Simple Summary
Spodoptera frugiperda of the family Noctuidae in the order Lepidoptera, is native to the Americas. It is characterized by its polyphagous nature and high reproductive capacity. S. frugiperda has caused significant socio-economic losses in many countries, primarily affecting Poaceae crops such as maize, rice, wheat, and sorghum. This study aims to analyze the members, differentiation, structure, and function of the JHBP gene family in S. frugiperda through bioinformatics approaches. Subsequently, we utilize real-time quantitative PCR (qPCR) to investigate the expression profiles of this gene family across different developmental stages and various tissues of S. frugiperda. The purpose of this analysis is to elucidate the potential roles of these genes, providing key target genes for future functional validation through gene knockdown and RNA interference (RNAi) techniques. This research offers insights that could contribute to the development of new pesticides.
Abstract
Juvenile hormone binding proteins (JHBPs) are carrier proteins that bind to juvenile hormone (JH) to form a complex, which then transports the JH to target organs to regulate insect growth and development. Through bioinformatics analysis, 76 genes encoding JHBP in S. frugiperda were identified from whole genome data (SfJHBP1-SfJHBP76). These genes are unevenly distributed across 8 chromosomes, with gene differentiation primarily driven by tandem duplication. Most SfJHBP proteins are acidic, and their secondary structures are mainly composed of α-helices and random coils. Gene structure and conserved motif analyses reveal significant variations in the number of coding sequences (CDS) and a high diversity in amino acid sequences. Phylogenetic analysis classified the genes into four subfamilies, with a notable presence of directly homologous genes between S. frugiperda and S. litura, suggesting a close relationship between the two species. RNA-seq data from public databases and qPCR of selected SfJHBP genes show that SfJHBP20, SfJHBP50, and SfJHBP69 are highly expressed at most developmental stages, while SfJHBP8 and SfJHBP14 exhibit specific expression during the pupal stage and in the midgut. These findings provide a theoretical basis for future studies on the biological functions of this gene family.
1. Introduction
The fall armyworm (Spodoptera frugiperda), of the Lepidoptera Noctuidae family, is indigenous to the Americas and known for its omnivorous habits and prolific breeding capabilities [1]. This species displays a broad host range, with a preference for attacking crops such as maize, rice, wheat, sorghum, and other cereals [2]. Noteworthy biological traits of S. frugiperda, including its rapid feeding and developmental rates, swift migration patterns, and advantageous hybrid characteristics, enhance its invasive potential. Its adaptability to a wide range of temperatures, host plants, and pesticide resistance facilitates rapid adaptation to new environments, leading to persistent agricultural damage [3]. S. frugiperda has inflicted substantial socio-economic losses in many countries, with estimates suggesting annual economic damages reaching billions of dollars in sub-Saharan Africa alone [4]. In 2017, the International Centre for Agricultural and Biological Sciences identified S. frugiperda as one of the top ten plant pests worldwide, and its invasion of China in 2019 marked a significant event. Since its incursion [5], the species has been observed annually in extensive areas of China.
JH is a regulatory substance present in insects, crustaceans, and certain plants that is crucial in controlling processes of growth and development [6,7]. During the larval phase, JH orchestrates growth and molting events by modulating specific gene expression patterns that endure into adulthood [8]. JH forms complexes with three categories of JHBPs within the hemolymph. These categories include lipoproteins, hexameric proteins, and low molecular weight proteins with a size of around 30 kDa [9,10]. The role of JHBP, particularly the JH-JHBP complex, in binding to membrane receptors to facilitate transportation has been elucidated, showing that JHBP is a pivotal component in the JH signaling cascade [11,12,13,14]. Additionally, JHBPs safeguard JH from enzymatic degradation by non-specific esterases [15], underscoring their indispensable nature in maintaining the integrity of JH signaling pathways.
The Takeout (TO) protein and JHBP both possess a shared domain that is thought to have the ability to attach to small lipophilic compounds, specifically JH. TO proteins were first discovered and designated in the fruit fly (Drosophila melanogaster). JHBPs and TO proteins belong to a superfamily of proteins [16]. TO proteins are prevalent in numerous insect species and play crucial roles in a multitude of physiological functions and behavioral patterns. Saito et al. identified nine BmJHBP genes in the silkworm EST database. These BmJHBP genes were expressed in various tissues and were regulated by developmental conditions [17]. Dauwalder et al. discovered through in situ hybridization that TO proteins are distributed in the head fat body of male D. melanogaster, but are absent in the head fat body of female D. melanogaster [18]. TO proteins are highly expressed in the head and abdomen of the green peach aphid (Myzus persicae) [19].
JH is an important insect hormone synthesized and secreted by the corpora allata (CA). It exerts unique biological effects within insects through the JHBP present in the hemolymph [20]. JHBP maintains JH at an appropriate titer in the hemolymph by binding or releasing JH, thereby achieving the function of protecting the embryo [21,22]. Insect diapause, depending on the developmental stage, can be classified into four types: embryonic diapause (egg diapause), larval diapause, pupal diapause, and adult diapause. In various studies on insect diapause, JH has been identified as an important hormone that regulates adult diapause, controlling developmental processes and promoting oocyte maturation during the adult stage [20]. The regulatory role of JH in insect growth and development has long been a focus of insect physiology research and has been successfully applied in pest control and beneficial insect utilization [23]. Insect reproductive diapause can be terminated by JH, as evidenced in studies on the black-legged melon leaf beetle (Aulacophora nigripennis) [24] and the rice bug (Scotinophara lurida) [25]. As the carrier or receptor of JH, JHBP plays a crucial role in the functioning of JH.
The structure and expression patterns of the JHBP gene have been elucidated in the tobacco hornworm (Manduca sexta) [26]. Xiuting He et al. cloned the JHBP gene from the small brown planthopper (Laodelphax striatellus), measuring gene expression across different larval stages. They discovered that the levels of gene expression were notably lower in all stages compared to the fourth instar larvae, and there was no distinction noted between the adult males and females [27]. Previous research has identified that the OfJHBP gene within the bamboo borer (Omphisa fuscidentalis) has the ability to impact larval diapause and serves as a key regulator in the processes of growth, development, and diapause [28]. In the greater wax moth (Galleria mellonella), JHBP mRNA levels peak during the final larval stage and decrease fivefold after pupation, indicating that JHBP can influence pupation [29]. Long Chen et al. cloned the JHBP gene from the Daursky beetle (Galeruca daurica), finding that GdJHBP is present in the hemolymph and expressed at various developmental stages, with higher expression before and after adult diapause, suggesting a significant role in adult summer diapause [30]. In the cotton bollworm (Helicoverpa armigera), HaJHBP expression is significantly suppressed under starvation conditions, leading to delayed larval development [31]. These studies collectively suggest that JHBP participates in regulating various stages of insect growth and development, and can also impact feeding, reproduction, mating, drug resistance, and immune responses [32,33].
