Nationwide Inventory of Mosquitoes and the Distribution of Invasive Aedes (Stegomyia) albopictus (Skuse, 1894) on the Islands of Sao Tome and Principe in Central Africa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
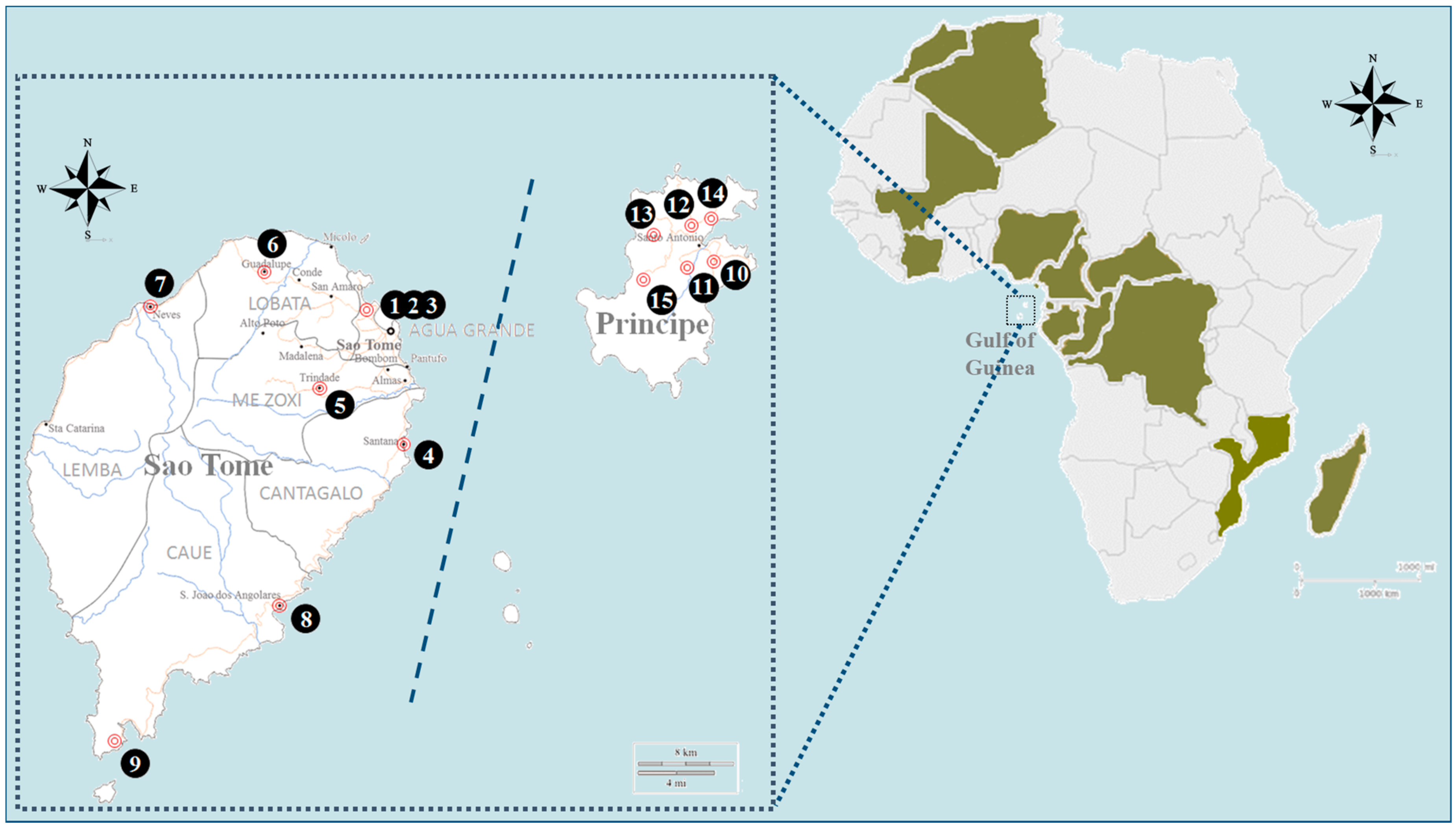
2.2. Mosquito Sampling
2.3. Molecular Analysis
2.4. PCR for Wolbachia-Specific Genes
3. Results
3.1. Identification of Ae. albopictus during Mosquito Surveys
3.2. Distribution of Ae. albopictus and its Breeding Sources
3.3. COI-Based Molecular Analysis
3.4. Wolbachia Infection in Ae. albopictus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Malaria Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 25 June 2024).
