1H NMR Profiling of Honey Bee Brains across Varying Ages and Seasons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Worker Bees
2.2. Brain Dissections
2.3. Metabolite Extraction
2.4. NMR Analysis
2.5. Data Processing and Statistical Analysis
3. Results
3.1. NMR Spectrum of Honey Bee Brains
3.2. Principal Component Analysis for Summer, Fall, and Winter Bees
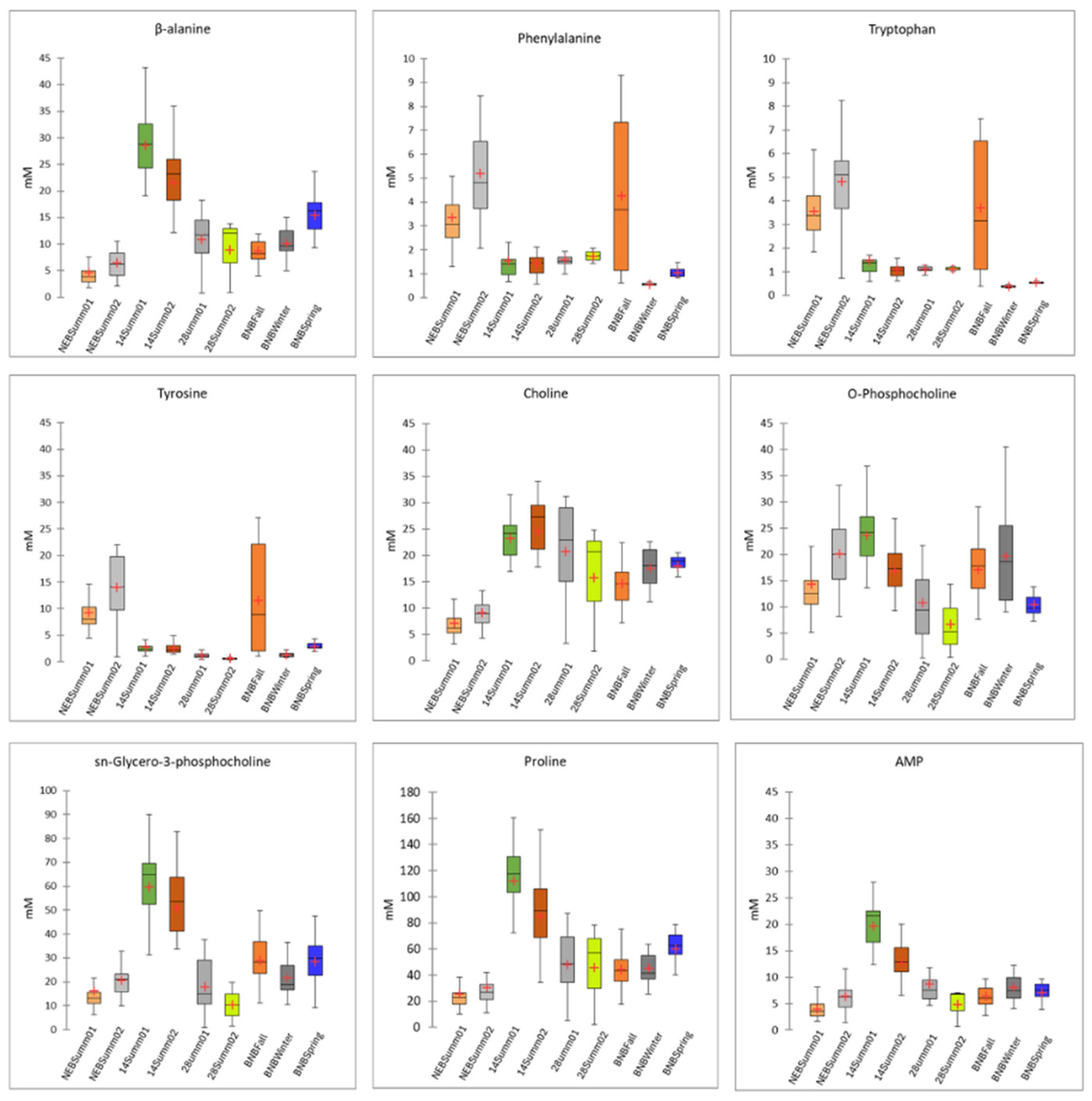
3.3. Statistically Significant Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Requier, F.; Pérez-Méndez, N.; Andersson, G.K.S.; Blareau, E.; Merle, I.; Garibaldi, L.A. Bee and Non-Bee Pollinator Importance for Local Food Security. Trends Ecol. Evol. 2023, 38, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A.; et al. Global Trends in the Number and Diversity of Managed Pollinator Species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony Losses, Managed Colony Population Decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.; DeGrandi-Hoffman, G.; Pruett, M.; Jones, V.P.; Corby-Harris, V.; Pireaud, J.; Curry, R.; Hopkins, B.; Northfield, T.D. Warmer Autumns and Winters Could Reduce Honey Bee Overwintering Survival with Potential Risks for Pollination Services. Sci. Rep. 2024, 14, 5410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Evans, J.D.; Robinson, G.E.; Berenbaum, M.R. Changes in Transcript Abundance Relating to Colony Collapse Disorder in Honey Bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2009, 106, 14790–14795. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Emwas, A.-H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-Based Metabolomics in Human Disease Diagnosis: Applications, Limitations, and Recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- de Falco, B.; Lanzotti, V. NMR Spectroscopy and Mass Spectrometry in Metabolomics Analysis of Salvia. Phytochem. Rev. 2018, 17, 951–972. [Google Scholar] [CrossRef]
- Pontes, J.G.M.; Brasil, A.J.M.; Cruz, G.C.F.; de Souza, R.N.; Tasic, L. NMR-Based Metabolomics Strategies: Plants, Animals and Humans. Anal. Methods 2017, 9, 1078–1096. [Google Scholar] [CrossRef]
- Pizzorno, M.C.; Field, K.; Kobokovich, A.L.; Martin, P.L.; Gupta, R.A.; Mammone, R.; Rovnyak, D.; Capaldi, E.A. Transcriptomic Responses of the Honey Bee Brain to Infection with Deformed Wing Virus. Viruses 2021, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Münch, D.; Amdam, G.V. The Curious Case of Aging Plasticity in Honey Bees. FEBS Lett. 2010, 584, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Page, R.E.; Peng, C.Y.-S. Aging and Development in Social Insects with Emphasis on the Honey Bee, Apis mellifera L. Exp. Gerontol. 2001, 36, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Omholt, S.W. The Regulatory Anatomy of Honeybee Lifespan. J. Theor. Biol. 2002, 216, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Honey Bee Biology, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2023; pp. 204–225. [Google Scholar]