This study investigated the bioinformatics of the SfJHBP gene family and its expression patterns in various developmental stages of S. frugiperda, as well as in different tissues of fifth instar larvae. The research analyzed the potential role of the gene and validated its function for future gene knockdown experiments and the application of RNAi technology. Understanding the control of S. frugiperda is crucial for identifying key target genes and guiding the development of new pesticides.
2. Materials and Methods
2.1. Identification and Chromosomal Localization of the JHBP Gene Family in S. frugiperda
Focusing on S. frugiperda as the research subject, the genomic sequence information file and annotation information file of the Zhejiang University version of S. frugiperda were obtained from the NCBI website (https://www.ncbi.nlm.nih.gov/ accessed on 12 February 2024). The HMM model file (PF06585) for the JHBP gene family was downloaded from the Pfam (version 37.0) database (http://pfam.xfam.org/ accessed on 15 February 2024) [34]. Using the software Tbtools (version 2.096) [35], the protein sequences of S. frugiperda were extracted and duplicate sequences were removed. These protein sequences were then examined for the presence of JHBP domains using tools such as HMMER (Version 3.0) [36], NCBI’s Conserved Domain Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi accessed on 17 February 2024) [36], and SMART (Version 9.0) (SMART: Main page (http://smart.embl-heidelberg.de/) accessed on 17 February 2024) [37], excluding those without JHBP domains.
The physicochemical properties of the JHBP gene family in S. frugiperda were analyzed using ExPaSy (https://web.expasy.org/protparam/ accessed on 19 February 2024) [38]. Subcellular localization was predicted using WOLFPSORT (https://wolfpsort.hgc.jp/ accessed on 22 February 2024) [39]. Secondary structures of the proteins were predicted using SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html accessed on 25 February 2024) [40]. Finally, the chromosomal distribution map of the JHBP gene family in S. frugiperda was created using TBtools (version 2.096) [35].
2.2. SfJHBP Gene Duplication and Ka/Ks Value Analysis
The software TBtools (version 2.096) [35] was used to analyze the duplication relationships among genes within the species and to calculate the non-synonymous substitution rate (Ka) and synonymous substitution rate (Ks). A Ka/Ks ratio greater than 1, less than 1, or equal to 1 indicates positive, negative, or neutral evolution, respectively [41]. The divergence time was calculated using the formula T = Ks/(2λ) (λ = 6.5 × 10−9) [42].
2.3. Structure and Conserved Motif Analysis of SfJHBP Genes
Motif analysis of S. frugiperda proteins was conducted using MEME (Version 5.5.5) (https://meme-suite.org/meme/tools/meme accessed on 3 March 2024) [43], with the number of motifs set to 10 and other parameters set to default. The gene structure and conserved motifs of the JHBP gene family in S. frugiperda, along with their phylogenetic tree, were integrated using TBtools (version 2.096) [35].
2.4. Phylogenetic and Synteny Analysis of the SfJHBP Gene Family
To investigate the evolutionary relationships between S. frugiperda and other species, genomic sequence and annotation files for Spodoptera litura Fabricius (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_002706865.2/ accessed on 7 March 2024) and Plutella xylostella Linnaeus (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_932276165.1/ accessed on 10 March 2024) were downloaded from the NCBI database. Using the HMM model file (PF06585) [34], JHBP protein sequences were extracted using TBtools (version 2.096) [35], and duplicates and sequences lacking JHBP domains were removed. The homologous JHBP protein sequences were then used to construct a phylogenetic tree in MEGA (Version 11.0.13) [44], and the tree was visualized using iTOL (Version 6.8.2): (https://itol.embl.de/ accessed on 20 March 2024) [45]. Synteny analysis was performed using TBtools (version 2.096) [35], based on gene location files on chromosomes and interspecies gene association files.
2.5. Analysis of Expression Patterns of the SfJHBP Gene Family in Diverse Developmental Stages and Tissues of S. frugiperda
Transcripts of the JHBP gene family are detected in various developmental stages of S. frugiperda including 1–6 instars, pupae, and both male and female adult stages, as well as different tissues such as midgut, head, cuticle, fat body, hemolymph, and malpighian tubule. The data set utilized in this study was obtained from the NCBI’s SRA database (https://www.ncbi.nlm.nih.gov/sra/?term= accessed on 26 March 2024), and the accession numbers are PRJNA590312 [46] and PRJNA1070356 [47] respectively; the data were processed using HeatMap of TBtools (version 2.096) [35] to obtain the SfJHBP gene family expression content heat map.
2.6. qPCR Expression Characteristics of Some SfJHBP Genes in Different Developmental Stages and Different Tissues of S. frugiperda
Insect Rearing
Larvae of S. frugiperda were gathered from the maize experimental field at Anhui Science and Technology University and then nurtured in a controlled climate chamber. They were fed fresh maize leaves within rearing containers, with adults receiving 10% honey water. Following several generations of rearing indoors, the insects were utilized for experiments. The conditions of the artificial climate chamber were set as follows: temperature (25 ± 1) °C, relative humidity (70 ± 5)%, and photoperiod 16:8 (L) hours.
Samples of S. frugiperda eggs (n = 100), 1st instar (n = 30), 2nd-6th instar larvae (n = 10), pupae (n = 5), male and female adults (n = 5), and different tissues of 5th instar larvae (fat body, Malpighian tubules, integument, midgut, head, hemolymph) (n = 15) were collected. Each sample was replicated three times, flash-frozen in liquid nitrogen, and stored at −80 °C for RNA extraction.
RNA extraction and reverse transcription we’re performed according to Lv et al. [48]. Primers for each specific gene were designed using Primer 3.0 [49], with GADPH as the reference gene (Table 1). qPCR was conducted according to Jin et al. [50], measuring the relative expression levels of 13 SfJHBP genes across various developmental stages and tissues(From the transcriptome data of various developmental phases and tissues of S. frugiperda, we selected 13 representative highly expressed genes). Each sample was tested in triplicate. The selected genes’ expression levels were calculated using the 2−ΔΔCT method [51], then analyzed with SPSS software (Version 28) [52]. Data visualization was done using GraphPad Prism (Version 9.5.0) [53].

Table 1.
Sequence of primers in this experiment.
