Expression and Functional Analysis of Two Cytochrome P450 Monooxygenase Genes and a UDP-Glycosyltransferase Gene Linked with Thiamethoxam Resistance in the Colorado Potato Beetle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Bioassay
2.3. RNA-Sequencing Data Analysis
2.4. Sequence and Phylogenetic Analysis
2.5. Preparation of Samples for Expression Analysis
2.6. Total RNA Isolation and cDNA Synthesis
2.7. Real-Time Quantitative PCR
2.8. RNA Interference
2.9. Statistical Analysis
3. Results
3.1. Resistance Levels of L. decemlineata Populations to Thiamethoxam
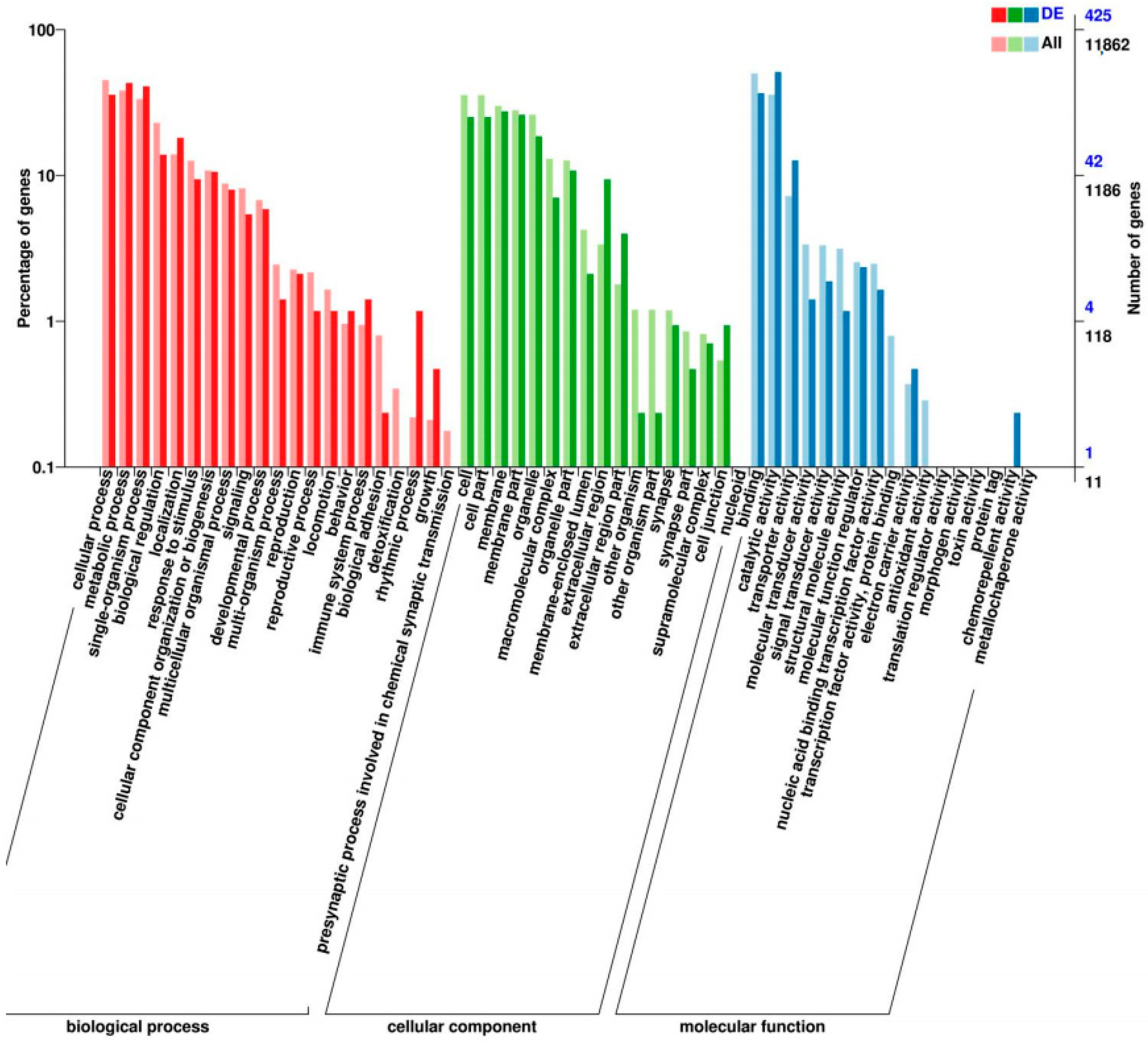
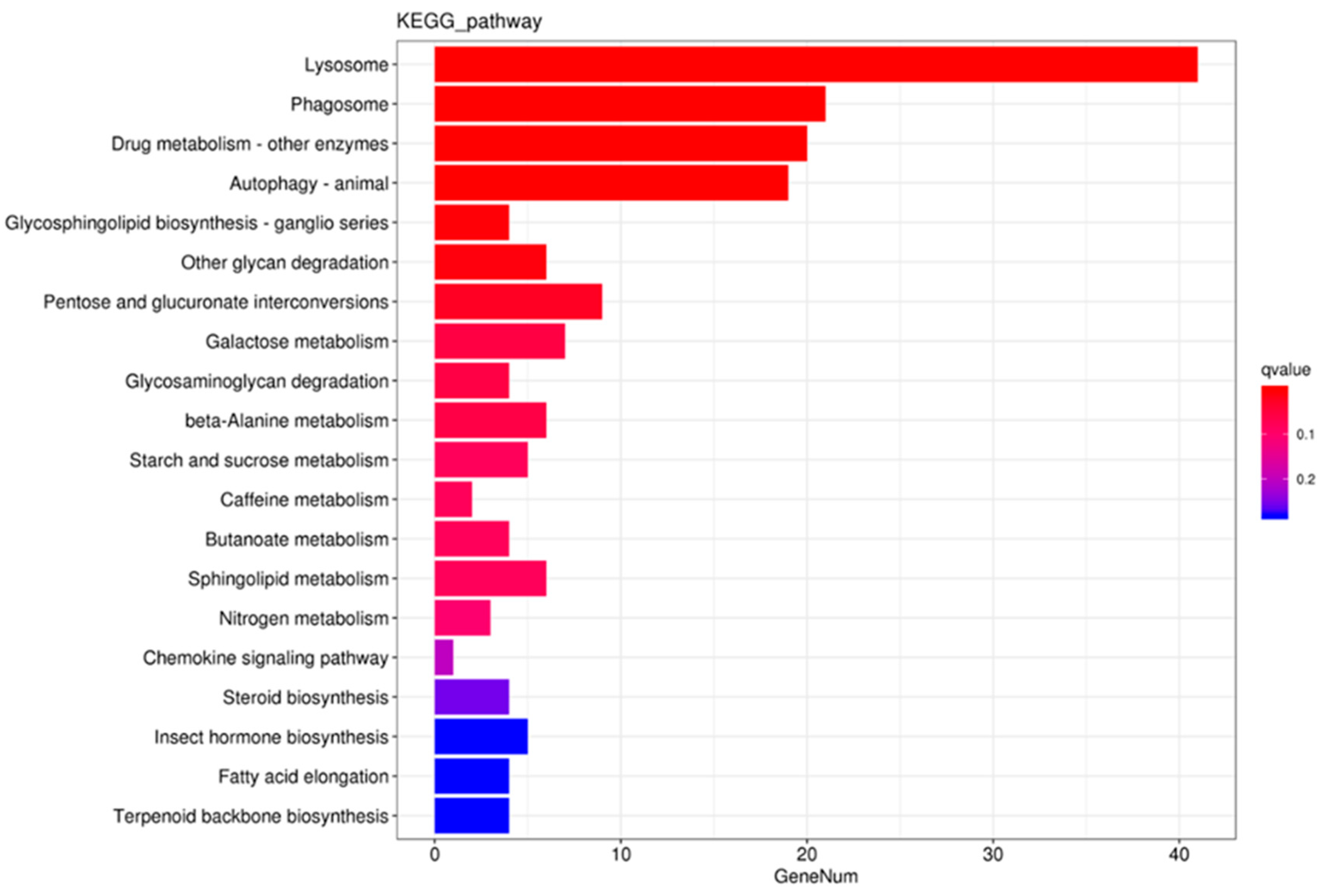
3.2. Transcriptome Analysis
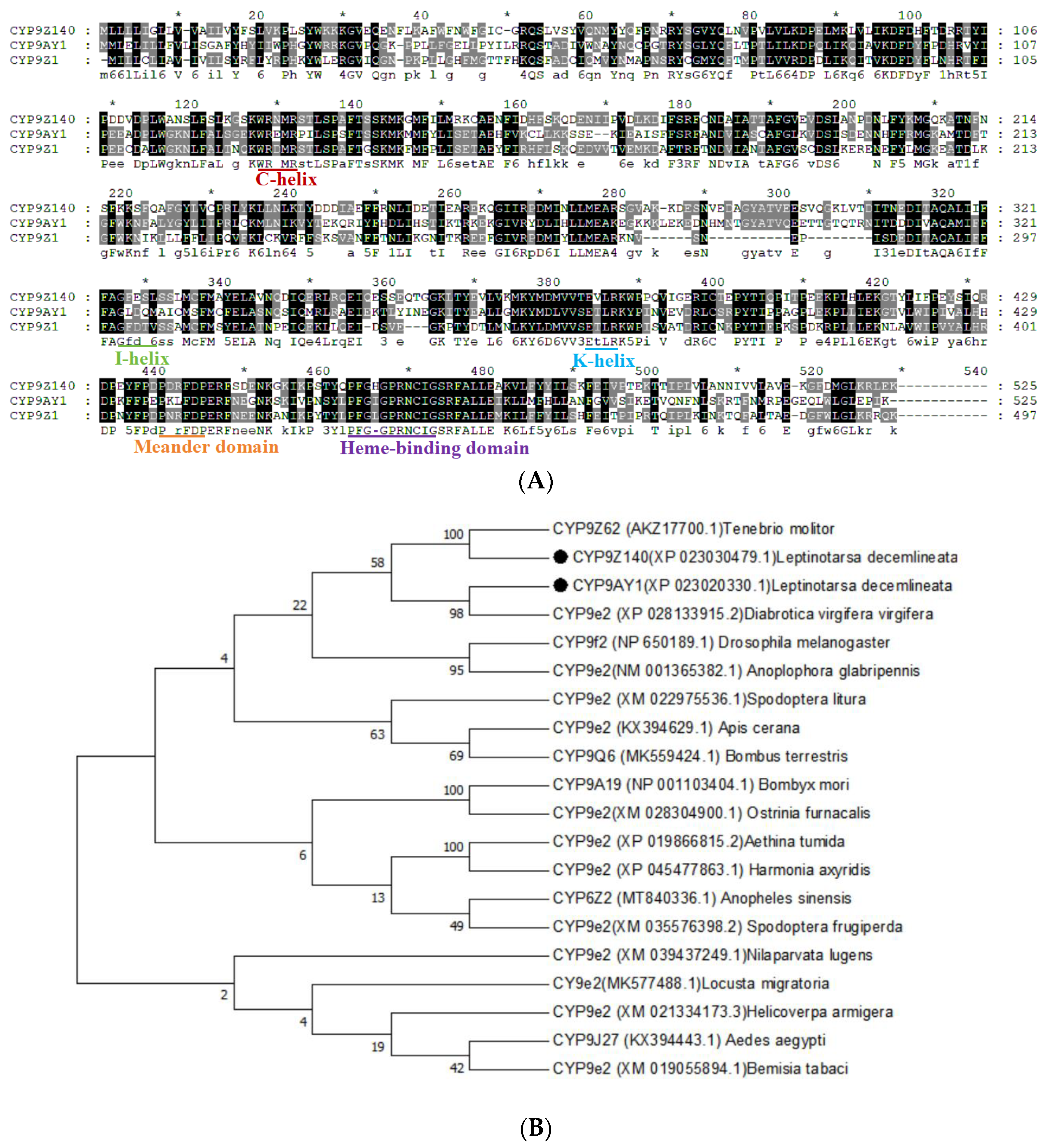
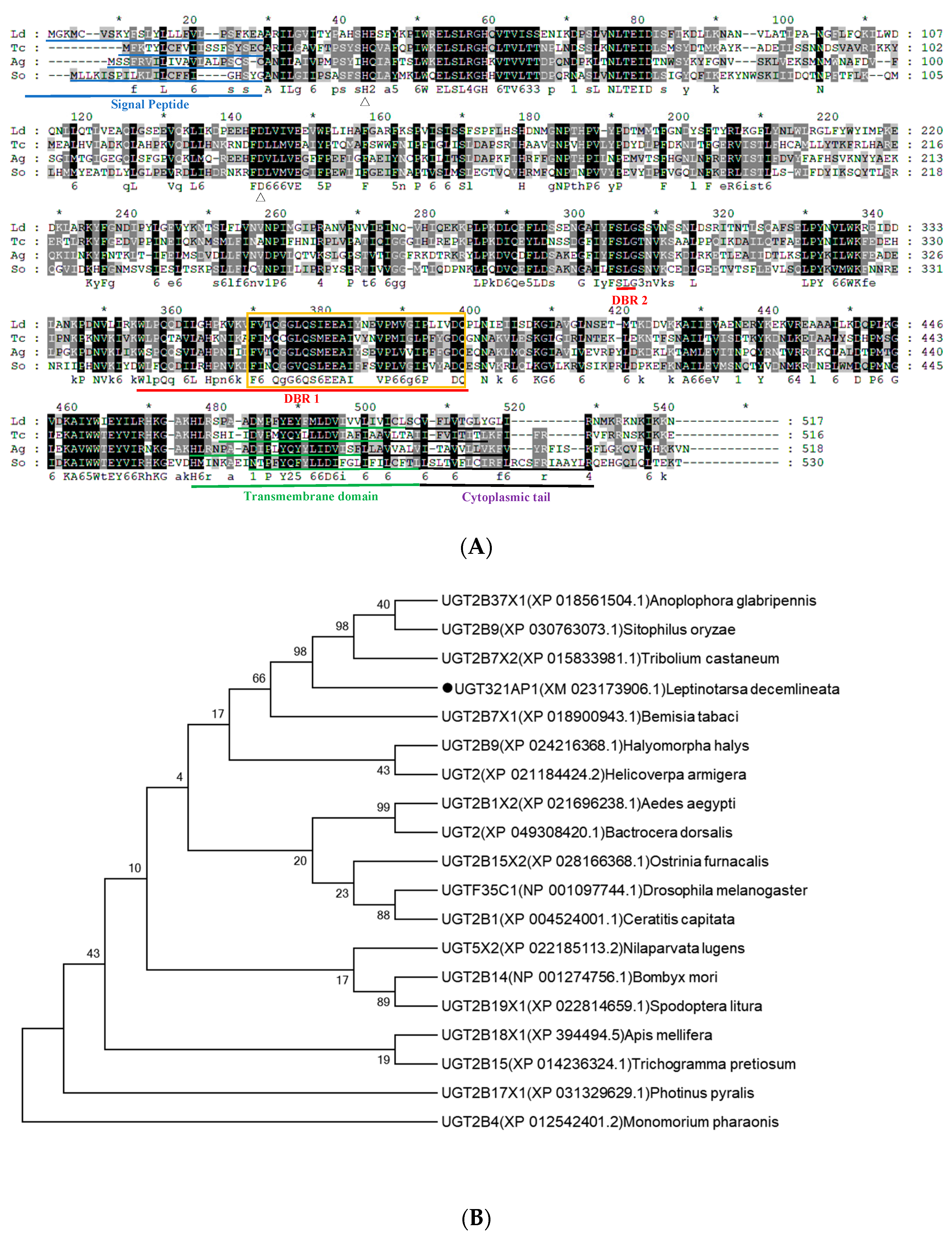
3.3. Gene Structure and Phylogenetic Analysis
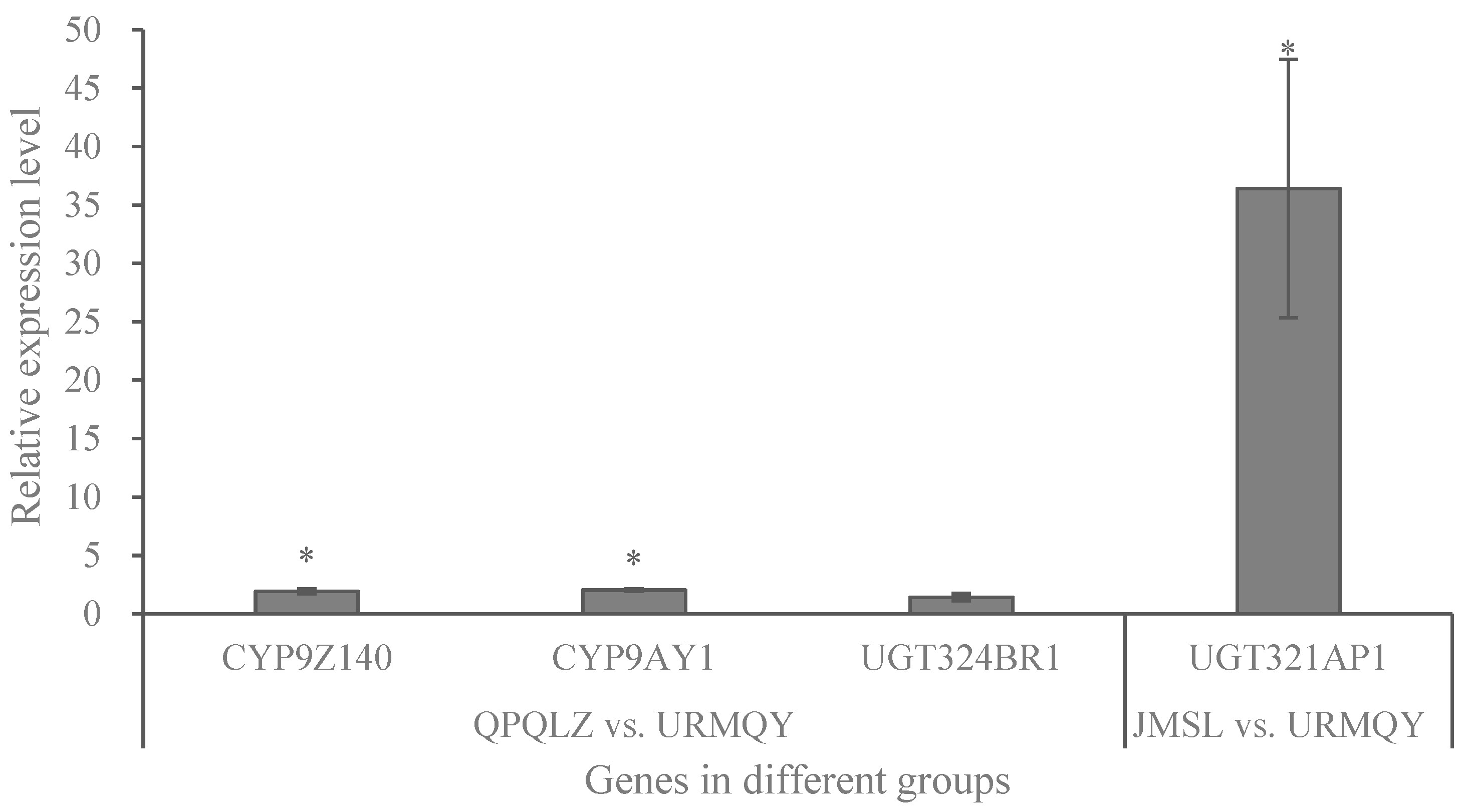
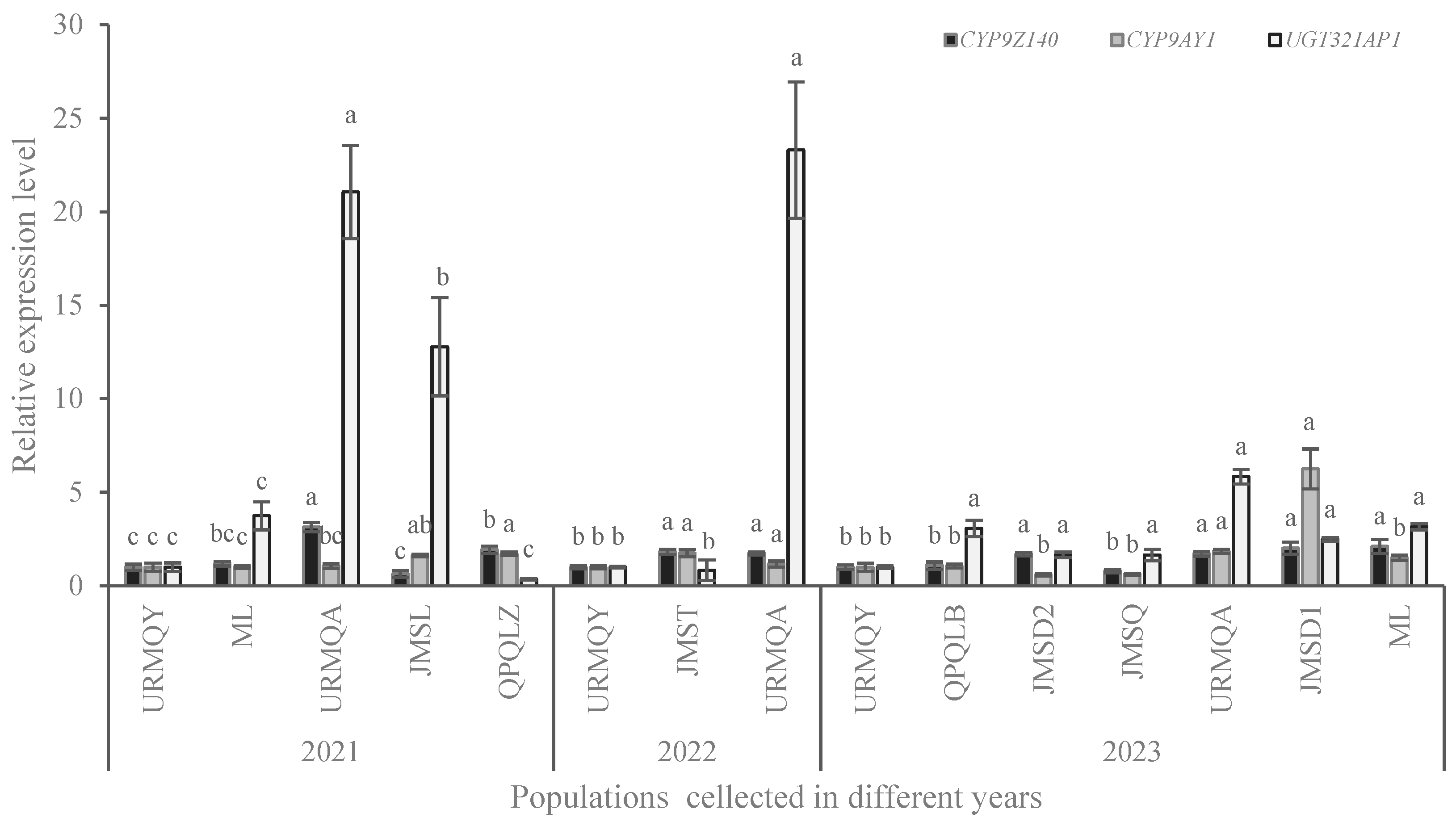
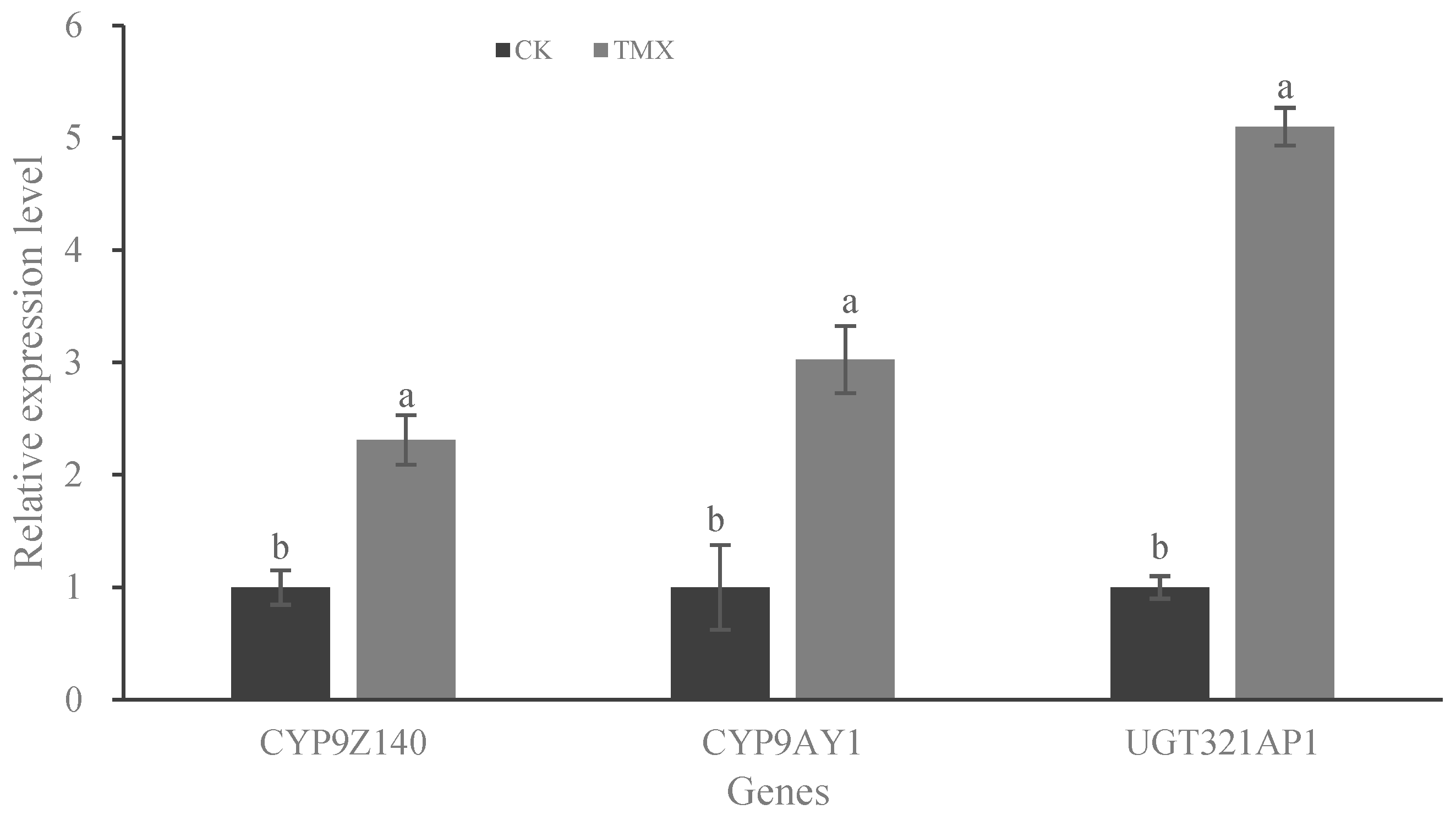
3.4. Expression Analysis of P450 and UGT Genes
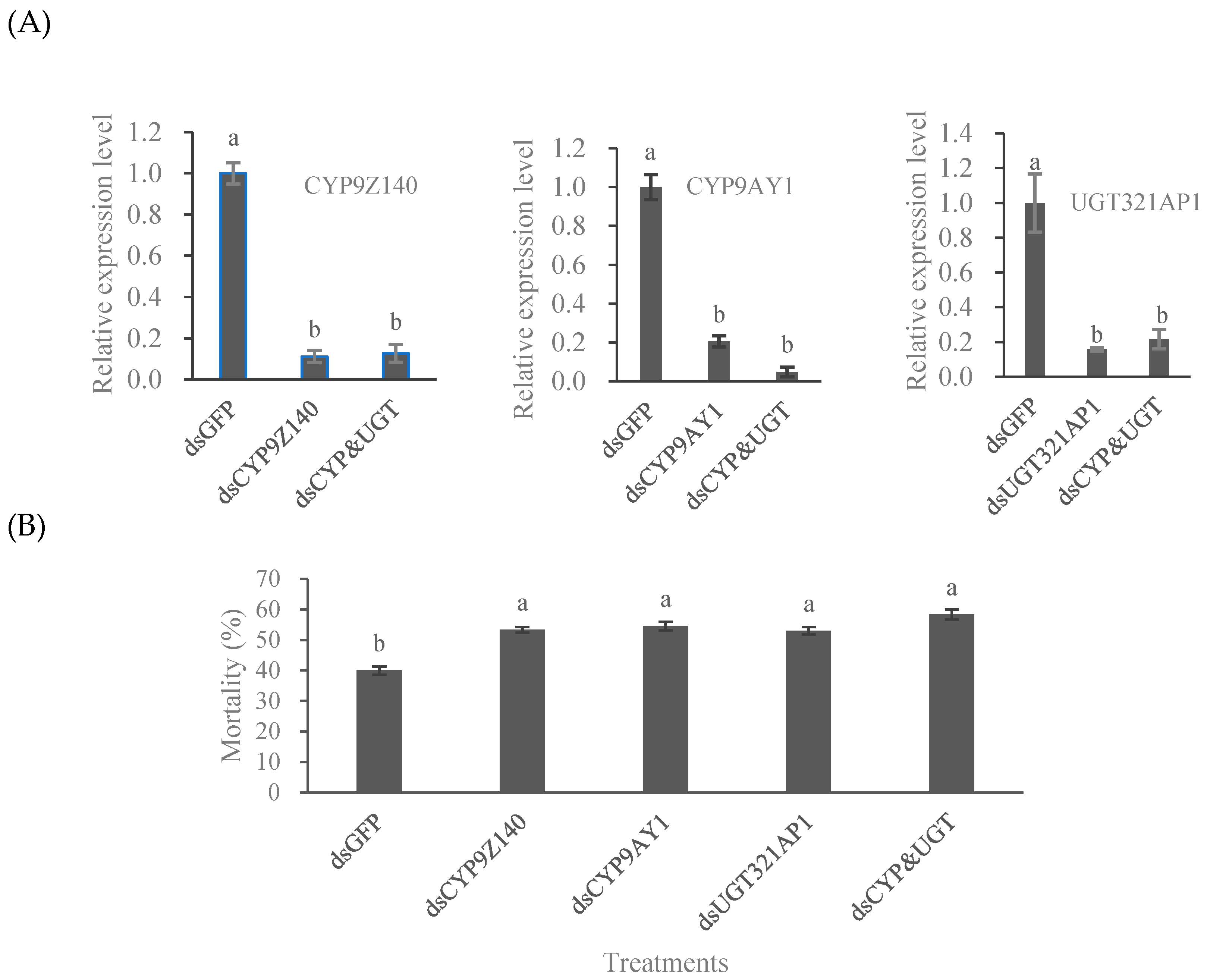
3.5. RNAi Effects of CYP9Z140, CYP9AY1, and UGT321AP1 on L. decemlineata
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.J.; Guo, W.C.; Li, G.Q.; Pan, H.P.; Chen, B.; Hou, W.W.; Li, S.H.; Gao, Y.L. Current status and prospects of the management of important insect pests on potato in China. Plant Protec. 2023, 49, 190–195. [Google Scholar]
- Liu, P.; Jiang, W.H.; Lu, W.P.; Li, G.Q. Susceptibility of Colorado potato beetle Leptinotarsa decemlineata adults from northern Xinjiang Uygur autonomous region to four neonicotinoids. Chin. J. Pestic. Sci. 2011, 13, 271–275. [Google Scholar]
- Shi, X.; Li, S.; Wang, Z.M.; Fu, K.Y.; Fu, W.J.; Jiang, W.H. Resistance monitoring to thiamethoxam and expression analysis of cytochrome P450 genes in Leptinotarsa decemlineata from Xinjiang. Sci. Agric. Sin. 2021, 54, 3004–3016. [Google Scholar] [CrossRef]