- Amarasinghe, A.; Kuritsk, J.N.; Letson, G.W.; Margolis, H.S. Dengue virus infection in Africa. Emerg. Infect. Dis. 2011, 17, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, L.; Winter, D.; Toancha, K.; Borges, A.; Gonçalves, A.; Santos, A.; do Nascimento, M.; Teixeira, N.; Sequeira, Y.S.; Lima, A.K.; et al. Phylogenomics of Dengue virus isolates causing dengue outbreak, São Tomé and Príncipe, 2022. Emerg. Infect. Dis. 2024, 30, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Paupy, C.; Grard, G.; Becquart, P.; Mombo, I.; Nso, B.B.; Kassa Kassa, F.; Nkoghe, D.; Leroy, E.M. Recent introduction and rapid dissemination of chikungunya virus and dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin. Infect. Dis. 2012, 55, e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.N.; Bessaud, M.; Pastorino, B.A.; Gravier, P.; Plumet, S.; Merle, O.L.; Moltini, I.; Coppin, E.; Tock, F.; Daries, W.; et al. Circulation of chikungunya virus in Gabon, 2006–2007. J. Med. Virol. 2008, 80, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.B.; Chang, C.A.; Hamel, D.J.; Mboup, S.; Ndiaye, D.; Imade, G.; Okpokwu, J.; Agbaji, O.; Bei, A.K.; Kanki, P.J. Continued transmission of Zika virus in humans in West Africa, 1992–2016. J. Infect. Dis. 2017, 215, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Koumavor, C.A.K.; Elguero, E.; Leroy, E.M. Potential association between Zika infection and microcephaly during 2007 fever outbreak, Gabon. Emerg. Infect. Dis. 2021, 27, 672–674. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P. Aedes albopictus and the world trade in used tires, 1988–1995: The shape of things to come? J. Am. Mosq. Control Assoc. 1998, 14, 83–94. [Google Scholar]
- Cornel, A.J.; Hunt, R.H. Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. J. Am. Mosq. Control Assoc. 1991, 7, 107–108. [Google Scholar]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; Fontenille, D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.; Cornel, A.J.; Melo, M.; Pereira, H.; Loiseau, C. First record of Aedes albopictus (Skuse 1894) on São tomé Island. Acta Trop. 2017, 171, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, C.; Melo, M.; Lee, Y.; Pereira, H.; Hanemaaijer, M.; Lanzaro, G.C.; Cornel, A.J. High endemism of mosquitoes on São Tomé and Príncipe Islands: Evaluating the general dynamic model in a worldwide island comparison. Insect Conserv. Divers. 2019, 12, 69–79. [Google Scholar] [CrossRef]
- Rader, J.A.; Serrato-Capuchina, A.; Anspach, T.; Matute, D.R. The spread of Aedes albopictus (Diptera: Culicidae) in the islands of São Tomé and Príncipe. Acta Trop. 2024, 251, 107106. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; da Cunha Ramos, H.; Capela, R.; Pires, C. Os mosquitos (Diptera: Culicidae) da Ilha de São Tomé. Garcia Orta Série Zool. 1998, 22, 1–20. [Google Scholar]
- Chen, Y.A.; Lien, J.C.; Tseng, L.F.; Cheng, C.F.; Lin, W.Y.; Wang, H.Y.; Tsai, K.H. Effects of indoor residual spraying and outdoor larval control on Anopheles coluzzii from São Tomé and Príncipe, two islands with pre-eliminated malaria. Malar. J. 2019, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Yellow Fever Expert Committee. Yellow fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e10016388. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; de Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region), 2nd ed.; Publications of the South African Institute for Medical Research: Johannesburg, South Africa, 1968; Volume 54, pp. 1–343. [Google Scholar]