3. Results
3.1. Identification of SfJHBP Gene Family and Chromosomal Localization Analysis
The genes discovered in this study have been given the names SfJHBP1 to SfJHBP76 in order of their placement on the chromosomes (Figure 1). The analysis revealed that the 76 JHBP genes exhibited an irregular distribution pattern. Chromosome 4 exhibited the largest quantity of genes (totaling 37). This was succeeded by chromosome 22, which contained 31 genes. Additionally, chromosomes 2 and 5 each had two genes, while chromosomes 7, 20, 23, and 25 each harbored a single gene. Notably, the SfJHBP genes on chromosomes 4 and 22 predominantly occurred in gene clusters.
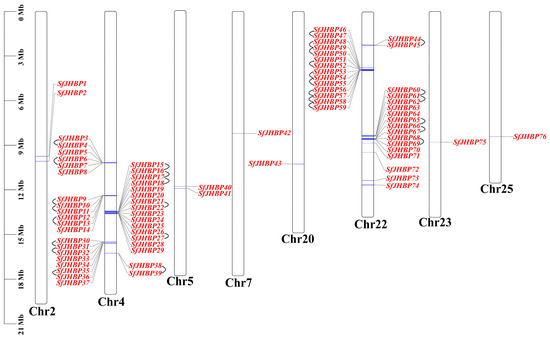
Figure 1.
Chromosomal localization of SfJHBP gene. Note: 76 SfJHBP genes are distributed on the 8 chromosomes of S. frugiperda. Chr represents the chromosome, and the genes connected by black lines represent gene pairs that are tandemly replicated on the same chromosome.
3.2. SfJHBP Protein Structure Analysis
3.2.1. Examination of the Physicochemical Characteristics and Subcellular Localization of the Proteins
By analyzing the physical and chemical properties of the protein, it was found that its amino acid length ranges from 189 aa to 544 aa, with a large length difference, and its molecular weight ranges from 21,806.51 Da to 60,148.08 Da. The amino acid length is proportional to its molecular weight; there are 66 SfJHBP proteins The sequence instability coefficient is less than 40, which means it is a stable protein. By analyzing the overall average hydrophobicity, 47 SfJHBP proteins have negative hydrophobicity. It is speculated that these 47 are hydrophilic proteins and the remaining 29 are hydrophobic proteins (Table S1).
Subcellular localization analysis identified 60 JHBP proteins in the extracellular region, two in the plasma membrane, eight in the endoplasmic reticulum, four in the cytoplasm, and two in the mitochondria. While the SfJHBP proteins exhibited diverse subcellular distributions, most were localized to the extracellular region, suggesting a primary functional role outside the cells.
Isoelectric point analysis revealed an isoelectric point below 7 for SfJHBP1-SfJHBP54, indicating an acidic nature, and above 7 for SfJHBP55-SfJHBP76, classified as alkaline proteins. This finding indicated that most members of this family were acidic.
3.2.2. Secondary Protein Structure
The structure of a protein molecule determines its function. As seen from the proportions listed in Table S2, the dominant secondary structure elements are α-helix > random coil > extended strand > β-turn. However, there are 17 proteins (SfJHBP24, SfJHBP31, SfJHBP32, SfJHBP35, SfJHBP36, SfJHBP42, SfJHBP46, SfJHBP49, SfJHBP55, SfJHBP62, SfJHBP64, SfJHBP67, SfJHBP68, SfJHBP70, SfJHBP73, SfJHBP74, SfJHBP76) where the random coil structure predominates. The proportion of β-turn structure is generally low, usually below 10%. It is hypothesized that α-helix and random coil structures may play dominant roles in the secondary structure of proteins, the extended strand structure might serve a secondary role, and the β-turn structure is likely to have a certain modifying function.
Nine SfJHBP protein pairs exhibited identical secondary structure ratios (SfJHBP3 and SfJHBP9; SfJHBP4 and SfJHBP10; SfJHBP5 and SfJHBP11; SfJHBP6 and SfJHBP12; SfJHBP7 and SfJHBP13; SfJHBP8 and SfJHBP14; SfJHBP23 and SfJHBP28; SfJHBP32 and SfJHBP36; SfJHBP73 and SfJHBP74; Table S2), indicating that two genes might encode the same protein.
3.3. Examination of SfJHBP Gene Duplication and Ka/Ks Ratios
A comprehensive analysis of the evolutionary relationships and chromosomal localization of family members identified 29 gene pairs involved in tandem replication events, as detailed in Table S3. This finding underscores the tandem replication mechanisms through which the JHBP gene family expansion predominantly occurred. Gene divergence occurred approximately 10.92–84.62 million years ago. Among the replicators, 20 exhibited Ka values smaller than Ks, while only one displayed a Ka value larger than Ks, suggesting a prevalent synonymous substitution pattern. Notably, the Ka/Ks ratio was less than 1.0 for 19 gene pairs, indicating intense purifying selection and minimal functional differentiation. Conversely, the Ka/Ks ratio exceeded 1.0 in two gene pairs, suggesting significant positive selection and pronounced functional divergence. Notably, eight gene pairs exhibited a Ka/Ks ratio that indicated substantial sequence divergence and a considerable evolutionary distance (NaN values detected).
3.4. Structure and Conserved Motif Analysis of SfJHBP Gene
Analysis of the structural distribution diagrams for the 76 JHBP gene introns and UTR regions demonstrated variations in both number and length (Figure 2). Notably, SfJHBP19 had three CDSs without UTR regions, SfJHBP17, SfJHBP55, and SfJHBP64 had five, and SfJHBP38 had six.
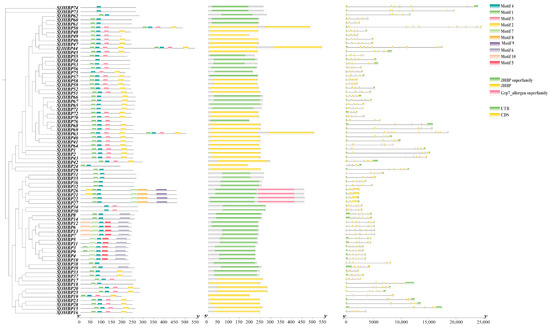
Figure 2.
Presents a schematic depiction of conserved motifs, domains, and the exon-intron architectures within the SfJHBP protein of S. frugiperda.
The analysis found that the JHBP proteins of S. frugiperda have a conserved motif with significant length variations, illustrating the relatively high diversity among JHBP family gene members. The distribution of these conserved motifs in S. frugiperda JHBP proteins was further analyzed (Figure 2). SfJHBP54, SfJHBP73 and SfJHBP74 all contain only one conserved motif.