- Liu, Z.W.; Williamson, M.S.; Lansdell, S.J.; Denholm, I.; Han, Z.J.; Millar, N.S. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc. Natl. Acad. Sci. USA 2005, 102, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Jouraku, A.; Kuwazaki, S.; Kanazawa, J.; Iwasa, T. The R81T mutation in the nicotinic acetylcholine receptor of Aphis gossypii is associated with neonicotinoid insecticide resistance with differential effects for cyano- and nitro-substituted neonicotinoids. Pestic. Biochem. Physiol. 2017, 143, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chen, J.H.; Li, C.G.; Wang, Q.; Guo, W.C.; Han, Z.J.; Jiang, W.H. The subunit gene Ldα1 of nicotinic acetylcholine receptors plays important roles in the toxicity of imidacloprid and thiamethoxam against Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2016, 127, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Li, S.; Shi, C.C.; Xie, L.J.; Fu, K.Y.; Jiang, W.H. The actions of neonicotinoid insecticides on nicotinic acetylcholine subunits Ldα1 and Ldα8 of Leptinotarsa decemlineata and assembled receptors. Insect Sci. 2022, 29, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.C.; Tian, Y.T.; Wang, Y.Q.; Guo, W.C.; Jiang, W.H. The interaction of nicotinic acetylcholine receptor subunits Ldα3, Ldα8 and Ldβ1 with neonicotinoids in Colorado potato beetle, Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2023, 195, 105558. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Z.; Scheibener, S.; Zhu, Y.C.; Portilla, M.; Reddy, G.V.P. Biochemical and molecular characterization of neonicotinoids resistance in the tarnished plant bug, Lygus lineolaris. Comp. Biochem. Phys. C 2024, 275, 109765. [Google Scholar] [CrossRef]
- Li, X.C.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Feyereisen, R.; Dermauw, W.; Leeuwen, T.V. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015, 121, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Karunker, I.; Morou, E.; Nikou, D.; Nauen, R.; Sertchook, R.; Stevenson, B.J.; Paine, M.J.I.; Morin, S.; Vontas, J. Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect. Biochem. Mol. Biol. 2009, 39, 697–706. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, Y.X.; Sun, H.H.; Liu, Z.W. Metabolic imidacloprid resistance in the brown planthopper, Nilaparvata lugens, relies on multiple P450 enzymes. Insect Biochem. Mol. Biol. 2016, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Xu, H.F.; Pan, Y.; Gao, X.W.; Xi, J.H.; Zhang, J.H.; Shang, Q.L. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shan, T.S.; Liu, Y.; Wang, C.C.; Shi, X.Y.; Gao, X.W. Identification and functional analysis of a cytochrome P450 gene involved in imidacloprid resistance in Bradysia odoriphaga Yang et Zhang. Pestic. Biochem. Physiol. 2019, 153, 129–135. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yang, H.L.; Dong, W.Y.; Gu, Z.X.; Wang, C.C.; Chen, A.Q.; Shi, X.Y.; Gao, X.W. Mutations in the nAChR β1 subunit and overexpression of P450 genes are associated with high resistance to thiamethoxam in melon aphid, Aphis gossypii Glover. Comp. Biochem. Physiol. Part B 2022, 258, 110682. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.G.; Hu, J.Y.; Yang, J.; Yin, C.; Du, T.H.; Huang, M.J.; Fu, B.L.; Gong, P.P.; Liang, J.J.; Liu, S.N.; et al. Cytochrome P450 CYP6DB3 was involved in thiamethoxam and imidacloprid resistance in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2023, 194, 105468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Moural, T.W.; Nelson, D.R.; Palli, S.R. A specialist herbivore pest adaptation to xenobiotics through up-regulation of multiple cytochrome P450s. Sci. Rep. 2016, 6, 20421. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Schoville, S.; Peterson, N.; Lan, Q.; Groves, R.L. Characterizing molecular mechanisms of imidacloprid resistance in melect populations of Leptinotarsa decemlineata in the central sands region of Wisconsin. PLoS ONE 2016, 11, e0147844. [Google Scholar] [CrossRef]
- Clements, J.; Sanchez-Sedillo, B.; Bradfield, A.C.; Groves, R.L. Transcriptomic analysis reveals similarities in genetic activation of detoxification mechanisms resulting from imidacloprid and chlorothalonil exposure. PLoS ONE 2018, 13, e0205881. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaplanoglu, E.; Chapman, P.; Scott, I.M.; Donly, C. Overexpression of a cytochrome P450 and a UDP-glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Rep. 2017, 7, 1762. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Pan, Y.; Tian, F.Y.; Li, J.Y.; Xu, H.F.; Liu, X.M.; Chen, X.W.; Gao, X.W.; Peng, T.F.; Bi, R.; et al. Functional validation of key cytochrome P450 monooxygenase and UDP-glycosyltransferase genes conferring cyantraniliprole resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2021, 176, 104879. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, Y.R.; Zhang, Y.; Yuan, J.J.; Zheng, X.B.; Cao, H.Y.; Qian, K.H.; Feng, J.M.; Tang, Y.X.; Chen, S.R.; et al. Uridine diphosphate glucosyltransferases are involved in spinosad resistance in western flower thrips Frankliniella occidentalis (Pergande). J. Hazard Mater. 2024, 466, 133575. [Google Scholar] [CrossRef]
- Du, T.H.; Fu, B.L.; Wei, X.G.; Yin, C.; Yang, J.; Huang, M.J.; Liang, J.J.; Gong, P.P.; Liu, S.N.; Xue, H.; et al. Knockdown of UGT352A5 decreases the thiamethoxam resistance in Bemisia tabaci (Hemiptera: Gennadius). Int. J. Biol. Macromol. 2021, 186, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Nauen, R.; Reitz, S.; Alyokhin, A.; Zhang, J.; Mota-Sanchez, D.; Kim, Y.; Palli, S.R.; Rondon, S.I.; Nault, B.A.; et al. The new kid on the block in insect pest management: Sprayable RNAi goes commercial. Sci. China Life Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gӧkce, A.; Aksoy, E.; Bakhsh, A. Downregulation of imidacloprid resistant genes alters the biological parameters in Colorado potato beetle, Leptinotarsa decemlineata (Say) (chrysomelidae: Coleoptera). Chemosphere 2020, 240, 124857. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Olson, J.M.; Sedillo, B.S.; Bradford, B.; Groves, R.L. Changes in emergence phenology, fatty acid composition, and xenobiotic-metabolizing enzyme expression is associated with increased insecticide resistance in the Colorado potato beetle. Arch. Insect Biochem. Physiol. 2020, 103, e21630. [Google Scholar] [CrossRef]
