The Diel Activity Pattern of Haemaphysalis longicornis and Its Relationship with Climatic Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
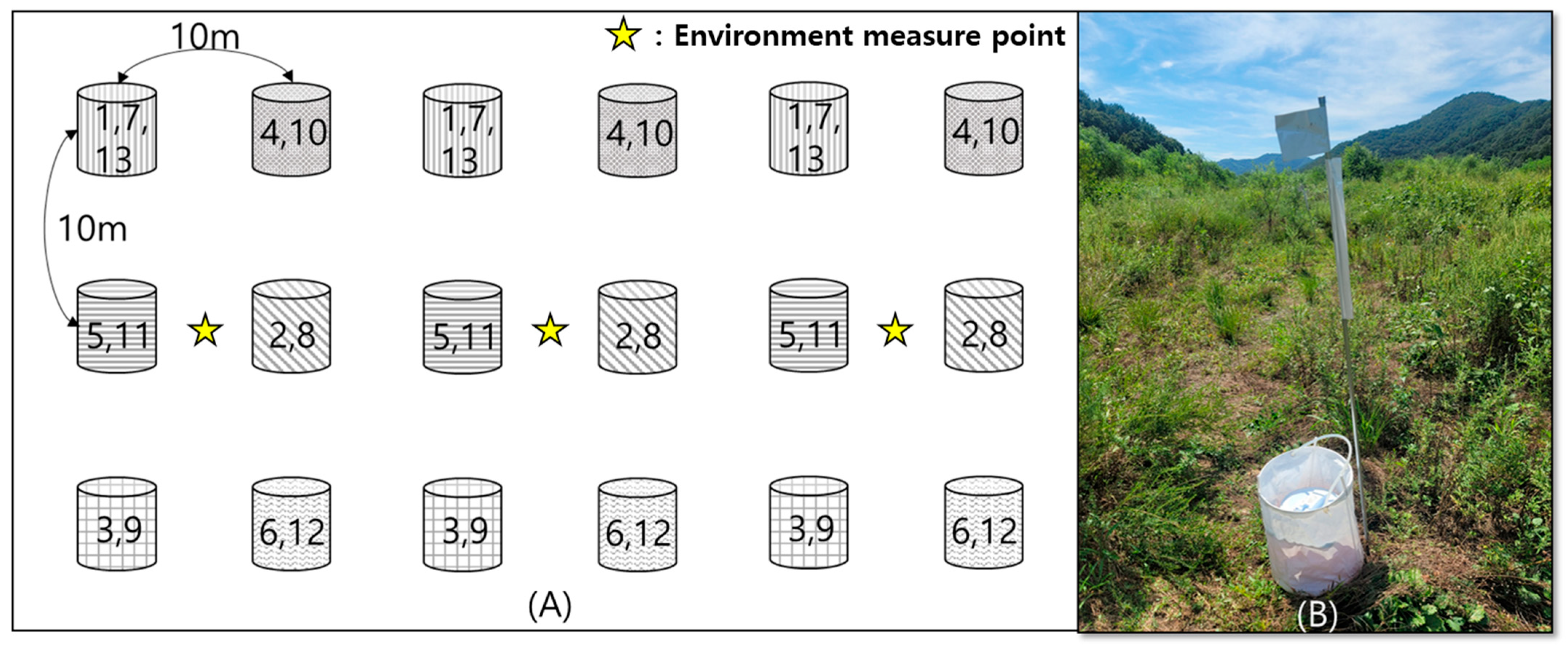
2.1. Study Site
2.2. Sampling Methods and Identification
2.3. Environmental Measurements
2.4. Statistical Analysis
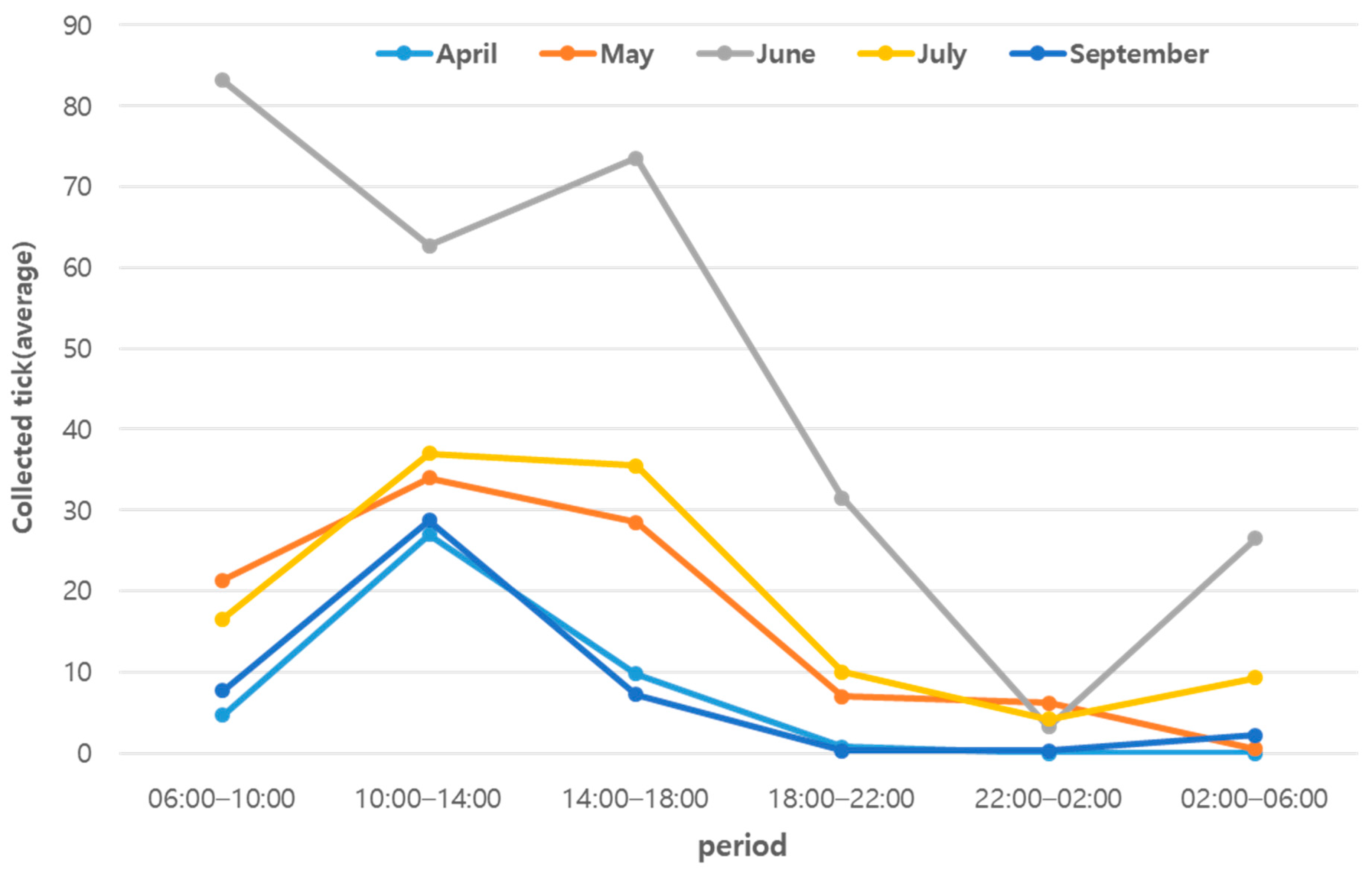
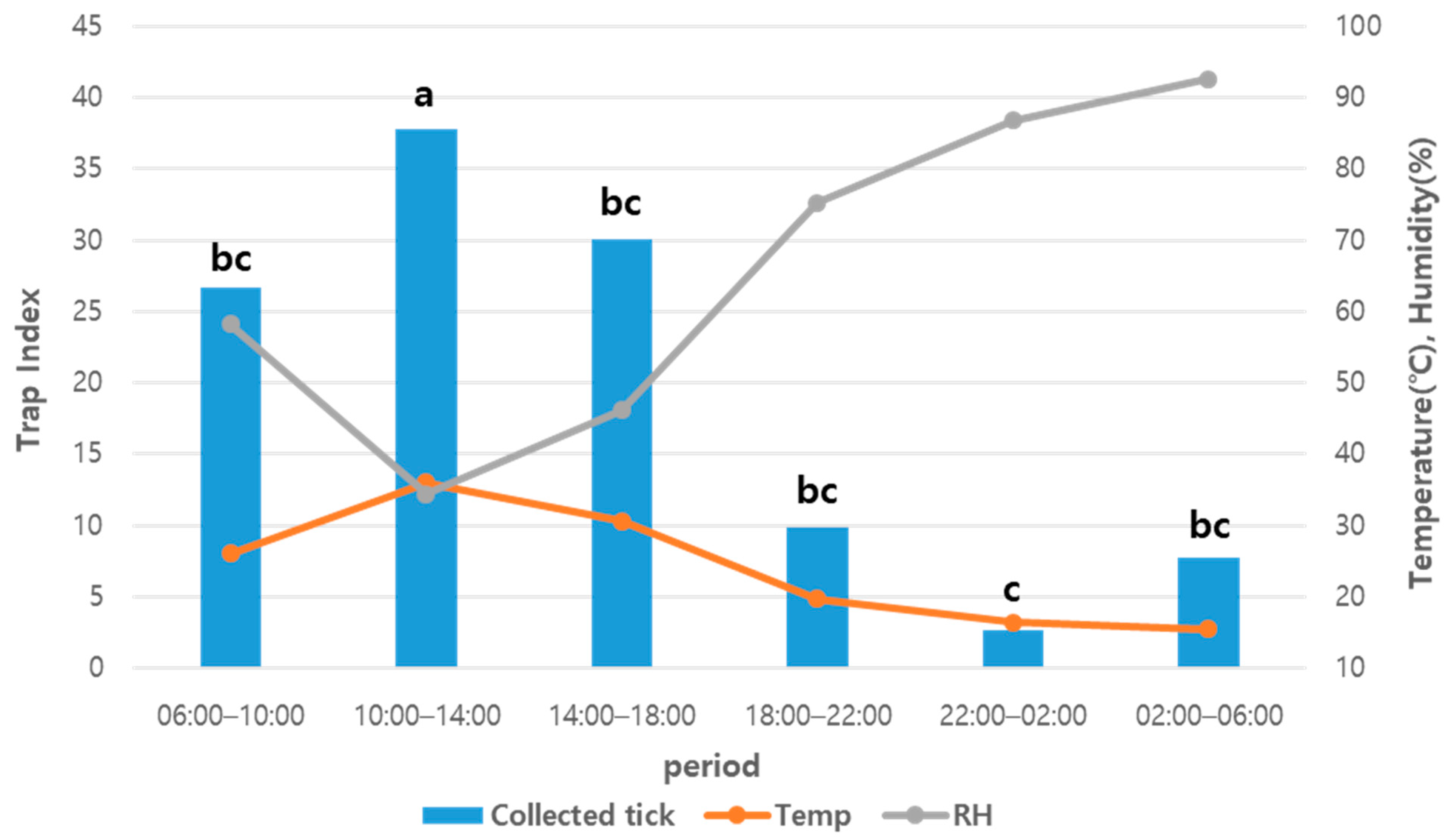
3. Result
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansfield, K.L.; Jizhou, L.; Phipps, L.P.; Johnson, N. Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 2017, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.-F.; Wang, Q.; Zhang, B.; Ji, P. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340.e1313. [Google Scholar] [CrossRef]
- Kodama, F.; Yamaguchi, H.; Park, E.; Tatemoto, K.; Sashika, M.; Nakao, R.; Terauchi, Y.; Mizuma, K.; Orba, Y.; Kariwa, H. A novel nairovirus associated with acute febrile illness in Hokkaido, Japan. Nat. Commun. 2021, 12, 5539. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kuwata, R.; Kimura, T.; Faizah, A.N.; Higa, Y.; Hayashi, T.; Sawabe, K.; Isawa, H. Toyo virus, a novel member of the Kaisodi group in the genus Uukuvirus (family Phenuiviridae) found in Haemaphysalis formosensis ticks in Japan. Arch. Virol. 