3.5. Cross-Species Evolutionary Analysis
The comprehensive NCBI database search identified 169 homolog sequences of JHBP, 76 from S. frugiperda, 40 from P. xylostella, and 53 from S. litura. The constructed phylogenetic tree (Figure 3) delineated four distinct groups: Group A, characterized by the lowest number of JHBP genes (two genes, one from S. frugiperda and one from S. litura); Group B, containing 46 genes (26 from S. frugiperda, six from P. xylostella, and 14 from S. litura); Group C, consisting of nine genes (five from S. frugiperda, one from P. xylostella, and three from S. litura); Group D was the largest group with 112 genes (44 from S. frugiperda, 33 from P. xylostella, and 35 from S. litura).
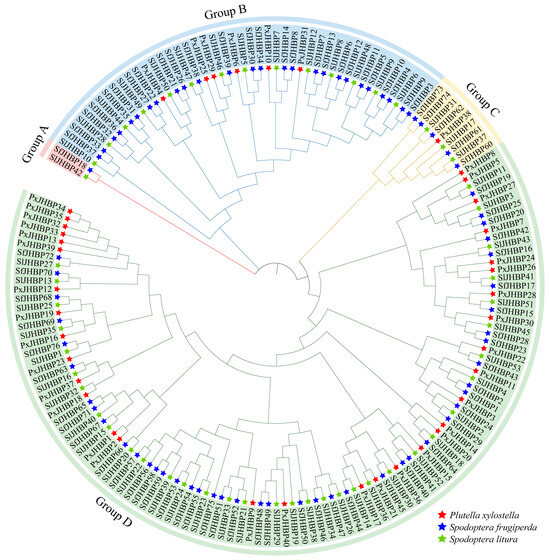
Figure 3.
Phylogenetic tree analysis of JHBP protein in S. frugiperda, S. litura and P. xylostella.
This research identified a collective of 33 pairs of genes that are orthologous across the different species, including 30 pairs between S. frugiperda and S. litura, 2 pairs between S. litura and P. xylostella, and 1 pair between S. frugiperda and P. xylostella; S. frugiperda and S. litura were more homologous genes with closer relationship, while S. frugiperda and P. xylostella were the least homologous genes with more distant relationship.
3.6. Cross-Species Covariance Analysis
The analysis revealed that S. frugiperda and P. xylostella exhibited 12 co-linear segments harboring JHBP genes, encompassing 23 gene pairs (Figure 4). S. frugiperda and S. litura displayed 14 co-linear segments with JHBP genes, comprising 36 gene pairs. These findings indicated a higher level of genomic homology between S. frugiperda and S. litura than between S. frugiperda and P. xylostella.
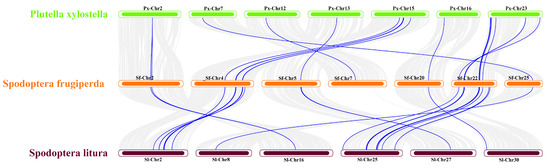
Figure 4.
Collinearity analysis of JHBP genes of S. frugiperda, S. litura and P. xylostella. Note: The figure shows the distribution of homologous genes in different species, and the blue line represents homologous genes between species.
3.7. SfJHBP Gene Family Expression at Various Developmental Stages
The data analysis indicated that SfJHBP60 and SfJHBP69 exhibited high expression levels across developmental stages (Figure 5), while six genes (SfJHBP17, SfJHBP24, SfJHBP29, SfJHBP33, SfJHBP37, and SfJHBP38) were poorly expressed in all developmental stages. Additionally, 27 genes (SfJHBP3, SfJHBP4, SfJHBP6, SfJHBP7, SfJHBP8, SfJHBP9, SfJHBP10, SfJHBP12, SfJHBP13, SfJHBP14, SfJHBP18, SfJHBP19, SfJHBP20, SfJHBP25, SfJHBP30, SfJHBP34, SfJHBP39, SfJHBP40, SfJHBP44, SfJHBP45, SfJHBP47, SfJHBP50, SfJHBP63, SfJHBP66, SfJHBP69, SfJHBP70, and SfJHBP76) were highly expressed in 1st to 5th instar larvae of S. frugiperda, 13 (SfJHBP18, SfJHBP19, SfJHBP21, SfJHBP26, SfJHBP43, SfJHBP44, SfJHBP45, SfJHBP50, SfJHBP60, SfJHBP73, SfJHBP74, and SfJHBP76) were highly expressed in the sixth instar larvae, pupae, and adult females and males.
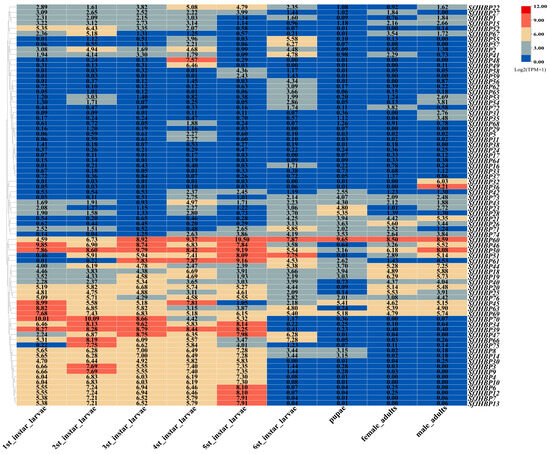
Figure 5.
Expression analysis of SfJHBP gene family in different developmental stage of S. frugiperda.
3.8. Expression Analysis of SfJHBP Gene Family in Different Tissues of S. frugiperda
As the findings, depicted in Figure 6, show, 13 genes (SfJHBP3, SfJHBP4, SfJHBP6, SfJHBP7, SfJHBP8, SfJHBP9, SfJHBP10, SfJHBP12, SfJHBP13, SfJHBP14, SfJHBP30, SfJHBP34, and SfJHBP39) were highly expressed in the midgut, 19 (SfJHBP1, SfJHBP2, SfJHBP20, SfJHBP25, SfJHBP40, SfJHBP41, SfJHBP42, SfJHBP44, SfJHBP46, SfJHBP47, SfJHBP50, SfJHBP51, SfJHBP60, SfJHBP62, SfJHBP66, SfJHBP69, SfJHBP70, SfJHBP75, and SfJHBP76) were highly expressed in the head and cuticle tissues, six (SfJHBP18, SfJHBP22, SfJHBP27, SfJHBP60, SfJHBP61, and SfJHBP71) were highly expressed in fat tissue, three (SfJHBP62, SfJHBP73, and SfJHBP74) were highly expressed in the hemolymph, and four (SfJHBP33, SfJHBP37, SfJHBP39, and SfJHBP71) were highly expressed in the Malpighian tubules.