- Yang, Z.F.; Yang, H.Y.; He, G.C. Cloning and characterization of two cytochrome P450 CYP6AX1 and CYP6AY1 cDNAs from Nilaparvata lugens Stl (Homoptera: Delphacidae). Arch. Insect Biochem. Physiol. 2010, 64, 88–99. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Deng, L.; Zhang, H.; Wang, Q.; Yin, M.; Song, P.; Elzaki, M.E.A.; Han, Z.; Wu, M. Imidacloprid is hydroxylated by Laodelphax striatellus CYP6AY3v2. Insect Mol. Biol. 2017, 26, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bouafoura, R.; Bastarache, P.; Ouédraogo, B.C.; Dumas, P.; Moffat, C.E.; Vickruck, J.L.; Morin, P.J. Characterization of insecticide response-associated transcripts in the Colo rado potato beetle: Relevance of selected cytochrome P450s and clothianidin. Insects 2022, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Guedes, R.N.C.; Aikins, M.J.; Perkin, L.; Chen, Z.; Phillips, T.W.; Zhu, K.Y.; Opit, G.P.; Hoon, K.; Sun, Y.M.; et al. Genes related to mitochondrial functions are differentially expressed in phosphine-resistant and -susceptible Tribolium castaneum. BMC Genom. 2015, 16, 968. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; He, X.J.; Wang, Z.L.; Yan, W.Y.; Zeng, Z.J.; Wu, X.B. Cloning and expression analysis of cytochrome CYP9E2 gene in the Chinese honeybee, Apis cerana cerana. Acta Entomol. Sin. 2016, 59, 1050–1057. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, K.; Kwon, D.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptome-based identification and characterization of genes commonly responding to five different insecticides in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2018, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Jia, Q.Q.; Zhang, X.B.; Zhang, X.Y.; Liu, S.N.; Park, Y.; Feyereisen, R.; Zhu, K.; Ma, E.; Zhang, J.Z.; et al. CYP303A1 has a conserved function in adult eclosion in Locusta migratoria and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2019, 113, 103210. [Google Scholar] [CrossRef] [PubMed]
