Fleischer, J.; Rausch, A.; Dietze, K.; Erler, S.; Cassau, S.; Krieger, J. A small number of malebiased candidate pheromone receptors are expressed in large subsets of the olfactory sensory neurons in the antennae of drones from the European honey bee Apis mellifera. Insect Sci. 2022, 29, 749–766. [Google Scholar] [CrossRef]
- Gong, Q.; Chen, H.; Farbman, A.I. Olfactory sensory axon growth and branching is influenced by sonic hedgehog. Dev. Dyn. 2009, 238, 1768–1776. [Google Scholar] [CrossRef]
- Henkin, R.I.; Abdelmeguid, M.; Knöppel, A.B. On the mechanism of smell loss in patients with Type II congenital hyposmia. Am. J. Otolaryngol. 2016, 37, 436–441. [Google Scholar] [CrossRef]
- Demirci, H.; Worden, F.; Nelson, C.C.; Elner, V.M.; Kahana, A. Efficacy of Vismodegib (Erivedge™) for basal cell carcinoma involving the orbit and periocular area. Ophthalm. Plast. Reconstruct. Surg. 2015, 31, 463. [Google Scholar]
- Maurya, D.K.; Bohm, S.; Alenius, M. Hedgehog signaling regulates ciliary localization of mouse odorant receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E9386–E9394. [Google Scholar] [CrossRef]
- Sanchez, G.M.; Alkhori, L.; Hatano, E.; Schultz, S.W.; Kuzhandaivel, A.; Jafari, S.; Granseth, B.; Alenius, M. Hedgehog signaling regulates the ciliary transport of odorant receptors in Drosophila. Cell Rep. 2016, 14, 464–470. [Google Scholar] [CrossRef]
- Claudianos, C.; Lim, J.; Young, M.; Yan, S.; Cristino, A.S.; Newcomb, R.D.; Gunasekaran, N.; Reinhard, J. Odor memories regulate olfactory receptor expression in the sensory periphery. Eur. J. Neurosci. 2014, 39, 1642–1654. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Bond, M.C.; Chen, M.; Ren, X.R.; Lyerly, H.K.; Barak, L.S.; Chen, W. Identification of select glucocorticoids as Smoothened agonists: Potential utility for regenerative medicine. Proc. Natl. Acad. Sci. USA 2010, 107, 9323–9328. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Rohatgi, R.; Siebold, C. Cholesterol access in cellular membranes controls Hedgehog signaling. Nat. Chem. Biol. 2020, 16, 1303–1313. [Google Scholar] [CrossRef]
- Yang, L.; Xie, G.; Fan, Q.; Xie, J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene 2010, 29, 469–481. [Google Scholar] [CrossRef]
- Sekulic, A.; Von Hoff, D. Hedgehog pathway inhibition. Cell 2016, 164, 831. [Google Scholar] [CrossRef]
- Bohl, S.R.; Bullinger, L.; Rücker, F.G. New targeted agents in acute myeloid leukemia: New hope on the rise. Int. J. Mol. Sci. 2019, 20, 1983. [Google Scholar] [CrossRef]
- Salaritabar, A.; Berindan-Neagoe, I.; Darvish, B.; Hadjiakhoondi, F.; Manayi, A.; Devi, K.P.; Barreca, D.; Orhan, I.E.; Süntar, I.; Farooqi, A.A.; et al. Targeting Hedgehog signaling pathway: Paving the road for cancer therapy. Pharmacol. Res. 2019, 141, 466–480. [Google Scholar] [CrossRef]
- Nation, J.L., Sr. Insect Physiology and Biochemistry; CRC Press: Boca Raton, FL, USA, 2022; Volume 5, p. 19. [Google Scholar]
- Liu, W.; Zhang, Y.; Dietrich, C.H.; Duan, Y. Comparative analysis of antennal fine structure of Goniagnathus punctifer, Stirellus yeongnamensis and Stirellus indrus (Hemiptera: Cicadellidae: Deltocephalinae). Zoomorphology 2020, 139, 461–469. [Google Scholar] [CrossRef]