2021, 166, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lv, X.-L.; Zhang, X.; Han, S.-Z.; Wang, Z.-D.; Li, L.; Sun, H.-T.; Ma, L.-X.; Cheng, Z.-L.; Shao, J.-W. Identification of a new orthonairovirus associated with human febrile illness in China. Nat. Med. 2021, 27, 434–439. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; De La Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slåtsve, A.M.; Stordal, F. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick-Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Changes in the geographic distribution of the blacklegged tick, Ixodes scapularis, in the United States. Ticks Tick-Borne Dis. 2023, 14, 102233. [Google Scholar] [CrossRef]
- Tufts, D.M.; Diuk-Wasser, M.A. First hemispheric report of invasive tick species Haemaphysalis punctata, first state report of Haemaphysalis longicornis, and range expansion of native tick species in Rhode Island, USA. Parasites Vectors 2021, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Fausett, E.; Kirstein, O.D.; Bellman, S.; Long, A.; Roeske, I.; Cheng, C.; Piantadosi, A.; Anderson, T.K.; Vazquez-Prokopec, G.M. Surveillance and detection of Haemaphysalis longicornis (Acari: Ixodidae) in protected areas from Georgia, USA. J. Med. Entomol. 2024, 61, tjae051. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Barker, S.; Cobos, M.E.; Barker, D.; Teo, E.; Foley, D.; Nakao, R.; Lawrence, K.; Heath, A.; Peterson, A.T. Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci. Rep. 2019, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Occi, J.; Bonilla, D.L.; Egizi, A.M.; Fonseca, D.M.; Mertins, J.W.; Backenson, B.P.; Bajwa, W.I.; Barbarin, A.M.; Bertone, M.A. Multistate infestation with the exotic disease–vector tick Haemaphysalis longicornis—United States, August 2017–September 2018. Morb. Mortal. Wkly. Rep. 2018, 67, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Heath, A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J. 2016, 64, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H.; Roberts, F.H.; Kohls, G.M.; Tipton, V.J. Review of Haemaphysalis (Kaiseriana) Longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 1968, 54, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Piedmonte, N.P.; Vinci, V.C.; Daniels, T.J.; Backenson, B.P.; Falco, R.C. Seasonal activity of Haemaphysalis longicornis (Acari: Ixodidae) in southern New York state. J. Med. Entomol. 2021, 58, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I. Modeling the Asian longhorned tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol. 2019, 56, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Tufts, D.M.; Goodman, L.B.; Benedict, M.C.; Davis, A.D.; VanAcker, M.C.; Diuk-Wasser, M. Association of the invasive Haemaphysalis longicornis tick with vertebrate hosts, other native tick vectors, and tick-borne pathogens in New York City, USA. Int. J. Parasitol. 2021, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; McKenna, D.; Piedmonte, N.; Vinci, V.; Egizi, A.M.; Backenson, B.; Falco, R.C. First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis. 2020, 70, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Egizi, A.M.; Robbins, R.G.; Beati, L.; Nava, S.; Occi, J.L.; Fonseca, D.M. A pictorial key to differentiate the recently detected exotic Haemaphysalis longicornis Neumann, 1901 (Acari, Ixodidae) from native congeners in North America. Zookeys 2019, 818, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.; Fryxell, T.R.; Hickling, G.J.; Vail, K.; Ivey, J. Asian Longhorned Tick. 2019. Available online: https://www.publichealthontario.ca/-/media/documents/F/2019/focus-on-asian-longhorned-tick.pdf (accessed on 12 May 2024).
