Predicting the Distribution of Neoceratitis asiatica (Diptera: Tephritidae), a Primary Pest of Goji Berry in China, under Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
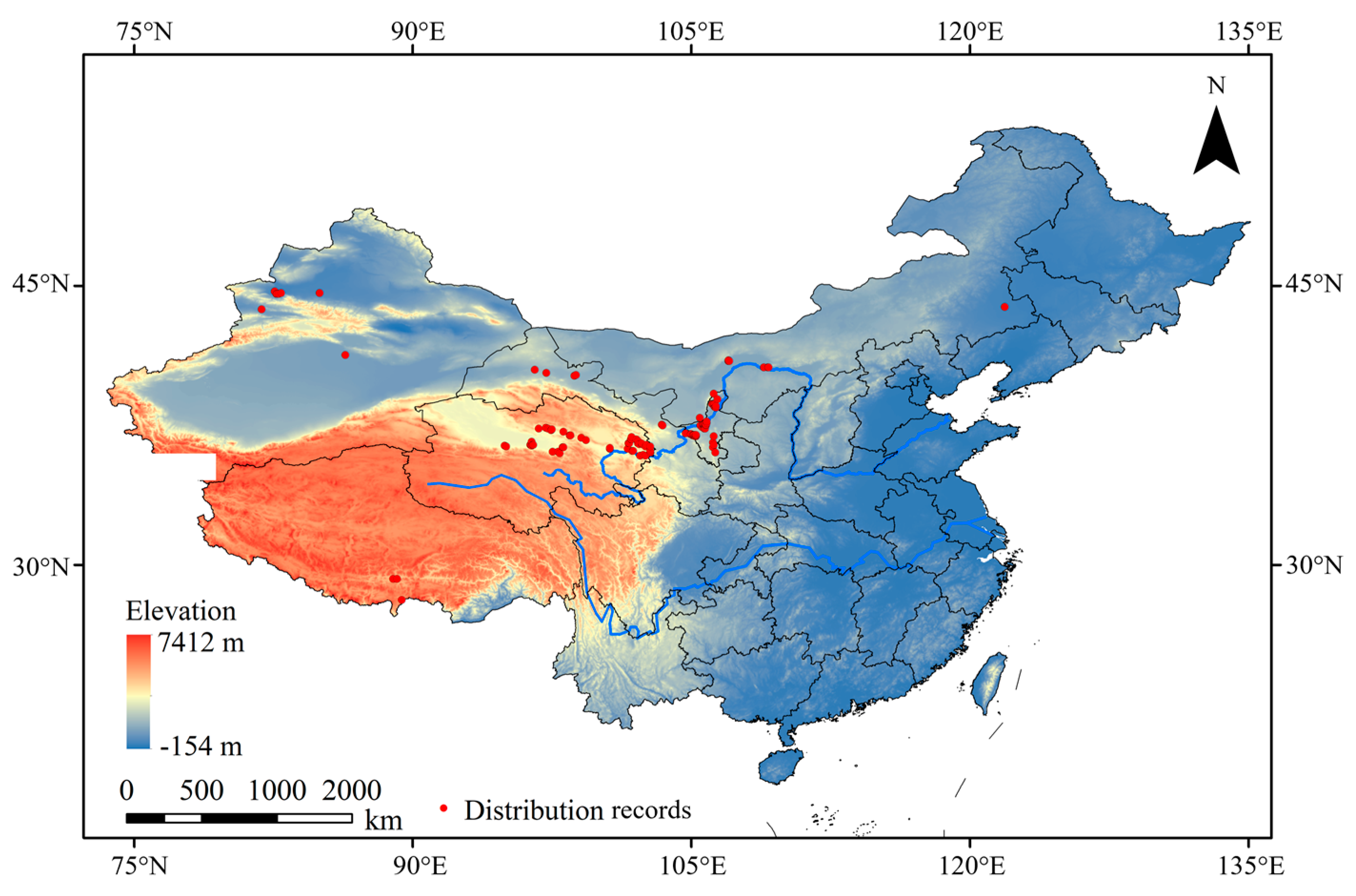
2.1. Acquisition and Processing of Distribution Records and Environmental Data
2.2. MaxEnt Model Optimization and Construction
2.3. MaxEnt Model Evaluation
2.4. Classification and Area Calculation of Suitable Habitats
2.5. Spatiotemporal Changes in Suitable Habitats
3. Results
3.1. Model Optimization and Evaluation
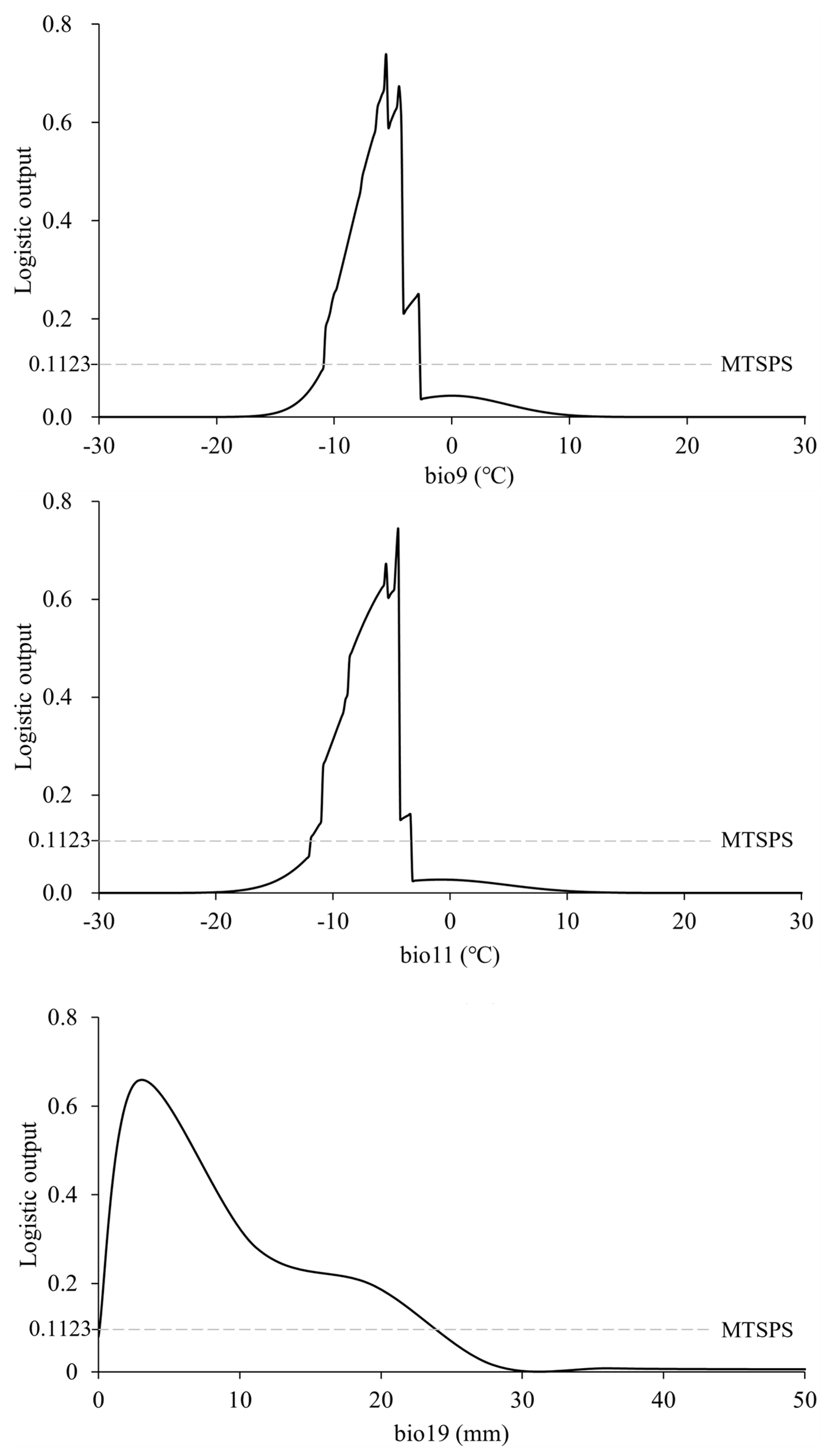
3.2. Dominant Environmental Factors
3.3. Suitable Habitats Under Different Climate Scenarios
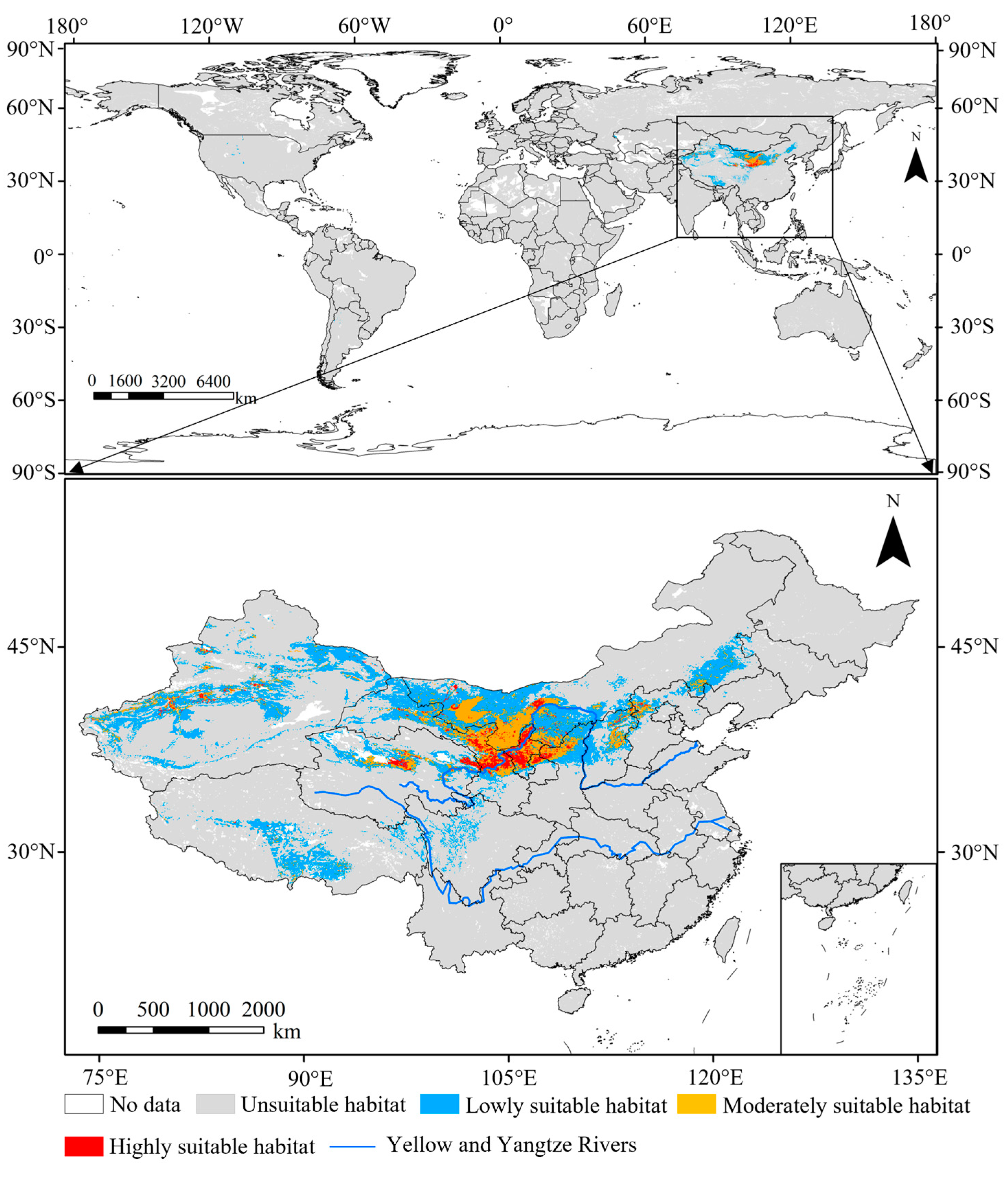
3.3.1. Current Suitable Habitats
3.3.2. Future Suitable Habitats
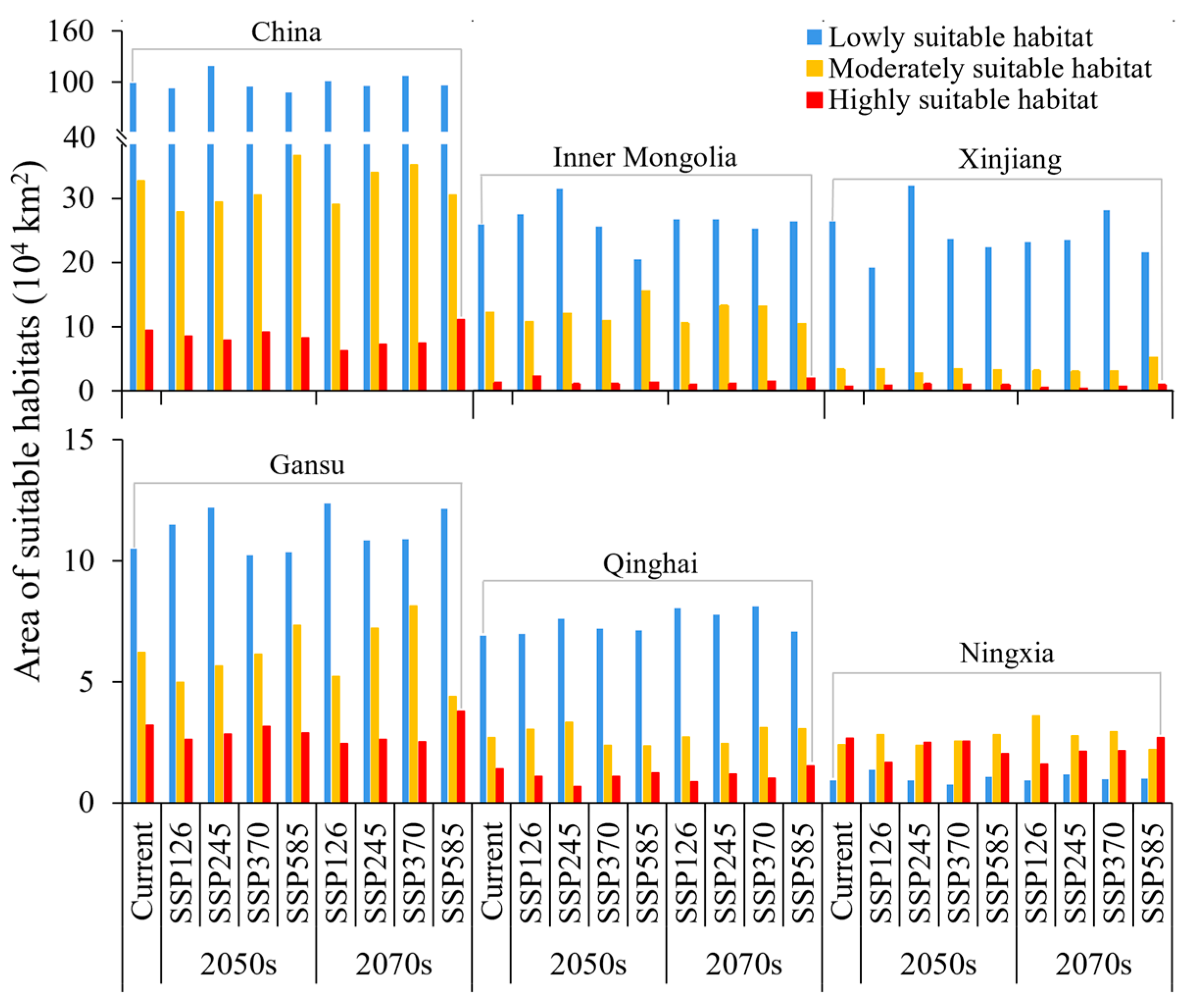
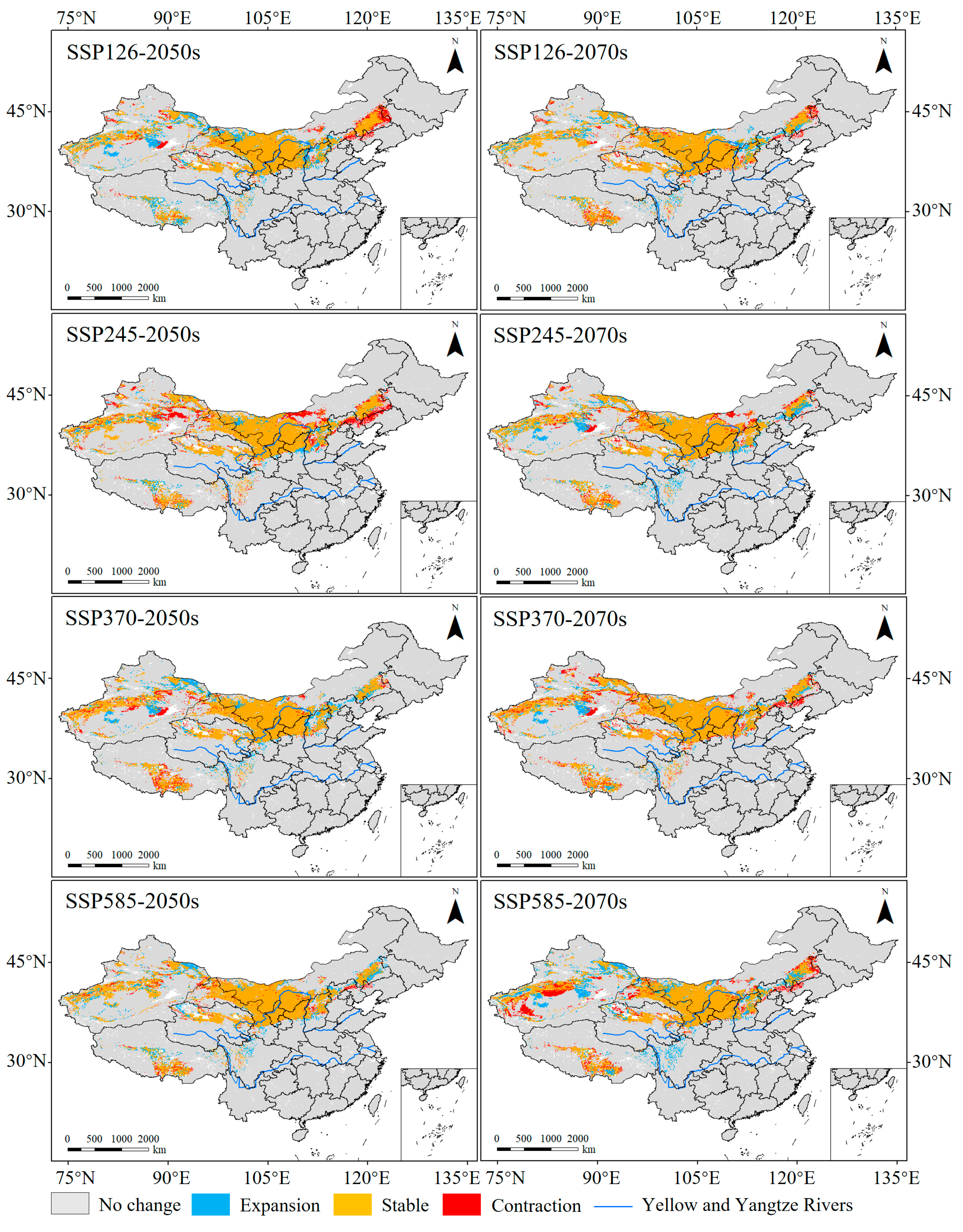
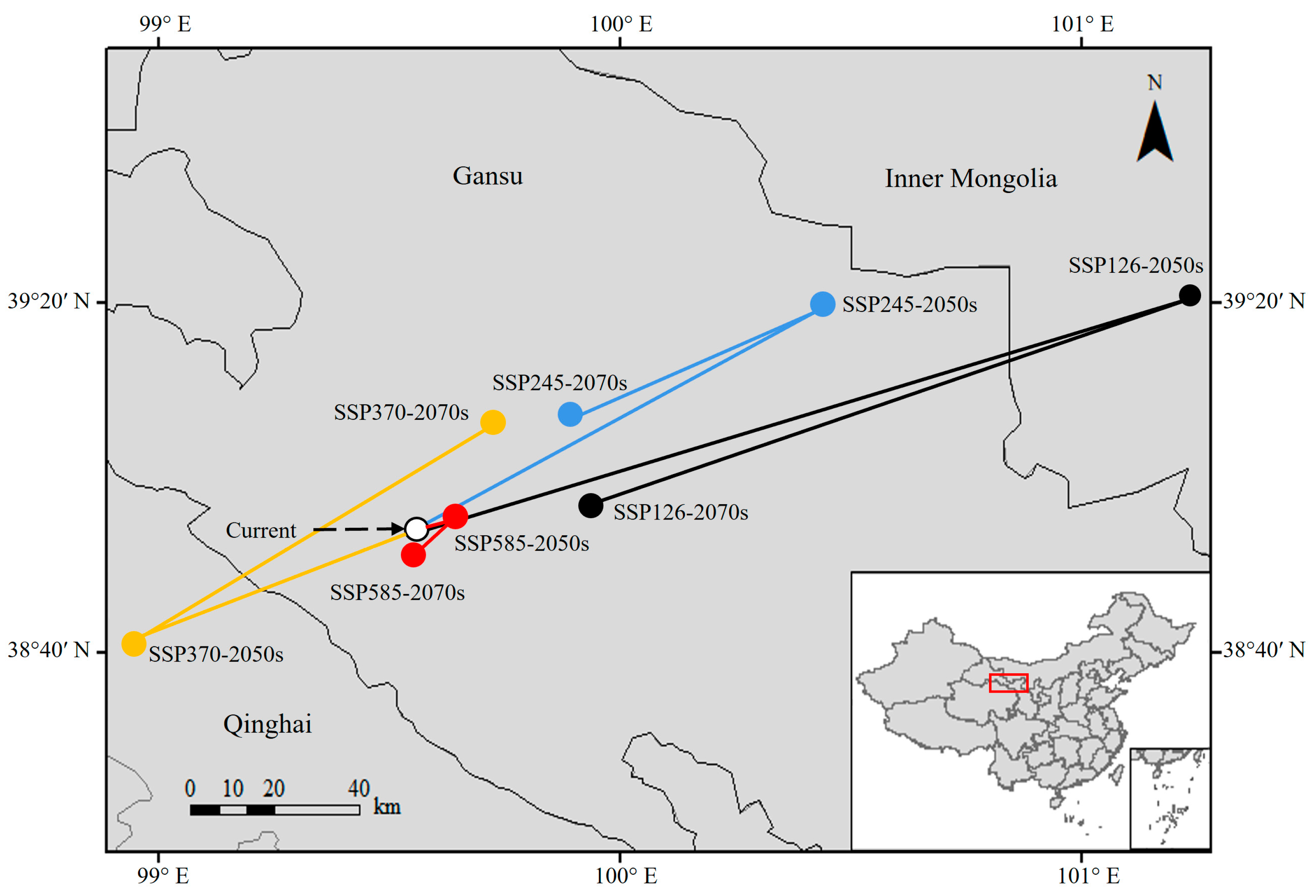
3.4. Spatiotemporal and Centroid Changes in the Suitable Habitats of N. asiatica
4. Discussion
4.1. Dominant Environmental Factors Influencing the Distribution of N. asiatica
4.2. Changes in Suitable Habitats of N. asiatica
4.3. Potential Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ring, M.J.; Lindner, D.; Cross, E.F.; Schlesinger, M.E. Causes of the global warming observed since the 19th century. Atmos. Clim. Sci. 2012, 2, 401. [Google Scholar] [CrossRef]