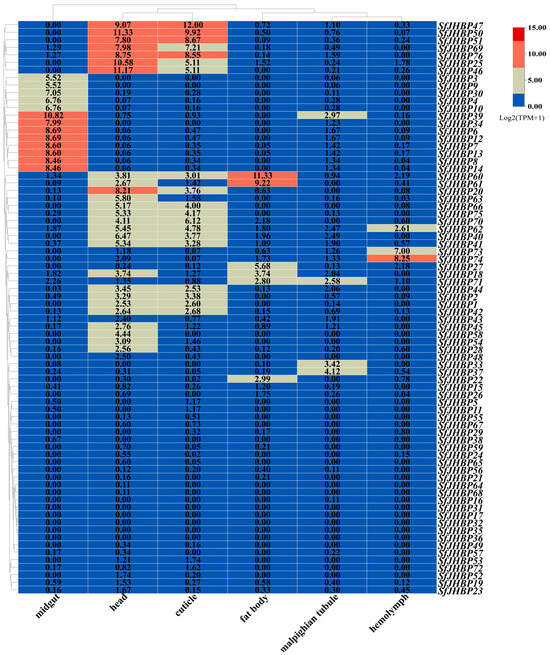
Figure 6.
Expression analysis of SfJHBP gene family in different organs of S. frugiperda.
3.9. qPCR Expression Analysis of Some SfJHBP Genes in Different Developmental Stages and Different Tissues of S. frugiperda
In the developmental stages of S. frugiperda, expression levels of 13 SfJHBP genes showed notable differences (Figure 7). Notably, SfJHBP8 and SfJHBP14 exhibited similar expression patterns, with peak expression during the pupa stage and minimal or absent expression in other phases. Additionally, the levels of SfJHBP18, SfJHBP19, SfJHBP20, SfJHBP40, SfJHBP50, SfJHBP66, SfJHBP69, and SfJHBP76 were elevated in the fifth instar compared to other stages. SfJHBP47 and SfJHBP60 had higher expression in the fourth instar than in different phases. SfJHBP25 had the highest expression during the first instar, followed by the fourth and fifth instars.
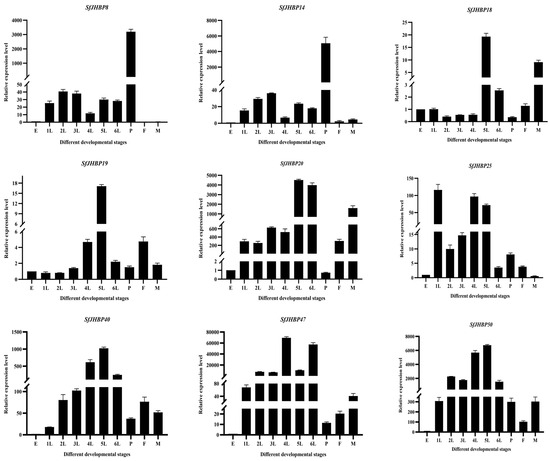
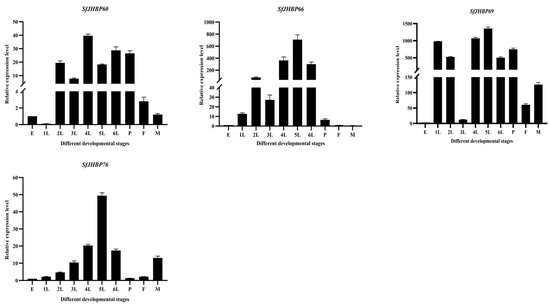
Figure 7.
Expression patterns of 13 SfJHBP genes at different developmental stages of S. frugiperda. Note: 1L: 1st instar 2L: 2nd instar 3L: 3rd instar 4L: 4th instar 5L: 5th instar 6L: 6th instar P: pupa stage F: female adult stage M: male adult stage Data points represent average Value ± standard deviation. In the expression of 13 SfJHBP genes in different developmental stages of S. frugiperda, we used the egg stage as the control group.
In summary, among these 13 genes, 8 SfJHBP genes are highly expressed in the fifth instar stage, followed by 2 genes in the fourth instar stage, while SfJHBP8 and SfJHBP14 are only specifically expressed in the pupal stage.
The levels of expression of 13 SfJHBP genes differ significantly among various tissues of S. frugiperda (Figure 8). Among these genes, SfJHBP8 and SfJHBP14 have similar expression patterns, with high levels only in the midgut and low levels in other tissues. On the other hand, SfJHBP18 shows the highest expression in the head, followed by the Malpighian tubules, with no expression in the hemolymph. SfJHBP20 and SfJHBP25 exhibit comparable expression patterns, with the head having the highest expression levels followed by the integument. In contrast, SfJHBP40, SfJHBP47, SfJHBP50, SfJHBP66, SfJHBP69, and SfJHBP76 display similar expression patterns, with the integument having the highest expression levels followed by the head. SfJHBP19 is expressed in all tissues, with high levels in the head, integument, and fat body, while SfJHBP60 shows high expression in the fat body followed by the head.
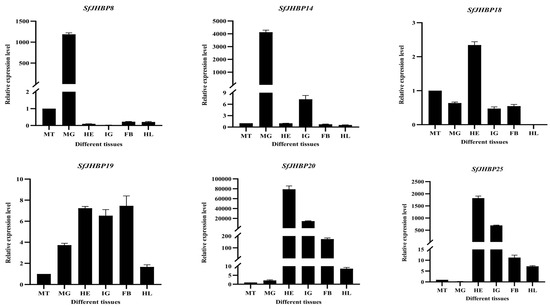
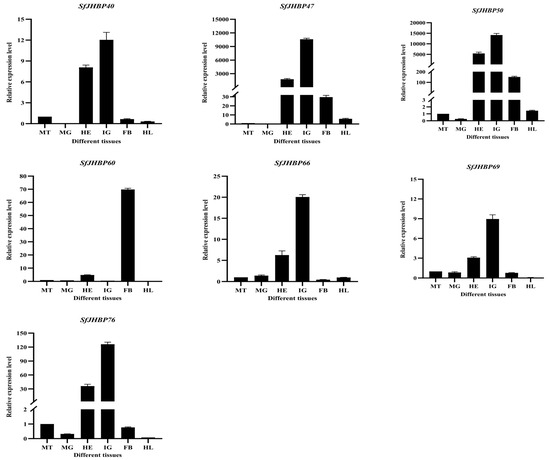
Figure 8.
Expression patterns of 13 SfJHBP genes in different tissues of S. frugiperda. Note: MT: Malpighian tube MG: midgut HE: head IG: integument FB: fat body HL: hemolymph. For the expression of 13 SfJHBP genes in different tissues of S. frugiperda, we used Malpighian tubules as the control group.Data points represent mean ± standard deviation.