- Yano, Y.; Shiraishi, S.; Uchida, T. Effects of temperature on development and growth in the tick, Haemaphysalis longicornis. Exp. Appl. Acarol. 1987, 3, 73–78. [Google Scholar] [CrossRef]
- Tufts, D.M.; VanAcker, M.C.; Fernandez, M.P.; DeNicola, A.; Egizi, A.; Diuk-Wasser, M.A. Distribution, host-seeking phenology, and host and habitat associations of Haemaphysalis longicornis ticks, Staten Island, New York, USA. Emerg. Infect. Dis. 2019, 25, 792. [Google Scholar] [CrossRef] [PubMed]
- Schappach, B.L.; Krell, R.K.; Hornbostel, V.L.; Connally, N.P. Exotic Haemaphysalis longicornis (Acari: Ixodidae) in the United States: Biology, ecology, and strategies for management. J. Integr. Pest Manag. 2020, 11, 21. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jung, M.; Kho, J.-W.; Song, H.; Moon, K.; Kim, Y.H.; Lee, D.-H. Characterization of overwintering sites of Haemaphysalis longicornis (Acari: Ixodidae) and tick infection rate with severe fever with thrombocytopenia syndrome virus from eight provinces in South Korea. Ticks Tick-Borne Dis. 2020, 11, 101490. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kaufman, P.E. Asian longhorned tick, Haemaphysalis longicornis Neumann (Arachnida: Acari: Ixodidae): EENY-739/IN1263, 8/2019. EDIS 2020, 2020. [Google Scholar] [CrossRef]
- Kang, J.-G.; Ko, S.; Smith, W.B.; Kim, H.-C.; Lee, I.-Y.; Chae, J.-S. Prevalence of Anaplasma, Bartonella and Borrelia Species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-M.; Kim, M.-S.; Park, M.-S.; Park, J.-H.; Chae, J.-S. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector-Borne Zoonotic Dis. 2003, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.S.; Jang, W.J.; Koh, S.E.; Park, T.K.; Kang, S.S.; Kim, B.J.; Kook, Y.H.; Park, K.H.; Lee, S.H. Identification of the Coxiella sp. detected from Haemaphysalis longicornis ticks in Korea. Microbiol. Immunol. 2004, 48, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Price, K.J.; Graham, C.B.; Witmier, B.J.; Chapman, H.A.; Coder, B.L.; Boyer, C.N.; Foster, E.; Maes, S.E.; Bai, Y.; Eisen, R.J. Borrelia burgdorferi sensu stricto DNA in field-collected Haemaphysalis longicornis ticks, Pennsylvania, United States. Emerg. Infect. Dis. 2021, 27, 608. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Z.; Kelly, P.; Li, J.; Ren, Y.; Wang, C. Borrelia miyamotoi sensu lato in Père David Deer and Haemaphysalis longicornis Ticks. Emerg. Infect. Dis. 2018, 24, 928. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, L.; Huang, X.; Ma, H.; Zhang, Y.; Du, Y.; Wang, P.; Tang, X.; Wang, H.; Kang, K. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: Discovery of a new bunyavirus. PLoS Pathog. 2011, 7, e1002369. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.-H.; Choe, P.G.; Kim, N.-J.; Lee, J.-K.; Oh, M.-d. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 2013, 19, 1892. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Ongkittikul, S.; Watanawong, R.; Rompho, P. Severe fever with thrombocytopenia syndrome virus: The first case report in Thailand. Bangk. Med. J. 2020, 16, 204. [Google Scholar]
- Tran, X.C.; Yun, Y.; Kim, S.-H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.-H.; Lee, K.H. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029. [Google Scholar] [CrossRef]
- Win, A.M.; Nguyen, Y.T.H.; Kim, Y.; Ha, N.-Y.; Kang, J.-G.; Kim, H.; San, B.; Kyaw, O.; Htike, W.W.; Choi, D.-O. Genotypic heterogeneity of Orientia tsutsugamushi in scrub typhus patients and thrombocytopenia syndrome co-infection, Myanmar. Emerg. Infect. Dis. 2020, 26, 1878. [Google Scholar] [CrossRef] [PubMed]
- KDCA (Korea Disease Control and Prevention Agency) Infectious Disease Web-Portal. Available online: http://www.kdca.go.kr/npt/biz/npp/nppMain.Do (accessed on 2 May 2024).