- Stange, E.E.; Ayres, M.P. Climate change impacts: Insects. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–7. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I. Climate change 2021–the physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; p. 2391. [Google Scholar] [CrossRef]
- Nedvěd, O. Temperature, effects on development and growth. In Encyclopedia of Insects; Academic Press: Amsterdam, The Netherlands, 2009; pp. 990–993. [Google Scholar] [CrossRef]
- Huang, J.; Li, J. Effects of climate change on overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepi-doptera: Noctuidae). Int. J. Biometeorol. 2015, 59, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Raza, M.M.; Khan, M.A.; Arshad, M.; Sagheer, M.; Sattar, Z.; Shafi, J.; Haq, E.U.; Ali, A.; Aslam, U.; Mushtaq, A. Impact of global warming on insects. Arch. Phytopathol. Plant Prot. 2015, 48, 84–94. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, P.; Xu, D.; Wang, R.; Sun, Q. Analysis of geographic distribution patterns of Lycium barbarum in the context of climate oscillations. Acta Bot. Boreal. Occident. Sin. 2022, 42, 2133–2142. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Duan, G.; Wang, Z.; Zhang, Y.; Fan, G. Potentially suitable habitats of Daodi goji berry in China under climate change. Front. Plant Sci. 2024, 14, 1279019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Xu, C.; Zhu, X.; Qiao, H.; Guo, K.; Xu, R.; Qiao, L.; Chen, J. Population dynamics and control strategies of major pests of wolfberry, Lycium barbarum. Mod. Chin. Med. 2017, 19, 1599–1604. [Google Scholar] [CrossRef]
- Yuan, X.; Qiao, H.; Yin, Z. Ovarian development and oviposition rhythm of Neoceratitis asiatica. Chin. J. Appl. Entomol. 2023, 60, 1458–1466. [Google Scholar] [CrossRef]
- Yin, Z. Biological characteristics and selection to host fruit of Neoceratitis asiatica. Master’s Thesis, Peking Union Medical College, Beijing, China, 2021. [Google Scholar] [CrossRef]
- Yin, X.; Wu, Y.; Zhao, W.; Zhao, F.; Sun, P.; Song, Y.; Qiu, L. Drought characteristics and sensitivity of potential evapotranspiration to climatic factors in the arid and semi-arid areas of northwest China. Hydrogeol. Eng. Geol. 2021, 48, 20–30. [Google Scholar] [CrossRef]
- Wei, H.; Qiao, H.; Liu, S.; Yuan, X.; Xu, C. Transcriptome-based selection and validation of reference genes for gene expression in goji fruit fly (Neoceratitis asiatica Becker) under developmental stages and five abiotic stresses. Int. J. Mol. Sci. 2022, 24, 451. [Google Scholar] [CrossRef]