- Fialho, M.D.C.Q.; Guss-Matiello, C.P.; Zanuncio, J.C.; Campos, L.A.O.; Serrão, J.E. A comparative study of the antennal sensilla in corbiculate bees. J. Apic. Res. 2014, 53, 392–403. [Google Scholar] [CrossRef]
- Liu, F.; Feng, X.X.; Zhu, S.L.; Huang, H.Y.; Chen, Y.D.; Pan, Y.F.; June, R.R.; Zheng, S.G.; Huang, J.L. Sonic hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via MAPK/ERK signaling pathway. Front. Immunol. 2018, 9, 2847. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kataoka, M. Rigidity of protein structure revealed by incoherent neutron scattering. Biochim. Biophys. Acta 2020, 1864, 129536. [Google Scholar] [CrossRef]
- Jacob, V.E. Current source density analysis of electroantennogram recordings: A tool for mapping the olfactory response in an insect antenna. Front. Cell Neurosc. 2018, 12, 287. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators exert natural selection on flower size and floral display in penstemon digitalis. N. Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee–flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]




| Primer Name | Primer Sequence (5′-3′) | Purpose |
|---|---|---|
| P1 | F: TCATTATTCCTAAAATTTCCCGCGT | Sequence amplification |
| F: CAAATCCCTGCAGCCCAAG | ||
| R: TGAATGAACCGCAAATCCCTG | ||
| P2 | F: ATTTCTAGCAAATGCTTAAAGCCTT | |
| R: GCACCTCTACGAGTAACTAACTTTGGTA | ||
| P3 | F: AAACATTTAATAATGCTGGTCGATT | |
| F: TTAATAATGCTGGTCGATTATCTATTAGT | ||
| R: TTTTATATTAAAAGTTTTTCGAAAAAGAA | ||
| Smo | F: TGGTGTTTGCCACTTGTCCT | qRT-PCR |
| R: ACCAAGCTCTGACTGCATGA | ||
| Arp1 | F: TGCTGCACTCGTAGTTGACAATGG | |
| R: ACCCTGGTGGCGTGGTCTTC | ||
| OR151 | F: AAACACCGGAAAGAGGTATGG | |
| R: TGCATCCAGCATACGAACAG | ||
| OR152 | F: CGCTCTTTTGCTCTCTGGTTCG | |
| R: TGATCAGCAGGCCAAGCATA | ||
| OR2 | F: CTCGTGGGCTCCTGTTCGCTTG | |
| R: CTGTTCCTTCGGGCTGCTCTGC |
| Compounds | CAS Number | Purity (%) | Origin |
|---|---|---|---|
| Linalool | 78-70-6 | >98 | Macklin |
| Myrcene | 123-35-3 | >90 | Macklin |
| 6-methyl-5-heptene-2-one (6-M) | 110-93-0 | >98 | Macklin |
| neral | 5392-40-5 | >97 | Macklin |
| 2-((4-Ethyl-5-(pyridin-3-yl)-4H-1,2,4-triazol-3-yl) thio)-N-(4-ethylphenyl) acetamidea (VUAA1) | 525582-84-7 | >97.5 | Macklin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wang, J.; Yu, D.; Zhang, Y.; Zhang, H.; Guo, Y. Expression and Functional Analysis of the Smo Protein in Apis mellifera. Insects 2024, 15, 555. https://doi.org/10.3390/insects15070555
Guo L, Wang J, Yu D, Zhang Y, Zhang H, Guo Y. Expression and Functional Analysis of the Smo Protein in Apis mellifera. Insects. 2024; 15(7):555. https://doi.org/10.3390/insects15070555
Chicago/Turabian StyleGuo, Lina, Jue Wang, Diandian Yu, Yu Zhang, Huiman Zhang, and Yuan Guo. 2024. "Expression and Functional Analysis of the Smo Protein in Apis mellifera" Insects 15, no. 7: 555. https://doi.org/10.3390/insects15070555
APA StyleGuo, L., Wang, J., Yu, D., Zhang, Y., Zhang, H., & Guo, Y. (2024). Expression and Functional Analysis of the Smo Protein in Apis mellifera. Insects, 15(7), 555. https://doi.org/10.3390/insects15070555