- Rueda, L. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa 2004, 589, 1–60. [Google Scholar] [CrossRef]
- Yen, T.Y.; Trovoada dos Santos Mde, J.; Tseng, L.F.; Chang, S.F.; Cheng, C.F.; Carvalho, A.V.; Shu, P.Y.; Lien, J.C.; Tsai, K.H. Seroprevalence of antibodies against dengue virus among pregnant women in the Democratic Republic of Sao Tome and Principe. Acta Trop. 2016, 155, 58–62. [Google Scholar] [CrossRef]
- Huang, Y. The subgenus Stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae). Zootaxa 2004, 700, 1–120. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Barr, A.R. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 1973, 22, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Bruno de Mesquita, A.J.V.H. Contribuição para o Estudo do Sazonismo em São Tomé. Bol. Agència Geral Colónias 1946, 22, 13–66. [Google Scholar]
- Gãndara, A. Subsídio para o estudo dos culicideos (Diptera) de São Tomé e Príncipe. An. Inst. Med. Trop. 1956, 13, 419–428. [Google Scholar]
- Lien, J.C.; Lin, C.C.; Lin, M.C.; Tseng, L.F. A new species of the genus Coquillettidia (Diptera, Culicidae) from the Democratic Republic of Sao Tome and Principe. J. Natl. Taiwan. Mus. 2008, 61, 55–59. [Google Scholar]
- Lin, C.C.; Tseng, L.F.; Lien, J.C. A new species of the genus Culiseta (Diptera, Culicidae) from the Democratic Republic of Sao Tome and Principe. J. Natl. Taiwan Mus. 2008, 61, 89–96. [Google Scholar]
- Lien, J.C.; Lin, C.C. Mimomyia (Etorleptiomyia) mediolineata (Theobald, 1904), a mosquito new to the Democratic Republic of Sao Tome and Principe, West Africa (Diptera, Culicidae). J. Natl. Taiwan Mus. 2009, 62, 65–74. [Google Scholar]
- Marshall, J.C.; Pinto, J.; Charlwood, J.D.; Gentile, G.; Santolamazza, F.; Simard, F.; Della Torre, A.; Donnelly, M.J.; Caccone, A. Exploring the origin and degree of genetic isolation of Anopheles gambiae from the islands of São Tomé and Príncipe, potential sites for testing transgenic-based vector control. Evol. Appl. 2008, 1, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.F.; Chang, W.C.; Ferreira, M.C.; Wu, C.H.; Rampão, H.S.; Lien, J.C. Rapid control of malaria by means of indoor residual spraying of alphacypermethrin in the Democratic Republic of São Tomé and Príncipe. Am. J. Trop. Med. Hyg. 2008, 78, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Ramos, H.C.; Pires, C.A. Sobre os vectores da malária em São Tomé e Príncipe. Garcia Horta Ser. Zool. 1990, 15, 135–152. [Google Scholar]
- Kamgang, B.; Acântara, J.; Tedjou, A.; Keumeni, C.; Yougang, A.; Ancia, A.; Bigirimana, F.; Clarke, S.E.; Gil, V.S.; Wondji, C. Entomological surveys and insecticide susceptibility profile of Aedes aegypti during the dengue outbreak in Sao Tome and Principe in 2022. PLoS Negl. Trop. Dis. 2024, 18, e0011903. [Google Scholar] [CrossRef]