In summary, among these 13 genes, 6 SfJHBP genes are highly expressed in the integument, 4 are highly expressed in the head, SfJHBP8 and SfJHBP14 are specifically expressed in the midgut, and SfJHBP60 is specifically expressed in the fat body.
4. Discussion
This study focuses on the JHBP gene family in S. frugiperda. We identified the members of this gene family and conducted analyses on their physicochemical properties, gene structure, subcellular localization, protein secondary structure, sequence characteristics, phylogenetic relationships, chromosomal localization, interspecies synteny, and gene expression. The analysis of JHBP gene family fills the gap of JHBP related research in S. frugiperda to some extent.
To date, some JHBP gene families have been identified in species such as the cypress looper (Dendrolimus houi Lajonquiere) [54] and the silkworm (Bombyx mori) [55] However, a comprehensive identification of JHBP genes in the genome of S. frugiperda has not been reported. In this study, 76 JHBP gene family members were identified at the whole-genome level in S. frugiperda (Table S1. SfJHBP genes are distributed across 8 chromosomes, and the number of JHBP family members varies among different species: 47 in D. houi [54] and 41 in B. mori [55]. This variation in the number of family members across species is likely a consequence of evolutionary processes, where gene family expansion or contraction occurs as an adaptive response to differing environmental conditions over long-term evolution.
Gene duplication is a critical mechanism for the evolution of genomes and species. This process is essential for the functional divergence of homologous genes and is a major factor in the development of new functional genes [56]. The different modes of gene duplication, such as segmental duplication, whole-genome duplication, tandem duplication, and transpositional duplication, play unique roles in shaping genetic diversity and complexity [57]. Through gene duplication, organisms can acquire novel traits and adapt to changing environments. This process not only contributes to the diversity of gene function but also plays a vital role in evolutionary processes. By understanding the various modes of gene duplication and their implications, valuable insights can be gained into the mechanisms driving genetic evolution. This study found that among the 76 SfJHBP genes, 29 gene duplication events occurred, all of which were tandem duplications, indicating that the expansion of SfJHBP genes was achieved through tandem duplication.
JH plays a pivotal role in regulating numerous essential life events in insects. Within the Lepidoptera order, which includes significant agricultural pests, the signaling of JH is tightly governed by species-specific JHBPs in the hemolymph. These JHBPs, characterized by their high-affinity and low molecular weight, are responsible for transporting JH from its site of synthesis to the target tissues. Hence, JHBPs show great potential as targets for creating innovative insect growth regulators (IGRs) and insecticides. JH are acyclic sesquiterpenoids that consist of α, β-unsaturated ester and terpene skeleton with a terminal epoxide. The hormone’s regulatory functions are dependent on both the ester and epoxide groups. JH plays a crucial role in controlling diverse processes like insect growth, development, and reproduction [6,7]. Through the analysis of SfJHBP gene family expression levels at various developmental stages, combining transcriptome data and qPCR analysis, revealed that SfJHBP20, SfJHBP50 and SfJHBP69 are significantly expressed in multiple developmental stages, indicating their pivotal role in growth and development.
Previous studies have shown that the expression levels of JHBPs in insects are consistent with changes in JH titer [58]. It is thus inferred that JHBPs in S. frugiperda exhibit similar expression patterns. Combining RNA-seq data from public databases and qPCR analysis of specific SfJHBP genes at different developmental stages, we found that SfJHBP18, SfJHBP19, SfJHBP20, SfJHBP25, SfJHBP40, SfJHBP50, SfJHBP66, and SfJHBP76 are most highly expressed at the 5th instar and decrease as the larvae progress to the 6th instar and pupate. This pattern reflects S. frugiperda’s varying needs for JH. As the larvae enter the 5th instar, their food intake significantly increases, and the expression of JHBPs rises, possibly regulating feeding behavior [17]; the significant reduction or absence of JH titer at the late 6th instar promotes molting and pupation [59], possibly related to internal and external morphological and structural reconstruction before eclosion [60]. This trend is similar to the declining expression of OfJHBP mRNA in O. fuscidentalis from the diapause to pupation stage, indicating JHBP’s crucial role before diapause pupation and eclosion. Furthermore, interference with JHBP in unmated female brown planthoppers (Nilaparvata lugens) significantly reduces their mating desire [61], suggesting a regulatory role of JHBP in adult reproductive behavior. In this study, the increased expression of SfJHBP18, SfJHBP19, SfJHBP20, SfJHBP40, SfJHBP47, and SfJHBP76 in adult insects may also be related to reproductive regulation, which requires further verification.
JH is vital for maintaining insect growth and morphology. A reduction in JH levels before molting can result in incomplete pupation [8]. Combining transcriptome data from different tissues and qPCR analysis of specific SfJHBP genes at various developmental stages revealed that the primary action sites of the SfJHBP gene family are the midgut, head, and integument tissues of S. frugiperda. The expression sites of JHBPs vary among different insects. For instance, GdJHBP is highly expressed in the thorax and abdomen of adult cypress looper beetles but low in the head [30]. In B.mori, JHBP is mainly expressed in the fat body of 4th instar larvae, with minor expression in the midgut, silk gland, and testis [17]. In the Italian honeybee (Apis mellifera), JHBP expression is detected only in the head and abdomen but not in other parts [62]. The cytoplasmic JHBP in the Chinese oak silkworm (Antheraea pernyi) is highly expressed in the Malpighian tubules and variably expressed in the hemolymph, fat body, silk gland, epidermis, testis, ovary, brain, and muscles [59]. The JHBP in O. fuscidentalis is highly expressed in the fat body of 5th instar larvae and lowly expressed in the brain, prothoracic gland, epidermis, gut, and silk gland [28]. These differences may be due to species variation, developmental stages, physiological conditions, or JHBP types.
The expression characteristics of the SfJHBP gene family in different developmental stages and tissues of S. frugiperda provide insights into the potential functions of specific genes. This information lays a theoretical foundation for future gene knockdown experiments to verify their functions and their efficacy in controlling S. frugiperda, offering references for developing new pesticides.