- Guo, S.; Liu, B.; He, J.; Zhao, Z.; Zhang, R.; Li, Z. Chromosome-level genome assembly of an important wolfberry fruit fly (Neoceratitis asiatica Becker). Sci. Data 2023, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Huang, R.; Meng, X.; Liang, Z. Studies on Lycium fruit fly, Neoceratitis asiatica (Becker). J. Plant Prot. 1963, 4, 387–398. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, J.; Xu, C.; Liu, S.; Guo, K.; Qiao, H. Morphological and developmental characteristics of preimaginal stages of lycium fruit fly Neoceratitis asiatica. Acta Phytophylacica Sin. 2021, 48, 830–838. [Google Scholar] [CrossRef]
- Dias, N.P.; Zotti, M.J.; Montoya, P.; Carvalho, I.R.; Nava, D.E. Fruit fly management research: A systematic review of monitoring and control tactics in the world. Crop Prot. 2018, 112, 187–200. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, D.; Yang, Y.; Li, Y.; Zhang, R.; Shi, A.; Xiao, C. Relative numbers of the fruit fly Bactrocera dorsalis, and natural enemies of insect pests, trapped on different colored sticky boards. Chin. J. Appl. Entomol. 2021, 58, 1176–1182. [Google Scholar] [CrossRef]
- Santana, P.A., Jr.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Uses and requirements of ecological niche models and related distributional models. Biodivers. Inf. 2006, 3, 59–72. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Waldock, C.; Stuart-Smith, R.D.; Albouy, C.; Cheung, W.W.L.; Edgar, G.J.; Mouillot, D.; Tjiputra, J.; Pellissier, L. A quantitative review of abundance-based species distribution models. Ecography 2022, 1, 2022. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Zhang, X. Prediction on potential establishment area of Aphis sp. in Ningxia based on MaxEnt model. Geogr. Gea. Inf. Sci. 2019, 35, 40–45. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Ma, J.; Zhang, X. Adaptability analysis of Aceria macrodonis Keifer in Ningxia based on Maxent model. J. Mt. Agric. Biol. 2022, 41, 66–70. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhang, R.; He, J.; Zhao, Z.; Wei, S.; Liu, L. Wolbachia infection of Neoceratitis asiatica (Diptera: Tephritidae). Flo. Entomol. 2019, 102, 125–129. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Ma, J.; Xia, D. Effect of soil moisture on the emergence of Neoceratitis asiatica. For. Sci. Technol. 2017, 5, 43–44. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; ENMeval, R.A. An R package for conducting spatially independent evaluations and estimating optimal model complexity for MAXENT ecological niche models. Methods Ecol. Evol. 2014, 12261, 1198–1205. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. Peerj 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhong, Q.; Shao, D.; Ren, Y.; Li, Q.; Wen, J.; Li, J. Potential suitable habitats of chili pepper in China under climate change. Plants 2024, 13, 1027. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef]
- Jaworski, T.; Hilszczanski, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the expected climate change. For. Res. Pap. 2013, 74, 345. [Google Scholar] [CrossRef]
- Chang, X.; Gao, H.; Chen, F. Effects of environmental moisture and precipitation on insects. Chin. J. Ecol. 2008, 4, 619–625. [Google Scholar] [CrossRef]
- Liu, W.; Meng, H.; Dong, B.; Fan, J.; Zhu, X.; Zhou, H. Predicting potential distribution of the Rhinoncus sibiricus under climatic in China using MaxEnt. PLoS ONE 2024, 19, e297126. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.F.G.; Honěk, A.; Keil, P.; Kotela, M.A.A.; Šizling, A.L.; Jarošík, V. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 2009, 23, 257–264. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Eskafi, F.M.; Fernandez, A. Larval-pupal mortality of mediterranean fruit fly (Diptera: Tephritidae) from interaction of soil, moisture, and temperature. Environ. Entomol. 1990, 6, 1666–1670. [Google Scholar] [CrossRef]
- Huang, J. Effects of soil temperature and snow cover on the mortality of overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Int. J. Biometeorol. 2016, 60, 977–989. [Google Scholar] [CrossRef]