- Coffinet, T.; Mourou, J.R.; Pradines, B.; Toto, J.C.; Jarjaval, F.; Amalvict, R.; Kombila, M.; Carnevale, P.; Pages, F. First record of Aedes albopictus in Gabon. J. Am. Mosq. Control Assoc. 2007, 23, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Laganier, R.; Nangouma, A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop. Med. Int. Health 2010, 15, 1185–1189. [Google Scholar] [CrossRef]
- Fontenille, D.; Toto, J.C. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg. Infect. Dis. 2001, 7, 1066–1067. [Google Scholar] [CrossRef]
- Kelvin, A.A. Outbreak of chikungunya in the Republic of Congo and the global picture. J. Infect. Dev. Ctries. 2011, 5, 441–444. [Google Scholar] [CrossRef]
- Savage, H.M.; Ezike, V.I.; Nwankwo, A.C.; Spiegel, R.; Miller, B. First record of breeding populations of Aedes albopictus in continental Africa: Implications for arboviral transmission. J. Am. Mosq. Control Assoc. 1992, 8, 101–103. [Google Scholar]
- Toto, J.C.; Abaga, S.; Carnevale, P.; Simard, F. First report of the oriental mosquito Aedes albopictus on the West African island of Bioko, Equatorial Guinea. Med. Vet. Entomol. 2003, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Ngoagouni, C.; Manirakiza, A.; Nakouné, E.; Paupy, C.; Kazanji, M. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl. Trop. Dis. 2013, 7, e2590. [Google Scholar] [CrossRef]
- Paupy, C.; Kassa Kassa, F.; Caron, M.; Nkoghé, D.; Leroy, E.M. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef]
- Agha, S.B.; Tchouassi, D.P. Urbanization of Aedes mosquito populations and evolution of arboviral disease risk in Africa. Curr. Opin. Insect Sci. 2022, 54, 100988. [Google Scholar] [CrossRef]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.P.; et al. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef]
- Stump, E.; Childs, L.M.; Walker, M. Parasitism of Aedes albopictus by Ascogregarina taiwanensis lowers its competitive ability against Aedes triseriatus. Parasites Vectors 2021, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Brengues, C.; Fontenille, D.; Njiokou, F.; Simard, F.; Paupy, C. Genetic structure of the tiger mosquito, Aedes albopictus, in Cameroon (Central Africa). PLoS ONE 2011, 6, e20257. [Google Scholar] [CrossRef]
- Kamgang, B.; Wilson-Bahun, T.A.; Irving, H.; Kusimo, M.O.; Lenga, A.; Wondji, C.S. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018, 3, 79. [Google Scholar] [CrossRef]
- Battaglia, V.; Agostini, V.; Moroni, E.; Colombo, G.; Lombardo, G.; Rambaldi Migliore, N.; Gabrieli, P.; Garofalo, M.; Gagliardi, S.; Gomulski, L.M.; et al. The worldwide spread of Aedes albopictus: New insights from mitogenomes. Front. Genet. 2022, 13, 931163. [Google Scholar] [CrossRef]
- Battaglia, V.; Gabrieli, P.; Brandini, S.; Capodiferro, M.R.; Javier, P.A.; Chen, X.G.; Achilli, A.; Semino, O.; Gomulski, L.M.; Malacrida, A.R.; et al. The worldwide spread of the tiger mosquito as revealed by mitogenome haplogroup diversity. Front. Genet. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Lo, E.; Hu, R.; Metzger, M.E.; Cummings, R.; Bonizzoni, M.; Fujioka, K.K.; Sorvillo, T.E.; Kluh, S.; Healy, S.P.; et al. Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS ONE 2013, 8, e68586. [Google Scholar] [CrossRef]