5. Conclusions
A total of 76 JHBP genes were identified from the genome of S. frugiperda. These genes are unevenly distributed across eight chromosomes. The differentiation of these genes primarily occurred through tandem duplication. The secondary structure of the proteins is predominantly composed of α-helices and random coils. There are a considerable number of orthologous genes shared between S. frugiperda and S. litura, indicating a close evolutionary relationship. Through qPCR analysis of 13 SfJHBP genes, it was found that SfJHBP20, SfJHBP50, and SfJHBP69 exhibit high expression levels at multiple developmental stages. Additionally, SfJHBP40, SfJHBP50, SfJHBP66, SfJHBP69, and SfJHBP76 are highly expressed in both the fifth instar larvae and the integument. SfJHBP8 and SfJHBP14 are specifically expressed during the pupal stage and in the midgut, while SfJHBP60 shows specific expression in the fat body. This study provides a basis for predicting the functions of JHBP genes in S. frugiperda, offering theoretical support for subsequent gene knockout experiments to validate their roles. Furthermore, the findings could inform strategies for controlling S. frugiperda and contribute to the development of new pesticides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15080573/s1. Table S1: Physical and chemical properties and subcellular localization of JHBP gene family in S. frugiperda; Table S2: Secondary structure analysis of the JHBP protein of S. frugiperda; Table S3: Analysis of gene replication events in SfJHBP family members of S. frugiperda.
Author Contributions
Conceptualization, D.W. and J.D.; methodology, D.W.; software, Y.L., K.Z. and T.W; validation, T.W., M.G., H.D. and H.Y.; formal analysis, H.Y. and H.D.; investigation, Y.L., T.W. and H.Y.; resources, D.W. and J.D.; data curation, Y.L., T.W. and K.Z.; writing—original draft preparation, Y.L., K.Z., T.W., M.G., H.D., H.Y., D.W. and J.D.; writing—review and editing, Y.L., K.Z., H.Y., D.W. and J.D.; visualization, Y.L., T.W. and H.Y.; supervision, D.W. and J.D.; project administration, Y.L.; funding acquisition, D.W., J.D. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Education Department of Anhui Province (2023AH051852), the Anhui Province International Joint Research Center of Forage Bio-breeding (No. AHIJRCFB202303), the Anhui Province Agricultural Germplasm Resource Bank (Nursery) Performance Award Project (Anhui Province Maize Germplasm Resource Bank 1025), the Transformation of high-yielding and stress-resistant corn varieties and precision cultivation technology for high planting density (2024ZH017), the Innovative Entrepreneurship Training Program for college students in Anhui Province (S202210879140), and the Key Discipline Construction Funds for Crop Science of Anhui Sciences and Technology University (No. XK-XJGF001).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank Anhui Science and Technology University forsupporting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.d.; Peterson, J.A.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.G. Overview of the invasion mechanism of fall armyworm Spodoptera frugiperda. J. Plant Prot. 2022, 49, 1313–1328. [Google Scholar]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Jia, H.-R.; Guo, J.-L.; Wu, Q.-L.; Hu, C.-X.; Li, X.-K.; Zhou, X.-Y.; Wu, K.-M. Migration of invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) across the Bohai Sea in northern China. J. Integr. Agric. 2021, 20, 685–693. [Google Scholar] [CrossRef]
- Truman, J.W.; Riddiford, L.M. The origins of insect metamorphosis. Nature 1999, 401, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I.; Granger, N.A.; Roe, R.M. The juvenile hormones: Historical facts and speculations on future research directions. Insect Biochem. Mol. Biol. 2000, 30, 617–644. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. Hormonal regulation of sequential larval cuticular gene expression. Arch. Insect Biochem. Physiol. 1986, 3, 75–86. [Google Scholar] [CrossRef]
- Kramer, K.; Dunn, P.; Peterson, R.; Seballos, H.; Sanburg, L.; Law, J. Purification and characterization of the carrier protein for juvenile hormone from the hemolymph of the tobacco hornworm Manduca sexta Johannson (Lepidoptera: Sphingidae). J. Biol. Chem. 1976, 251, 4979–4985. [Google Scholar] [CrossRef]
- De Kort, C.; Koopmanschap, A. High molecular transport proteins for JH-III in insect hemolymph. Experientia 1986, 42, 834–836. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Muszyńska-Pytel, M.; Grzelak, K.; Kochman, M. Biosynthesis and degradation of juvenile hormone in corpora allata and imaginal wing discs of Galleria mellonella (L.). Insect Biochem. 1987, 17, 249–254. [Google Scholar] [CrossRef]
- Trowell, S.C. High affinity juvenile hormone carrier proteins in the haemolymph of insects. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 795–807. [Google Scholar] [CrossRef]
- Kochman, M.; Wieczorek, E. Proteins involved in juvenile hormone signal transmission. In Insects: Chemical, Physiological and Environmental Aspects; Konopinska, D., Goldsworthy, G., Nachman, R.J., Nawrot, J., Orchard, I., Rosinski, G., Sobótka, W., Eds.; University of Wrocław Press: Wrocław, Poland, 1995; pp. 92–118. [Google Scholar]
- Sok, A.J.; Andruszewska, G.; Niewiadomska-Cimicka, A.; Grad, I.; Rymarczyk, G.; Pajdzik, D.; Orłowski, M.; Schmidt, M.T.; Grajek, W.; Ożyhar, A. Regulatory elements in the juvenile hormone binding protein gene from Galleria mellonella—Topography of binding sites for Usp and EcRDBD. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2008, 1779, 390–401. [Google Scholar] [CrossRef]
- Dębski, J.; Wysłouch-Cieszyńska, A.; Dadlez, M.; Grzelak, K.; Kłudkiewicz, B.; Kołodziejczyk, R.; Lalik, A.; Ożyhar, A.; Kochman, M. Positions of disulfide bonds and N-glycosylation site in juvenile hormone binding protein. Arch. Biochem. Biophys. 2004, 421, 260–266. [Google Scholar] [CrossRef]
- Sarov-Blat, L.; So, W.V.; Liu, L.; Rosbash, M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell 2000, 101, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Su, Z.H.; Emi, A.; Mita, K.; Takeda, M.; Fujiwara, Y. Cloning and expression analysis of takeout/JHBP family genes of silkworm, Bombyx mori. Insect Mol. Biol. 2006, 15, 245–251. [Google Scholar] [CrossRef]
- Dauwalder, B.; Tsujimoto, S.; Moss, J.; Mattox, W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002, 16, 2879–2892. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.; Dombrovsky, A.; Raccah, B.; Sherman, A. A microarray approach identifies ANT, OS-D and takeout-like genes as differentially regulated in alate and apterous morphs of the green peach aphid Myzus persicae (Sulzer). Insect Biochem. Mol. Biol. 2006, 36, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Orth, A.P.; Lan, Q.; Goodman, W.G. Ligand regulation of juvenile hormone binding protein mRNA in mutant Manduca sexta. Mol. Cell. Endocrinol. 1999, 149, 61–69. [Google Scholar] [CrossRef]