- Miao, D.; Liu, M.-J.; Wang, Y.-X.; Ren, X.; Lu, Q.-B.; Zhao, G.-P.; Dai, K.; Li, X.-L.; Li, H.; Zhang, X.-A. Epidemiology and ecology of severe fever with thrombocytopenia syndrome in China, 2010–2018. Clin. Infect. Dis. 2021, 73, e3851–e3858. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Li, A.; Wang, S.; Li, D. Epidemiological characteristics of severe fever with thrombocytopenia syndrome from 2010 to 2019 in Mainland China. Int. J. Environ. Res. Public Health 2021, 18, 3092. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, K.; Tomozane, M.; Sano, C.; Ohta, R. Clinical Presentation and Mortality of Severe Fever with Thrombocytopenia Syndrome in Japan: A Systematic Review of Case Reports. Int. J. Environ. Res. Public Health 2022, 19, 2271. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, K.; Mitoma, S.; Norimine, J.; Shimojima, M.; Kuroda, Y.; Hinoura, T. Seroprevalence for severe fever with thrombocytopenia syndrome virus among the residents of Miyazaki, Japan: An epidemiological study. J. Infect. Chemother. 2024, 30, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; Campbell, S.R. Ambient air temperature as a predictor of activity of adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 1994, 31, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Kleinjan, J.E.; Schoeler, G.B. Diel activity of nymphal Dermacentor occidentalis and Ixodes pacificus (Acari: Ixodidae) in relation to meteorological factors and host activity periods. J. Med. Entomol. 1995, 32, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Dusbabek, F. Micrometeorological and microhabitat factors affecting maintenance and dissemination of tick-borne diseases in the environment. Ecol. Dyn. Tick-Borne Zoonoses 1994, 91, 138. [Google Scholar]
- Bartosik, K.; Wiśniowski, Ł.; Buczek, A. Questing behavior of Dermacentor reticulatus adults (Acari: Amblyommidae) during diurnal activity periods in eastern Poland. J. Med. Entomol. 2012, 49, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Meteorologically mediated diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Entomol. 2003, 40, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mejlon, H.A. Diel activity of Ixodes ricinus Acari: Ixodidae at two locations near Stockholm, Sweden. Exp. Appl. Acarol. 1997, 21, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-G.; Noh, B.-E.; Lee, H.S.; Kim, T.-K.; Song, B.-G.; Lee, H.I. Nationwide temporal and geographical distribution of tick populations and phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in ticks in Korea, 2020. Microorganisms 2021, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu islands. Brigh. Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1. [Google Scholar]
- Kim, H.G.; Jung, M.; Lee, D.-H. Seasonal activity of Haemaphysalis longicornis and Haemaphysalis flava (Acari: Ixodida), vectors of severe fever with thrombocytopenia syndrome (SFTS) virus, and their SFTS virus harboring rates in Gyeonggi Province, South Korea. Exp. Appl. Acarol. 2022, 87, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kim-Jeon, M.-D.; Jegal, S.; Jun, H.; Jung, H.; Park, S.H.; Ahn, S.K.; Lee, J.; Gong, Y.W.; Joo, K.; Kwon, M.J. Four year surveillance of the vector hard ticks for SFTS, Ganghwa-do, Republic of Korea. Korean J. Parasitol. 2019, 57, 691. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Han, S.-Y.; Sung, S.-H.; Jung, E.-Y.; Kim, J.-H.; Lee, S.-J.; Yoo, S.-S. Survey on tick distribution and tick-borne pathogens in Daejeon and adjacent areas in South Korea. Ticks Tick-Borne Dis. 2021, 12, 101711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.N.; Dubinina, H.V. Abiotic parameters and diel and seasonal activity of Borrelia-infected and uninfected Ixodes persulcatus (Acarina: Ixodidae). J. Med. Entomol. 2000, 37, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S. The impact of tick ecology on pathogen transmission dynamics. In Ticks: Biology, Disease and Control; Cambridge University Press: Cambridge, UK, 2008; pp. 40–72. [Google Scholar]