- Effler, P.V.; Pang, L.; Kitsutani, P.; Vorndam, V.; Nakata, M.; Ayers, T.; Elm, J.; Tom, T.; Reiter, P.; Rigau-Perez, J.G.; et al. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 2005, 11, 742–749. [Google Scholar] [CrossRef]
- Pereira-Dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A systematic review: Is Aedes albopictus an efficient bridge vector for zoonotic arboviruses? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill. 2017, 22, 17-00646. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Ba, Y.; Faye, O.; Soumare, M.L.; Dia, I.; Sall, A.A. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 493–498. [Google Scholar] [CrossRef]
- Gubler, D.J.; Nalim, S.; Tan, R.; Saipan, H.; Sulianti Saroso, J. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 1979, 28, 1045–1052. [Google Scholar] [CrossRef]
- Gaye, A.; Wang, E.; Vasilakis, N.; Guzman, H.; Diallo, D.; Talla, C.; Ba, Y.; Dia, I.; Weaver, S.C.; Diallo, M. Potential for sylvatic and urban Aedes mosquitoes from Senegal to transmit the new emerging dengue serotypes 1, 3 and 4 in West Africa. PLoS Negl. Trop. Dis. 2019, 13, e0007043. [Google Scholar] [CrossRef]
- Jiolle, D.; Moltini-Conclois, I.; Obame-Nkoghe, J.; Yangari, P.; Porciani, A.; Scheid, B.; Kengne, P.; Ayala, D.; Failloux, A.B.; Paupy, C. Experimental infections with Zika virus strains reveal high vector competence of Aedes albopictus and Aedes aegypti populations from Gabon (Central Africa) for the African virus lineage. Emerg. Microbes Infect. 2021, 10, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Nchoutpouen, E.; Simard, F.; Paupy, C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasites Vectors 2012, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Pages, F.; Jarjaval, F.; Failloux, A.B. Introduction of Aedes albopictus in Gabon: What consequences for dengue and chikungunya transmission? Trop. Med. Int. Health 2008, 13, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Kitrayapong, P.; Baimai, V.; O’Neill, S.L. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 2002, 66, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, M.S.; Arias-Goeta, C.; Di Genua, C.; Failloux, A.B.; Sinkins, S.P. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits chikungunya virus. PLoS Negl. Trop. Dis. 2013, 7, e2152. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, M.S.; Arias-Goeta, C.; Failloux, A.B.; Sinkins, S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA 2012, 109, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.H.; Lien, J.C.; Huang, C.G.; Wu, W.J.; Chen, W.J. Molecular (sub) grouping of endosymbiont Wolbachia infection among mosquitoes of Taiwan. J. Med. Entomol. 2004, 41, 677–683. [Google Scholar] [CrossRef]
- Pinchoff, J.; Silva, M.; Spielman, K.; Hutchinson, P. Use of effective lids reduces presence of mosquito larvae in household water storage containers in urban and peri-urban Zika risk areas of Guatemala, Honduras, and El Salvador. Parasites Vectors 2021, 14, 167. [Google Scholar] [CrossRef]
- Kusumawathie, P.H.; Yapabandarab, A.M.; Jayasooriya, G.A.; Walisinghe, C. Effectiveness of net covers on water storage tanks for the control of dengue vectors in Sri Lanka. J. Vector Borne Dis. 2009, 46, 160–163. [Google Scholar] [PubMed]