- Beirne, B. Effects of precipitation on corp insects. Can. Entomol. 1970, 11, 1360–1373. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Berzitis, E.A.; Minigan, J.N.; Hallett, R.H.; Newman, J.A. Climate and host plant availability impact the future distribution of the bean leaf beetle (Cerotoma trifurcata). Glob. Change Biol. 2014, 20, 2778–2792. [Google Scholar] [CrossRef]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Suitable areas of Phakopsora pachyrhizi, Spodoptera exigua, and their host plant Phaseolus vulgaris are projected to reduce and shift due to climate change. Theor. Appl. Climatol. 2019, 135, 409–424. [Google Scholar] [CrossRef]
- Chen, I.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, D.; Zhuo, Z. Predicting the impact of climate change on the geographical distribution of Leafhopper, Cicadella viridis in China through the MaxEnt model. Insects 2023, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, X.; Fei, Y. Predicting the current and future distribution of Monochamus carolinensis (Coleoptera: Cerambycidae) based on the maximum entropy. Pest Manag. Sci. 2023, 12, 5393–5404. [Google Scholar] [CrossRef] [PubMed]



| Factor | Description | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| bio9 | Mean temperature of driest quarter (°C) | 31.8 | 51.9 |
| bio19 | Precipitation of coldest quarter (mm) | 27.6 | 2.5 |
| bio11 | Mean temperature of coldest quarter (°C) | 18.4 | 12.4 |
| bio12 | Annual precipitation (mm) | 6.3 | 2.5 |
| bio2 | Mean diurnal range (°C) | 5.4 | 0.2 |