- Fieler, A.M.; Rosendale, A.J.; Farrow, D.W.; Dunlevy, M.D.; Davies, B.; Oyen, K.; Xiao, Y.; Benoit, J.B. Larval thermal characteristics of multiple ixodid ticks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110939. [Google Scholar] [CrossRef] [PubMed]
- Heath, A. The temperature and humidity preferences of Haemaphysalis longicornis, Ixodes holocyclus and Rhipicephalus sanguineus (Ixodidae): Studies on eggs. Int. J. Parasitol. 1979, 9, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Heath, A. The temperature and humidity preferences of Haemaphysalis longicornis, Ixodes holocyclus and Rhipicephalus sanguineus (Ixodidae): Studies on engorged larvae. Int. J. Parasitol. 1981, 11, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T. Influence of aggregation and relative humidity on longevity of unfed bush tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae). J. Parasitol. 2008, 94, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Knülle, W.; Rudolph, D. Humidity relationships and water balance of ticks. In Physiology of Ticks; Elsevier: Amsterdam, The Netherlands, 1982; pp. 43–70. [Google Scholar]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- FUJIMOTO, K. Effect of photoperiod on the host attachment and development in the tick, Haemaphysalis longicornis. Med. Entomol. Zool. 1995, 46, 345–348. [Google Scholar] [CrossRef]
- Nielebeck, C.; Kim, S.H.; Pepe, A.; Himes, L.; Miller, Z.; Zummo, S.; Tang, M.; Monzón, J.D. Climatic stress decreases tick survival but increases rate of host-seeking behavior. Ecosphere 2023, 14, e4369. [Google Scholar] [CrossRef]
- Burtis, J.C.; Sullivan, P.; Levi, T.; Oggenfuss, K.; Fahey, T.J.; Ostfeld, R.S. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasites Vectors 2016, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Dubie, T.R.; Turner, J.; Noden, B.H. Questing behavior and analysis of tick-borne bacteria in Ixodes scapularis (Acari: Ixodidae) in Oklahoma. J. Med. Entomol. 2018, 55, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.J.; Foré, S.A.; Kim, H.-J. Seasonal changes in questing efficiency of wild Amblyomma americanum (Acari: Ixodidae) nymphs. Ticks Tick-Borne Dis. 2022, 13, 101988. [Google Scholar] [CrossRef] [PubMed]
- Di, C. Exploring Local Environmental Factors Influencing Geographic Distribution of Black-Legged Tick Questing Activity. Master’s Thesis, CUNY Hunter College, New York, NY, USA, 2019. [Google Scholar]
- Jensen, P. Host seeking activity of Ixodes ricinus ticks based on daily consecutive flagging samples. Exp. Appl. Acarol. 2000, 24, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Influence of meso-and microscale habitat structure on focal distribution of sympatric Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Adalsteinsson, S.A.; D’Amico, V.; Shriver, W.G.; Brisson, D.; Buler, J.J. Scale-dependent effects of nonnative plant invasion on host-seeking tick abundance. Ecosphere 2016, 7, e01317. [Google Scholar] [CrossRef] [PubMed]
- Arsnoe, I.M.; Hickling, G.J.; Ginsberg, H.S.; McElreath, R.; Tsao, J.I. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS ONE 2015, 10, e0127450. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Pathogens manipulating tick behavior—Through a glass, darkly. Pathogens 2020, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports Edinburgh: London, UK, 2003; Volume 74. [Google Scholar]