- Harwood, J.F.; Farooq, M.; Turnwall, B.T.; Richardson, A.G. Evaluating liquid and granular Bacillus thuringiensis var. israelensis broadcast applications for controlling vectors of dengue and chikungunya viruses in artificial containers and tree holes. J. Med. Entomol. 2015, 52, 663–667. [Google Scholar] [PubMed]


| Genus | Mosquito Species | Year/Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 1 | 2004 | 2005 | 2006 1 | 2010 | 2011 | 2012 | 2015 1 | 2016 | ||
| Aedes | Ae. (Stegomyia) aegypti | + | + | + | + | + | + | + | + | + |
| Ae. (Stegomyia) albopictus | − | − | − | − | − | − | − | + | + | |
| Ae. (Stegomyia) africanus | − | − | − | − | − | − | − | − | − | |
| Ae. (Neomelaniconion) circumluteolus | + | + | + | + | − | + | − | + | − | |
| Ae. (Aedimorphus) gandarai | − | + | + | + | − | − | − | − | − | |
| Ae. (Aedimorphus) nigricephalus | + | + | + | + | + | + | + | + | − | |
| Anopheles | An. (Anopheles) coustani | − | + | + | + | − | + | + | + | + |
| An. (Cellia) funestus | − | − | − | − | − | − | − | − | − | |
| An. (Cellia) coluzzii | + | + | + | + | + | + | + | + | + | |
| An. (Anopheles) paludis | − | − | − | − | − | − | − | − | − | |
| An. (Cellia) pharoensis | − | − | − | − | − | − | − | − | − | |
| Culex | Cx. (Culex) annulioris | − | − | − | + | − | − | − | − | − |
| Cx. (Culex) antennatus | + | + | + | + | − | + | + | + | + | |
| Cx. (Culiciomyia) cambournaci | + | + | + | + | − | − | − | + | − | |
| Cx. (Culiciomyia) cinerellus | + | + | + | + | + | + | + | + | + | |
| Cx. (Culex) decens | + | + | + | + | + | + | + | + | − | |
| Cx. (Eumelanomyia) inconspicuosus | + | + | + | + | − | + | − | + | + | |
| Cx. (Culex) invidiosus | − | + | + | + | − | + | − | + | + | |
| Cx. (Culiciomyia) macfiei | − | − | − | + | − | − | − | − | − | |
| Cx. (Eumelanomyia) micolo | − | − | − | + | − | − | + | + | − | |
| Cx. (Culiciomyia) nebulosus | − | + | − | + | − | − | − | − | − | |
| Cx. (Culex) pipiens | − | − | − | − | − | − | − | − | − | |
| Cx. (Culex) poicilipes 3 | − | − | + | − | − | − | − | − | − | |
| Cx. (Culex) quinquefasciatus | + | + | + | + | + | + | + | + | + | |
| Cx. (Culex) rima | − | − | − | − | − | − | − | − | − | |
| Cx. (Culex) tamsi | + | − | − | + | − | − | − | − | − | |
| Cx. (Culex) thalassius | − | + | + | + | − | + | + | + | − | |
| Cx. (Lutzia) tigripes | + | + | + | + | − | + | + | + | − | |
| Coquillettidia | Cq. saotomensis 2 | − | − | + | − | − | − | − | − | − |
| Culiseta | Cs. (Theomyia) fraseri | − | − | − | − | − | − | − | − | − |
| Cs. (Theomyia) wui 2 | + | − | − | − | − | − | − | − | − | |
| Eretmapodites | Er. chrysogaster | − | + | + | + | − | − | − | − | − |
| Mansonia | Ma. (Mansonioides) africana | − | − | − | − | − | − | − | − | − |
| Ma. (Coquillettidia) annetti 3 | − | − | − | + | − | − | − | − | − | |
| Mimomyia | Mi. (Etoreptiomyia) mediolineata 3 | + | + | + | + | + | + | + | + | − |
| Polyleptiomyia | Po. gandarai | − | − | − | − | − | − | − | + | + |
| Toxorhynchites | Tx. (Toxorhynchites) brevipalpis conradti | − | − | − | + | − | − | − | − | − |
| Tx. (Toxorhynchites) capelai | − | + | − | − | − | − | − | − | − | |