- Orth, A.P.; Tauchman, S.J.; Doll, S.C.; Goodman, W.G. Embryonic expression of juvenile hormone binding protein and its relationship to the toxic effects of juvenile hormone in Manduca sexta. Insect Biochem. Mol. Biol. 2003, 33, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Riddiford, L.M. Insect juvenile hormone action as a potential target of pest management. J. Pestic. Sci. 2006, 31, 77–84. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanaka, K. Hormonal control of diapause and overwintering traits in a leaf beetle, Aulacophora nigripennis. Physiol. Entomol. 2000, 25, 337–345. [Google Scholar] [CrossRef]
- Cho, J.R.; Lee, M.; Kim, H.S.; Boo, K.S. Effect of the juvenile hormone analog, fenoxycarb on termination of reproductive diapause in Scotinophara lurida (Burmeister) (Heteroptera: Pentatomidae). J. Asia-Pac. Entomol. 2007, 10, 145–150. [Google Scholar] [CrossRef]
- Orth, A.; Doll, S.; Goodman, W. Sequence, structure and expression of the hemolymph juvenile hormone binding protein gene in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2003, 33, 93–102. [Google Scholar] [CrossRef] [PubMed]
- He, X. The DNA Cloning and RNA Interference of Some Functional Genes in Laodelphax striatellus (Fallen). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2011. [Google Scholar]
- Ritdachyeng, E.; Manaboon, M.; Tobe, S.S.; Singtripop, T. Molecular characterization and gene expression of juvenile hormone binding protein in the bamboo borer, Omphisa fuscidentalis. J. Insect Physiol. 2012, 58, 1493–1501. [Google Scholar] [CrossRef]
- Parkitna, J.R.; Ozyhar, A.; Wisniewski, J.R.; Kochman, M. Cloning and Sequence Analysis of Galleria mellonella Juvenile Hormone Binding Protein A Search for Ancestors and Relatives. Biol. Chem. 2002, 383, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Tan, Y.; Pang, B.; Xin, B. Cloning and Expression Profiling of the JHBP Gene, GdJHBP, in Galeruca daurica. Chin. J. Appl. Entomol. 2020, 57, 623–631. [Google Scholar]
- Zhang, W.; Liang, G.; Ma, L.; Jiang, T.; Xiao, H. Dissecting the role of juvenile hormone binding protein in response to hormone and starvation in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 1411–1417. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Li, F.; Li, G.; Dong, S. Cloning and Expression Dynamics of Juvenile Hormone Binding Protein Gene in Laodelphax striatellus. J. Nanjing Agric. Univ. 2012, 35, 59–64. [Google Scholar]
- Fujimoto, Z.; Suzuki, R.; Shiotsuki, T.; Tsuchiya, W.; Tase, A.; Momma, M.; Yamazaki, T. Crystal structure of silkworm Bombyx mori JHBP in complex with 2-methyl-2, 4-pentanediol: Plasticity of JH-binding pocket and ligand-induced conformational change of the second cavity in JHBP. PLoS ONE 2013, 8, e56261. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Bonthala, V.S.; Muthamilarasan, M.; Pandey, G.; Khan, Y.; Prasad, M. Genome-wide development of transposable elements-based markers in foxtail millet and construction of an integrated database. DNA Res. 2015, 22, 79–90. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C. The genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, e38. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular evolutionary genetics analysis software for microcomputers. Bioinformatics 1994, 10, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Ye, X.; Xu, H.; Mei, Y.; Yang, Y.; Chen, X.; Yang, Y.; Liu, T.; Yu, Y.; Yang, W. The genetic adaptations of fall armyworm Spodoptera frugiperda facilitated its rapid global dispersal and invasion. Mol. Ecol. Resour. 2020, 20, 1050–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, R.; Gao, J.; Xiao, X.; Yin, X.; Hu, S.; Zhang, Y.; Liang, P.; Gu, S. Two cuticle-enriched chemosensory proteins confer multi-insecticide resistance in Spodoptera frugiperda. Int. J. Biol. Macromol. 2024, 266, 130941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Meng, J.Y.; Ruan, H.Y.; Yang, C.L.; Zhang, C.Y. Expression stability of candidate RT-qPCR housekeeping genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2021, 108, e21831. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.; Basu, C. Real-time PCR (qPCR) primer design using free online software. Biochem. Mol. Biol. Educ. 2011, 39, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, F.; Huang, W.; Sun, Q.; Huang, X. Identification of reliable reference genes for qRT-PCR in the ephemeral plant Arabidopsis pumila based on full-length transcriptome data. Sci. Rep. 2019, 9, 8408. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hinton, P.R.; McMurray, I.; Brownlow, C. SPSS Explained; Routledge: Oxfordshire, UK, 2014. [Google Scholar]
- Swift, M.L. GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Z.; Zhao, Z.; He, H.; Lin, X.; Liang, G. Identification and biological analysis of JHBP gene family members within Dendrolimus houi. J. For. Environ. 2024, 44, 95–104. [Google Scholar]
- Li, W.; Cheng, T.; Hu, W.; Peng, Z.; Liu, C.; Xia, Q. Genome-wide identification and analysis of JHBP-domain family members in the silkworm Bombyx mori. Mol. Genet. Genom. 2016, 291, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Innan, H.; Kondrashov, F. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010, 11, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Freeling, M. Bias in plant gene content following different sorts of duplication: Tandem, whole-genome, segmental, or by transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-Q.; Tang, F.; Gong, S.-Q.; Luo, J.-Y.; Yuan, R.; Cao, C.-W. Cloning of juvenile hormone binding protein genes in Lymantria dispar and its response to CO2 stresses. J. Environ. Entomol. 2019, 41, 1339–1347. [Google Scholar]
- Li, Y.; Peng, M.; Chen, M.; Li, Y.; Zhang, Q.; Jiang, X.; Liu, Y. Characterization of insect cytosolic juvenile hormone binding protein gene: Highly homology with vertebrate glyoxalase domain containing protein 4. Biochem. Syst. Ecol. 2015, 58, 227–234. [Google Scholar] [CrossRef]
- Qian, J. Study on the Cloning and Expression Patterns of JH-Related Enzyme Genes in Ulomoides Dermestoides. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2016. [Google Scholar]
- Su, Q.; Lv, J.; Li, W.-X.; Sun, J.-W.; Li, S.-H.; Zhang, W.-Q. Identification of putative abdominal vibration-related genes through transcriptome analyses in the brown planthopper (Nilaparvata lugens). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100856. [Google Scholar] [CrossRef]
- Hagai, T.; Cohen, M.; Bloch, G. Genes encoding putative Takeout/juvenile hormone binding proteins in the honeybee (Apis mellifera) and modulation by age and juvenile hormone of the takeout-like gene GB19811. Insect Biochem. Mol. Biol. 2007, 37, 689–701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).