- Ji, S.-R.; Byun, H.-R.; Rieu, M.-S.; Han, S.-W.; Nam, H.-Y.; Seo, S.; Park, S.-Y.; Kang, H.-Y.; Choi, C.-Y.; Cho, S.-Y. First detection of Bandavirus dabieense in ticks collected from migratory birds in the Republic of Korea. Acta Trop. 2024, 257, 107279. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.-B.; Cho, Y.-S.; Cho, Y.-K.; Kang, J.-G.; Shin, N.-S.; Chae, J.-S. Epidemiological investigation of tick species from near domestic animal farms and cattle, goat, and wild boar in Korea. Korean J. Parasitol. 2019, 57, 319. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Kim, H.; Won, S.; Kim, H.-C.; Chong, S.-T.; Klein, T.A.; Kim, K.-G.; Seo, H.-Y.; Chae, J.-S. Ticks collected from wild and domestic animals and natural habitats in the Republic of Korea. Korean J. Parasitol. 2014, 52, 281. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Chong, S.T.; Sames, W.J.; Nunn, P.V.; Wolf, S.P.; Robbins, R.G.; Klein, T.A. Tick surveillance of small mammals captured in Gyeonggi and Gangwon Provinces, Republic of Korea, 2004–2008. Syst. Appl. Acarol. 2010, 15, 100–108. [Google Scholar] [CrossRef]
- Thompson, A.T.; White, S.A.; Shaw, D.; Garrett, K.B.; Wyckoff, S.T.; Doub, E.E.; Ruder, M.G.; Yabsley, M.J. A multi-seasonal study investigating the phenology, host and habitat associations, and pathogens of Haemaphysalis longicornis in Virginia, USA. Ticks Tick-Borne Dis. 2021, 12, 101773. [Google Scholar] [CrossRef] [PubMed]
- Léger, E.; Vourc’h, G.; Vial, L.; Chevillon, C.; McCoy, K.D. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 2013, 59, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]

| April | May | June | July | September | Total | |
|---|---|---|---|---|---|---|
| Adults | ||||||
| Daytime | 6.7 ± 23.3 | 2.4 ± 4.0 | 14.3 ± 20.5 | 14.1 ± 8.7 | 2.4 ± 2.8 | 8.0 ± 15.2 |
| Nighttime | 0.1 ± 0.3 | 0.9 ± 1.7 | 5.6 ± 6.0 | 3.5 ± 3.2 | 0.4 ± 0.7 | 2.1 ± 3.4 |
| Nymphs | ||||||
| Daytime | 7.2 ± 5.8 | 25.5 ± 39.7 | 58.8 ± 88.5 | 15.6 ± 20.9 | 12.1 ± 10.5 | 23.8 ± 47.5 |
| Nighttime | 0.2 ± 0.4 | 3.7 ± 6.2 | 14.8 ± 23.1 | 4.3 ± 5.8 | 0.5 ± 0.9 | 4.7 ± 12.0 |
| Total | ||||||
| Daytime | 13.8 ± 25.9 | 27.9 ± 43.1 | 73.1 ± 105.9 | 29.7 ± 24.6 | 14.5 ± 13.0 | 31.8 ± 57.0 |
| Nighttime | 0.3 ± 0.6 | 4.6 ± 7.5 | 20.4 ± 28.1 | 7.8 ± 8.3 | 0.9 ± 1.3 | 6.9 ± 15.1 |
| Date | Time Period | Temperature (Mean) | Relative Humidity (Mean) | |||||
|---|---|---|---|---|---|---|---|---|
| 06:00–10:00 | 10:00–14:00 | 14:00–18:00 | 18:00–22:00 | 22:00–02:00 | 02:00–06:00 | |||
| 27 April | 4.7 ± 5.1 | 27.0 ± 43.4 | 9.8 ± 7.0 | 0.8 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.7–36.5 (17.8 °C) | 11.0–100.0 (61.5%) |
| 18 May | 21.3 ± 39.1 | 34.0 ± 61.0 | 28.5 ± 31.4 | 7.0 ± 6.5 | 6.2 ± 10.9 | 0.5 ± 0.5 | 9.5–34.1 (20.7 °C) | 16.3–85.4 (46.9%) |
| 20 June | 83.2 ± 93.0 | 62.7 ± 140.8 | 73.5 ± 96.9 | 31.5 ± 28.4 | 3.2 ± 4.0 | 26.5 ± 36.1 | 17.7–42.1 (28.4 °C) | 28.3–100.0 (68.0%) |
| 28 July | 16.5 ± 8.1 | 37.0 ± 26.5 | 35.5 ± 31.3 | 10.0 ± 11.7 | 4.2 ± 2.3 | 9.3 ± 8.2 | 21.3–40.3 (28.2 °C) | 39.5–100.0 (84.1%) |
| 14 September | 7.7 ± 10.3 | 28.7 ± 9.9 | 7.2 ± 3.3 | 0.3 ± 0.5 | 0.3 ± 0.5 | 2.2 ± 1.7 | 19.0–36.1 (25.2 °C) | 37.2–88.0 (67.5%) |
| Total Mean | 26.7 ± 51.5 | 37.8 ± 68.5 | 30.9 ± 50.6 | 9.9 ± 17.4 | 2.8 ± 5.5 | 7.7 ± 18.5 | 5.7–42.1 (24.0 °C) | 11.0–100.0 (65.6%) |
| TEMP | HUMD | LIT | |
|---|---|---|---|
| Temperature (TEMP) | |||
| Relative humidity (HUMD) | −0.593 * | ||
| Light intensity (LIT) | 0.802 * | −0.649 * | |
| Collected total tick (CTT) | 0.679 * | −0.361 * | 0.572 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, B.-E.; Kim, G.-h.; Lee, H.S.; Kim, H.; Lee, H.-I. The Diel Activity Pattern of Haemaphysalis longicornis and Its Relationship with Climatic Factors. Insects 2024, 15, 568. https://doi.org/10.3390/insects15080568
Noh B-E, Kim G-h, Lee HS, Kim H, Lee H-I. The Diel Activity Pattern of Haemaphysalis longicornis and Its Relationship with Climatic Factors. Insects. 2024; 15(8):568. https://doi.org/10.3390/insects15080568
Chicago/Turabian StyleNoh, Byung-Eon, Gi-hun Kim, Hak Seon Lee, Hyunwoo Kim, and Hee-Il Lee. 2024. "The Diel Activity Pattern of Haemaphysalis longicornis and Its Relationship with Climatic Factors" Insects 15, no. 8: 568. https://doi.org/10.3390/insects15080568