| Uranotaenia | Ur. (Uranotaenia) alboabdominalis 3 | + | − | − | − | − | − | − | − | − |
| Ur. (Uranotaenia) balfouri | + | + | + | + | + | + | + | + | + | |
| Ur. (Pseudoficalbia) capelai | − | + | + | − | − | − | − | − | − | |
| Ur. (Uranotaenia) fraseri 3 | + | − | − | + | − | − | − | − | − | |
| Ur. (Pseudoficalbia) micromelas | − | + | + | + | − | − | − | + | + | |
| Ur. (Pseudoficalbia) principiensis | − | + | − | − | − | − | − | − | − | |
| Total species | 16 | 23 | 21 | 27 | 8 | 15 | 13 | 20 | 12 | |
| Mosquitoes | 2015 | 2016 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AG 1 | CT 2 | MZ 3 | LO 4 | LE 5 | CU 6 | PR 7 | Total | AG 1 | CT 2 | MZ 3 | LO 4 | LE 5 | CU 6 | PR 7 | Total | |
| Aedes | ||||||||||||||||
| Ae. (Stegomyia) albopictus | 56 | 8 | 34 | 6 | 0 | 15 | 0 | 119 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| Ae. (Stegomyia) aegypti | 22 | 0 | 5 | 2 | 0 | 1 | 0 | 30 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Ae. (Aedimorphus) nigricephalus | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ae. (Neomelaniconion) circumluteolus | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anopheles | ||||||||||||||||
| An. (Cellia) coluzzii | 1001 | 148 | 24 | 194 | 169 | 203 | 58 | 1797 | 548 | 70 | 31 | 86 | 142 | 183 | 515 | 1575 |
| An. coustani | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Culex | ||||||||||||||||
| Cx. antennatus | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cx. (Culiciomyia) cambournaci | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cx. (Culiciomyia) cinerellus | 170 | 23 | 0 | 10 | 2 | 23 | 0 | 228 | 3 | 21 | 1 | 7 | 2 | 2 | 0 | 36 |
| Cx. decens | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cx. (Eumelanomyia) inconspicuosus | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cx. invidiosus | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Cx. (Eumelanomyia) micolo | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cx. quinquefasciatus | 3029 | 60 | 5 | 32 | 66 | 23 | 0 | 3215 | 23 | 0 | 0 | 0 | 2 | 0 | 0 | 25 |
| Cx. thalassius | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lutzia | ||||||||||||||||
| Lutzia (Metalutzia) tigripes | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mimomyia | ||||||||||||||||
| Mi. (Etorleptiomyia) mediolineata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polyleptiomyia | ||||||||||||||||
| Po. gandarai | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Uranotaenia | ||||||||||||||||
| Ur. balfouri | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Ur. (Pseudoficalbia) micromelas | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Total | 4419 | 239 | 68 | 244 | 237 | 265 | 58 | 5530 | 619 | 91 | 32 | 93 | 146 | 185 | 515 | 1681 |
| Region | District | Locality | Type of Artificial Container | Containers with Larvae/Containers with Water (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | ||||||||||
| Aal 1 | Aae 2 | Ani 3 | Aal 1 | Aae 2 | Ani 3 | Aga 4 | Aci 5 | ||||
| Sao Tome | Agua Grande | Ponte Graca | Waste cans, water-storage tanks | 20/156 (12.8) | 3/156 (1.9) | 1/156 (0.6) | 64/180 (35.6) | 12/180 (6.7) | 3/180 (1.7) | 6/180 (3.3) | 0/180 (0) |