- Du, T.H.; Yin, C.; Gui, L.Y.; Liang, J.J.; Liu, S.N.; Fu, B.L.; He, C.; Yang, J.; Wei, X.G.; Gong, P.P.; et al. Over-expression of UDP-glycosyltransferase UGT353G2 confers resistance to neonicotinoids in whitefly (Bemisia tabaci). Pestic. Biochem. Physiol. 2023, 196, 105635. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Gong, Y.H.; Ali, S.; Hou, M.L. Mechanisms of resistance to thiamethoxam and dinotefuran compared to imidacloprid in the brown planthopper: Roles of cytochrome P450 monooxygenase and a P450 gene CYP6ER1. Pestic. Biochem. Physiol. 2018, 150, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Schoville, S.; Peterson, N.; Huseth, A.S.; Lan, Q.; Groves, R.L. RNA interference of three up-regulated transcripts associated with insecticide resistance in an imidacloprid resistant population of Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2017, 135, 35–40. [Google Scholar] [CrossRef]
- Kaplanoglu, E.; Scott, I.M.; Vickruck, J.; Donly, C. Role of CYP9E2 and a long non-coding RNA gene in resistance to a spinosad insecticide in the Colorado potato beetle, Leptinotarsa decemlineata. PLoS ONE 2024, 19, e0304037. [Google Scholar] [CrossRef]

| Sampling Date | Population | Sampling Location | Co-Ordinates |
|---|---|---|---|
| June 2021 | QPQLZ | Development zone of Zakuqiniulu Town, Qapqal County, Yili Prefecture | 43.50 N, 81.17 E |
| June 2021 | ML | Dongcheng Town, Mulei County, Changji Prefecture | 43.83 N, 90.29 E |
| June 2021 | JMSL | Louzhuangzi Village, Jimsar County, Changji Prefecture | 43.46 N, 89.8 E |
| June 2021 | URMQA | Anningqu Town, Xinshi District, Urumqi City | 43.57 N, 87.28 E |
| July 2021 | URMQY | Yongfeng Town, Urumqi County, Urumqi City | 43.40 N, 87.19 E |
| July 2022 | JMST | Taiping Village, Jimsar County, Changji Prefecture | 43.77 N, 89.16 E |
| July 2022 | URMQA | Anningqu town, Xinshi District, Urumqi City | 43.57 N, 87.28 E |
| June 2023 | URMQA | Anningqu Town, Xinshi District, Urumqi City | 43.57 N, 87.28 E |
| June 2023 | JMSQ | Quanzijie Town, Jimsar County, Changji Prefecture | 43.83 N, 87.62 E |
| June 2023 | ML | Dongcheng Town, Mulei County, Changji Prefecture | 43.83 N, 90.29 E |
| July 2023 | QPQLB | Development zone of Ba Town, Qapqal County, Yili Prefecture | 43.84 N, 81.15 E |