- Huang, Z.-Y.; Robinson, G.E. Seasonal Changes in Juvenile Hormone Titers and Rates of Biosynthesis in Honey Bees. J. Comp. Physiol. B 1995, 165, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal Control of the Yolk Precursor Vitellogenin Regulates Immune Function and Longevity in Honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kalcic, F.; Duarte, I.F.; Titera, D.; Kamler, M.; Mrna, P.; Hyrsl, P.; Danihlik, J.; Dobes, P.; Kunc, M.; et al. 1H NMR Profiling of Honey Bee Bodies Revealed Metabolic Differences between Summer and Winter Bees. Insects 2022, 13, 193. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Aoki, M.; Takada, M.; Kanou, Y.; Sasagawa, H.; Kitagawa, Y.; Kadowaki, T. The Changes of Gene Expression in Honeybee (Apis mellifera) Brains Associated with Ages. J. Zool. 2004, 21, 23–28. [Google Scholar] [CrossRef]
- Wolschin, F.; Münch, D.; Amdam, G. Structural and Proteomic Analyses Reveal Regional Brain Differences during Honeybee Aging. J. Exp. Biol. 2009, 212, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Kreibich, C.D.; Amdam, G.V.; Münch, D. Metabolic Enzymes in Glial Cells of the Honeybee Brain and Their Associations with Aging, Starvation and Food Response. PLoS ONE 2018, 13, e0198322. [Google Scholar] [CrossRef]
- Behrends, A.; Scheiner, R. Learning at Old Age: A Study on Winter Bees. Front. Behav. Neurosci. 2010, 4, 1563. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.-C.; Krekling, T.; Amdam, G.V. Cellular Senescence in Honey Bee Brain Is Largely Independent of Chronological Age. Exp. Gerontol. 2006, 41, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Łoś, A.L.; Bieńkowska, M.A.; Strachecka, A. Honey bee (Apis mellifera) as an Alternative Model Invertebrate Organism. Medycyna Weterynarynjna 2019, 75, 93–106. [Google Scholar]
- Lee, H.-Y.; Lee, S.-H.; Min, K.-J. Insects as a Model System for Aging Studies. Entomol. Res. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Quigley, T.P.; Amdam, G.V.; Rueppell, O. Honeybee Workers as Models of Aging. In Handbook of Models for Human Aging, 2nd ed.; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 533–547. [Google Scholar] [CrossRef]
- Morfin, N.; Fillier, T.; Pham, T.H.; Goodwin, P.; Thomas, R.; Guzman, E. First Insights into the Honey Bee (Apis mellifera) Brain Lipidome and Its Neonicotinoid-Induced Alterations Associated with Reduced Self-Grooming Behavior. J. Adv. Res. 2022, 37, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R. Revealing the Metabolome of Animal Tissues Using 1H Nuclear Magnetic Resonance Spectroscopy. In Metabolomics: Methods and Protocols; Weckwerth, W., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 229–246. [Google Scholar] [CrossRef]
- O’Hara, L.; Longstaffe, J.G. 1H-Nuclear Magnetic Resonance Metabolomics Analysis of Arabidopsis thaliana Exposed to Perfluorooctanoic Acid and Perfluoroctanesulfonic Acid. Environ. Toxicol. Chem. 2023, 42, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: A Unified LC-MS Workflow for Global Metabolomics. Nat. Commun. 2024, 15, 3675. [Google Scholar] [CrossRef]
- Lumivero. XLSTAT Statistical and Data Analysis Solution. 2024. Available online: https://www.xlstat.com/en (accessed on 21 May 2024).