| Compo de Milho | Discarded plastic containers, ovitraps placed at sentinel sites | 16/150 (10.7) | 1/150 (0.7) | 3/150 (2) | 45/179 (25.1) | 21/179 (11.7) | 0/179 (0) | 0/179 (0) | 0/179 (0) | ||
| Boa Morte | Used tires, water-storage tanks | 20/158 (12.7) | 13/158 (8.2) | 0/158 (0) | 24/151 (15.9) | 14/151 (9.3) | 0/151 (0) | 0/151 (0) | 3/151 (2.0) | ||
| Cantagalo | Santana | Used tires, discarded tools, water-storage tanks, washbasins | nd | nd | nd | 22/186 (11.8) | 0/186 (0) | 0/186 (0) | 0/186 (0) | 0/186 (0) | |
| MeZoxi | Trindade | Waste cans | nd | nd | nd | 42/181 (23.2) | 0/181 (0) | 0/181 (0) | 2/181 (1.1) | 1/181 (0) | |
| Lobata | Guadalupe | Used tires | 7/178 (3.9) | 0/178 (0) | 0/178 (0) | 23/186 (12.4) | 0/186 (0) | 0/186 (0) | 0/186 (0) | 0/186 (0) | |
| Lemba | Neves | Used tires | nd | nd | nd | 45/185 (24.3) | 0/185 (0) | 0/185 (0) | 0/185 (0) | 0/185 (0) | |
| Caue | Sao Joao dos Angolares | Used tires | nd | nd | nd | 26/197 (13.2) | 2/197 (1.0) | 0/197(0) | 0/197 (0) | 0/197 (0) | |
| Porto Alegre | Used tires | nd | nd | nd | 6/118 (5.1) | 0/118 (0) | 0/118 (0) | 1/118 (0.8) | 0/118 (0) | ||
| Total | 64/642 (10.0) | 17/642 (2.6) | 4/642 (0.6) | 297/1563 (19.0) | 49/1563 (3.1) | 3/1563 (0.2) | 9/1563 (0.6) | 3/1563 (0.2) | |||
| Principe | Príncipe | Santo Cristo | Water-storage tanks | nd | nd | nd | 13/176 (7.4) | 0/176 (0) | 0/176 (0) | 0/176 (0) | 0/176 (0) |
| Porto Real | Used tires | nd | nd | nd | 25/191 (13.1) | 0/191 (0) | 0/191 (0) | 0/191 (0) | 0/191 (0) | ||
| Aeroporto | Used tires, leakage of water tower | nd | nd | nd | 114/250 (45.6) | 0/250 (0) | 0/250 (0) | 0/250 (0) | 0/250 (0) | ||
| Ponta do Sol | Waste cans, water-storage tanks | nd | nd | nd | 7/146 (4.8) | 0/146 (0) | 0/146 (0) | 0/146 (0) | 0/146 (0) | ||
| Picao | Water-storage tanks | nd | nd | nd | 20/203 (9.9) | 0/203 (0) | 0/203 (0) | 0/203 (0) | 0/203 (0) | ||
| Sao Joaquim | Buckets | nd | nd | nd | 12/169 (7.1) | 0/169 (0) | 0/169 (0) | 0/169 (0) | 0/169 (0) | ||
| Total | nd | nd | nd | 191/1135 (16.8) | 0/1135 (0) | 0/1135 (0) | 0/1135 (0) | 0/1135 (0) | |||
| Total | 64/642 (10.0) | 17/642 (2.6) | 4/642 (0.6) | 488/2698 (18.1) | 49/2698 (1.8) | 3/2698 (0.1) | 9/2698 (3.3) | 3/2698 (0.1) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-Y.; Cheng, C.-F.; Tseng, L.-F.; Carvalho, R.M.C.d.A.; Tsai, K.-H. Nationwide Inventory of Mosquitoes and the Distribution of Invasive Aedes (Stegomyia) albopictus (Skuse, 1894) on the Islands of Sao Tome and Principe in Central Africa. Insects 2024, 15, 560. https://doi.org/10.3390/insects15080560
Yen T-Y, Cheng C-F, Tseng L-F, Carvalho RMCdA, Tsai K-H. Nationwide Inventory of Mosquitoes and the Distribution of Invasive Aedes (Stegomyia) albopictus (Skuse, 1894) on the Islands of Sao Tome and Principe in Central Africa. Insects. 2024; 15(8):560. https://doi.org/10.3390/insects15080560
Chicago/Turabian StyleYen, Tsai-Ying, Chien-Fu Cheng, Lien-Fen Tseng, Ronalg Mendes Costa d’ Assunção Carvalho, and Kun-Hsien Tsai. 2024. "Nationwide Inventory of Mosquitoes and the Distribution of Invasive Aedes (Stegomyia) albopictus (Skuse, 1894) on the Islands of Sao Tome and Principe in Central Africa" Insects 15, no. 8: 560. https://doi.org/10.3390/insects15080560