| July 2023 | JMSD1 | Dayou Town, Jimsar County, Changji Prefecture | 43.50 N, 89.03 E |
| August 2023 | JMSD2 | Dayou Town, Jimsar County, Changji Prefecture | 43.80 N, 89.04 E |
| Gene | GenBank Accession | Primer Sequence (5′–3′) | Product Size (bp) | Application |
|---|---|---|---|---|
| CYP9Z140 | XP_023030479.1 | F: TAACGAGTTTAGCGTCAG | 1881 | Cloning |
| R: CAATTGTTAATATGGAAGAC | ||||
| CYP9AY1 | XP_023020330.1 | F: TCGGTGGAATACCCATAT | 1916 | |
| R: CAAACCAAATCCAAAACA | ||||
| UGT321AP1 | XM_023173906.1 | F: TCGAAACAGTGTTGGATATT | 1663 | |
| R: AGTTTGACATGGCAACTTAG | ||||
| CYP9Z140 | F: ACATGGCCCGAGGAATTGTA | 157 | qPCR | |
| R: TTTTCAACGGCAAGGACCAC | ||||
| CYP9AY1 | F: CATTCGGCATTGGTCCAAGA | 163 | ||
| R: CCTTCTGGGCGCATATTGAA | ||||
| UGT321AP1 | F: CATCAGGAAATGGCTACCGC R: AGACCCACAGCTATGCCTTT | 189 | ||
| RPL4 | EB761170 | F: AAAGAAACGAGCATTGCCCTTCC | 119 | |
| R: TTGTCGCTGACACTGTAGGGTTGA | ||||
| EF1α | EB754313 | F: AAGGTTCCTTCAAGTATGCGTGGG | 184 | |
| R: GCACAATCAGCTTGCGATGTACCA | ||||
| CYP9Z140 | F: AGATCAGCAAACAGCCAGTAGTCAC | 394 | RNAi | |
| R: TATTAGCCCACAATGGATCAACATC | ||||
| CYP9AY1 | F: TCGCAAATGATGTTATAGCTTCTTG | 231 | ||
| R: ATGGTACTATGGATGAGGTCGTGAA | ||||
| UGT321AP1 | F: CGTCGCTGGTTAATCTCA R: GGGTGCGTAGGGTTGC | 337 |
| Year | Population | Slope ± SE | LD50 (µg/Beetle)/(95% FL) | Resistance Ratio |
|---|---|---|---|---|
| 2021 | URMQY | 2.1167 ± 0.0528 | 0.0311 (0.0238–0.0408) | 1.00 |
| QPQLZ | 1.7757 ± 0.1442 | 0.2592 (0.1797–0.3741) | 8.33 | |
| JMSL | 3.0319 ± 0.2467 | 0.2234 (0.1870–0.2669) | 7.18 | |
| URMQA | 2.0484 ± 0.0546 | 0.0944 (0.0717–0.1243) | 3.04 | |
| ML | 2.5969 ± 0.1200 | 0.0679 (0.0549–0.0842) | 2.18 | |
| 2022 | URMQA | 1.5184 ± 0.0336 | 0.2963 (0.1505–0.5834) | 9.52 |
| JMST | 2.6984 ± 0.1913 | 0.1006 (0.0823–0.1228) | 3.23 | |
| 2023 | ML | 1.0797 ± 0.0213 | 0.2309 (0.1057–0.5044) | 7.42 |
| JMSD1 | 1.9694 ± 0.1116 | 0.2072 (0.1445–0.2970) | 6.66 | |
| URMQA | 1.5521 ± 0.0887 | 0.1440 (0.0838–0.2083) | 4.63 | |
| JMSQ | 3.5502 ± 0.1599 | 0.1310 (0.1068–0.1485) | 4.21 | |