| t_bs | Topsoil base saturation (%) | 3.4 | 1.3 |
| t_gravel | Topsoil gravel content (%) | 3.3 | 1 |
| bio15 | Precipitation seasonality (mm) | 1.7 | 0.5 |
| t_silt | Topsoil silt fraction (%) | 0.9 | 0.8 |
| t_caco3 | Topsoil calcium carbonate (%) | 0.8 | 0.7 |
| bio14 | Precipitation of driest month (mm) | 0.2 | 26 |
| t_caso4 | Topsoil gypsum (%) | 0.2 | 0.2 |
| t_cec_soil | Topsoil cec (soil) (cmol/kg) | 0 | 0.1 |
| t_cec_clay | Topsoil cec (clay) (cmol/kg) | 0 | 0 |
| t_clay | Topsoil clay fraction (%) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Fan, G.; Deng, C.; Duan, G.; Li, J. Predicting the Distribution of Neoceratitis asiatica (Diptera: Tephritidae), a Primary Pest of Goji Berry in China, under Climate Change. Insects 2024, 15, 558. https://doi.org/10.3390/insects15080558
Song Z, Fan G, Deng C, Duan G, Li J. Predicting the Distribution of Neoceratitis asiatica (Diptera: Tephritidae), a Primary Pest of Goji Berry in China, under Climate Change. Insects. 2024; 15(8):558. https://doi.org/10.3390/insects15080558
Chicago/Turabian StyleSong, Zhongkang, Guanghui Fan, Changrong Deng, Guozhen Duan, and Jianling Li. 2024. "Predicting the Distribution of Neoceratitis asiatica (Diptera: Tephritidae), a Primary Pest of Goji Berry in China, under Climate Change" Insects 15, no. 8: 558. https://doi.org/10.3390/insects15080558