- McDevitt, J.C.; Gupta, R.A.; Dickinson, S.G.; Martin, P.L.; Rieuthavorn, J.; Freund, A.; Pizzorno, M.C.; Capaldi, E.A.; Rovnyak, D. Methodology for Single Bee and Bee Brain 1H-NMR Metabolomics. Metabolites 2021, 11, 864. [Google Scholar] [CrossRef]
- Lou, H. Dopamine Precursors and Brain Function in Phenylalanine Hydroxylase Deficiency. Acta Paediatr. 1994, 83, 86–88. [Google Scholar] [CrossRef]
- Pryor, A.; Hart, S.; Berry, M.D. Synthesis and Neurochemistry of Trace Amines. In Trace Amines and Neurological Disorders; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 27–43. [Google Scholar] [CrossRef]
- Vleugels, R.; Verlinden, H.; Vanden Broeck, J. Serotonin, Serotonin Receptors and their Actions in Insects. Neurotransmitter 2015, 2, e314. [Google Scholar] [CrossRef]
- Nouvian, M.; Mandal, S.; Jamme, C.; Claudianos, C.; d’Ettorre, P.; Reinhard, J.; Barron, A.B.; Giurfa, M. Cooperative Defence Operates by Social Modulation of Biogenic Amine Levels in the Honey Bee Brain. Proc. Biol. Sci. B 2018, 285, 20172653. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, B. Cellular Physiology of the Honey Bee Brain. In Honeybee Neurobiology and Behavior; Galizia, C., Eisenhardt, D., Giurfa, M., Eds.; Springer: New York, NY, USA, 2012; pp. 185–198. [Google Scholar] [CrossRef]
- Bombardi, C.; Salamanca, G.; Tagliavia, C.; Grandis, A.; Mille, F.; De Iorio, M.G.; Minozzi, G. Immunohistochemical Distribution of Serotonin Transporter (SERT) in the Optic Lobe of the Honeybee, Apis mellifera. Animals 2022, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.J.; Robinson, G.E. Biogenic Amines and Division of Labor in Honey Bee Colonies: Behaviorally Related Changes in the Antennal Lobes and Age-Related Changes in the Mushroom Bodies. J. Comp. Physiol. A 1999, 184, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Harris, J.L.; Otis, G.W. Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux 2001, 48, 88–93. [Google Scholar] [CrossRef]
- Hammer, M.; Menzel, R. Learning and Memory in the Honeybee. J. Neurosci. 1995, 15, 1617–1630. [Google Scholar] [CrossRef]
- Frontali, N. Acetylcholine Synthesis in the Housefly Head. J. Insect Physiol. 1958, 1, 319–326. [Google Scholar] [CrossRef]
- Gauthier, M.; Grünewald, B. Neurotransmitter Systems in the Honey Bee Brain: Functions in Learning and Memory. In Honeybee Neurobiology and Behavior; Galizia, C., Eisenhardt, D., Giurfa, M., Eds.; Springer: New York, NY, USA, 2012; pp. 155–169. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat Body—Multifunctional Insect Tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Wyatt, G.R. The Biochemistry of Insect Hemolymph. Annu. Rev. Entomol. 1961, 6, 75–102. [Google Scholar] [CrossRef]
- Bouchebti, S.; Bodner, L.; Bergman, M.; Magory Cohen, T.; Levin, E. The Effects of Dietary Proline, β-Alanine, and γ-Aminobutyric Acid (GABA) on the Nest Construction Behavior in the Oriental Hornet (Vespa Orientalis). Sci. Rep. 2022, 12, 7449. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of Sublethal Doses of Clothianidin and/or V. destructor on Honey Bee (Apis mellifera) Self-Grooming Behavior and Associated Gene Expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef]
- Erez, T.; Bonda, E.; Kahanov, P.; Rueppell, O.; Wagoner, K.; Chejanovsky, N.; Soroker, V. Multiple Benefits of Breeding Honey Bees for Hygienic Behavior. J. Invertebr. Pathol. 2022, 193, 107788. [Google Scholar] [CrossRef]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Bini, L.; Puglia, M.; Bianchi, L.; Pacini, E. Amino Acids and Protein Profile in Floral Nectar: Much More than a Simple Reward. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 475–481. [Google Scholar] [CrossRef]