| JMSD2 | 1.9202 ± 0.0577 | 0.0946 (0.0630–0.1422) | 3.04 | |
| QPQLB | 1.3473 ± 0.0956 | 0.0690 (0.0355–0.1319) | 2.22 |
| Samples | Clean Reads | Clean Bases | GC Content (%) | Q30 (%) |
|---|---|---|---|---|
| URMQY | 26,114,887 | 7,748,786,787 | 41.23 | 94.17 |
| JMSL | 24,582,542 | 7,263,561,882 | 40.52 | 93.39 |
| QPQLZ | 21,000,522 | 6,225,521,885 | 40.94 | 94.16 |
| Gene Function | Gene ID | URMQY vs. JMSL | URMQY vs. QPQLZ | ||
|---|---|---|---|---|---|
| log2FC | FDR Value | log2FC | FDR Value | ||
| CYP4C1-like | 111503441 | 1.47 | 0.0018 | ||
| CYP12a5 | 111514589 | 1.64 | 0.0167 | ||
| CYP9e2-like | 111508872 | 1.30 | 0.0035 | ||
| CYP9e2-like | 111518298 | 2.28 | 1.44 × 10−17 | ||
| CYP9e2-like | 111508919 | 1.56 | 5.11 × 10−7 | ||
| CYP4V2-like | 111510743 | 1.46 | 0.0453 | ||
| CYP6a13 | 111506689 | 1.33 | 0.0004 | ||
| CYP4c3-like | 111504218 | 1.47 | 0.0132 | ||
| UGT2B4-like | 111517685 | 2.43 | 0.004 | ||
| UGT2B7-like | 111518183 | 2.50 | 0.008 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tian, Y.; Zhou, D.; Fang, J.; Cao, J.; Shi, C.; Lei, Y.; Fu, K.; Guo, W.; Jiang, W. Expression and Functional Analysis of Two Cytochrome P450 Monooxygenase Genes and a UDP-Glycosyltransferase Gene Linked with Thiamethoxam Resistance in the Colorado Potato Beetle. Insects 2024, 15, 559. https://doi.org/10.3390/insects15080559
Wang Y, Tian Y, Zhou D, Fang J, Cao J, Shi C, Lei Y, Fu K, Guo W, Jiang W. Expression and Functional Analysis of Two Cytochrome P450 Monooxygenase Genes and a UDP-Glycosyltransferase Gene Linked with Thiamethoxam Resistance in the Colorado Potato Beetle. Insects. 2024; 15(8):559. https://doi.org/10.3390/insects15080559
Chicago/Turabian StyleWang, Yaqi, Yitong Tian, Dongdi Zhou, Jiayi Fang, Jingwei Cao, Chengcheng Shi, Yixuan Lei, Kaiyun Fu, Wenchao Guo, and Weihua Jiang. 2024. "Expression and Functional Analysis of Two Cytochrome P450 Monooxygenase Genes and a UDP-Glycosyltransferase Gene Linked with Thiamethoxam Resistance in the Colorado Potato Beetle" Insects 15, no. 8: 559. https://doi.org/10.3390/insects15080559