- González-Tokman, D.; Villada-Bedoya, S. Physiological Mechanisms of Heat Tolerance. In Effects of Climate Change on Insects: Physiological, Evolutionary, and Ecological Responses; González-Tokman, D., Dáttilo, W., Eds.; Oxford Academic: Oxford, UK, 2024; pp. 51–64. [Google Scholar] [CrossRef]
- Tiedje, K.E.; Stevens, K.; Barnes, S.; Weaver, D.F. Beta-Alanine as a Small Molecule Neurotransmitter. Neurochem. Int. 2010, 57, 177–188. [Google Scholar] [CrossRef]
- Galizia, C.G.; Eisenhardt, D.; Giurfa, M. (Eds.) Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- True, J.R.; Yeh, S.-D.; Hovemann, B.T.; Kemme, T.; Meinertzhagen, I.A.; Edwards, T.N.; Liou, S.-R.; Han, Q.; Li, J. Drosophila Tan Encodes a Novel Hydrolase Required in Pigmentation and Vision. PLoS Genet. 2005, 1, e63. [Google Scholar] [CrossRef]
- Suh, J.; Jackson, F.R. Drosophila Ebony Activity Is Required in Glia for the Circadian Regulation of Locomotor Activity. Neuron 2007, 55, 435–447. [Google Scholar] [CrossRef]
- Bogo, G.; Bortolotti, L.; Sagona, S.; Felicioli, A.; Galloni, M.; Barberis, M.; Nepi, M. Effects of Non-Protein Amino Acids in Nectar on Bee Survival and Behavior. J. Chem. Ecol. 2019, 45, 278–285. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Poirot, E.; Flores-Mosquera, M. GABA, a Non-Protein Amino Acid Ubiquitous in Food Matrices. Cogent Food Agric. 2018, 4, 1534323. [Google Scholar] [CrossRef]
- Gemelli, T.; de Andrade, R.B.; Rojas, D.B.; Zanatta, Â.; Schirmbeck, G.H.; Funchal, C.; Wajner, M.; Dutra-Filho, C.S.; Wannmacher, C.M.D. Chronic Exposure to β-Alanine Generates Oxidative Stress and Alters Energy Metabolism in Cerebral Cortex and Cerebellum of Wistar Rats. Mol. Neurobiol. 2018, 55, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Getz, M.; Rangamani, P.; Ghosh, P. Regulating Cellular Cyclic Adenosine Monophosphate: “Sources,” “Sinks,” and Now, “Tunable Valves”. WIREs Sist. Biol. Med. 2020, 12, e1490. [Google Scholar] [CrossRef] [PubMed]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Stauch, K.L.; Petrascheck, M.; Benton, H.P.; Epstein, A.A.; Fang, M.; Gorantla, S.; Tran, M.; Hoang, L.; Kurczy, M.E.; et al. Metabolic Drift in the Aging Brain. Aging 2016, 8, 1000–1020. [Google Scholar] [CrossRef]



| NEB Marking and Collection | 14-Day– Old Bee Collection | 28-Day– Old Bee Collection | Brood Nest Bees | Number of Colonies |
|---|---|---|---|---|
| 24 June 2019 (NEBSumm01) | 8 July 2019 (14Summ01) | 22 July 2019 (28Summ01) | NA | 30 |
| 24 June 2019 (NEBSumm02) | 7 August 2019 (14Summ02) | 21 August 2019 (28Summ02) | NA | 28 |
| NA | NA | NA | 17 September 2019 (BNBFall) | 26 |
| NA | NA | NA | 24 February 2020 (BNBWinter) | 17 |
| NA | NA | NA | 8 May 2020 (BNBSpring) | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E.; Legge, N.; Longstaffe, J. 1H NMR Profiling of Honey Bee Brains across Varying Ages and Seasons. Insects 2024, 15, 578. https://doi.org/10.3390/insects15080578
Morfin N, Goodwin PH, Guzman-Novoa E, Legge N, Longstaffe J. 1H NMR Profiling of Honey Bee Brains across Varying Ages and Seasons. Insects. 2024; 15(8):578. https://doi.org/10.3390/insects15080578
Chicago/Turabian StyleMorfin, Nuria, Paul H. Goodwin, Ernesto Guzman-Novoa, Nicole Legge, and James Longstaffe. 2024. "1H NMR Profiling of Honey Bee Brains across Varying Ages and Seasons" Insects 15, no. 8: 578. https://doi.org/10.3390/insects15080578








