Chlorantraniliprole Enhances Cellular Immunity in Larvae of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Insecticides
2.2. Larval Toxicity Bioassay
2.3. Hemolymph Collection
2.4. Hemocyte Assays
2.5. Total Hemocyte Counts
2.6. Phagocytosis Assay
2.7. Nodulation Assay
2.8. Encapsulation Assay
2.9. Plasmatocyte-Spreading Assay
2.10. Hemocyte Cytoskeleton Assay
2.11. Statistical Analyses
3. Results
3.1. Larval Toxicity Bioassay
3.2. Hemocyte Types
3.3. Hemocyte Morphology
3.4. Total Hemocyte Counts
3.5. Phagocytosis Assay
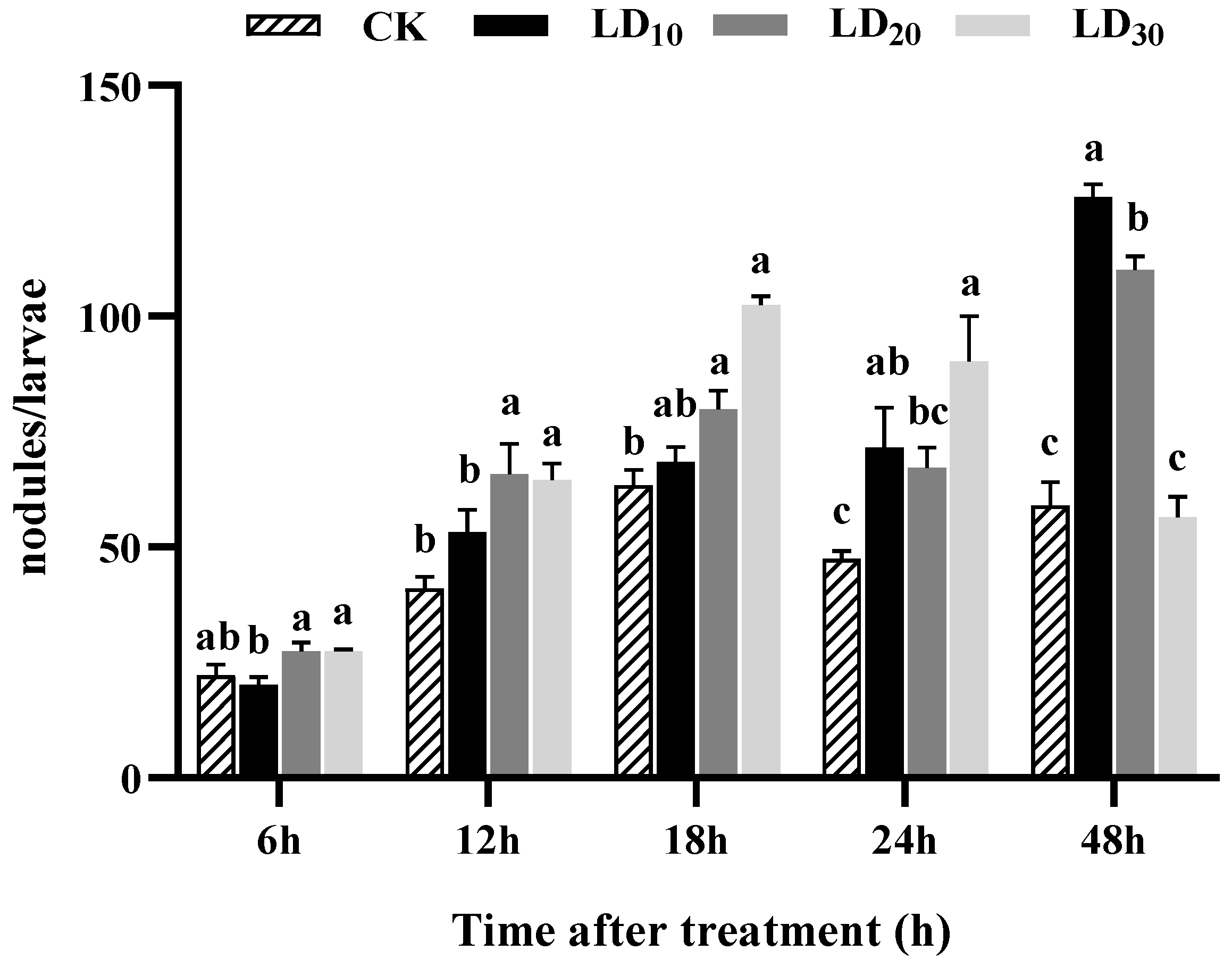
3.6. Nodulation Assay
3.7. Encapsulation Assay
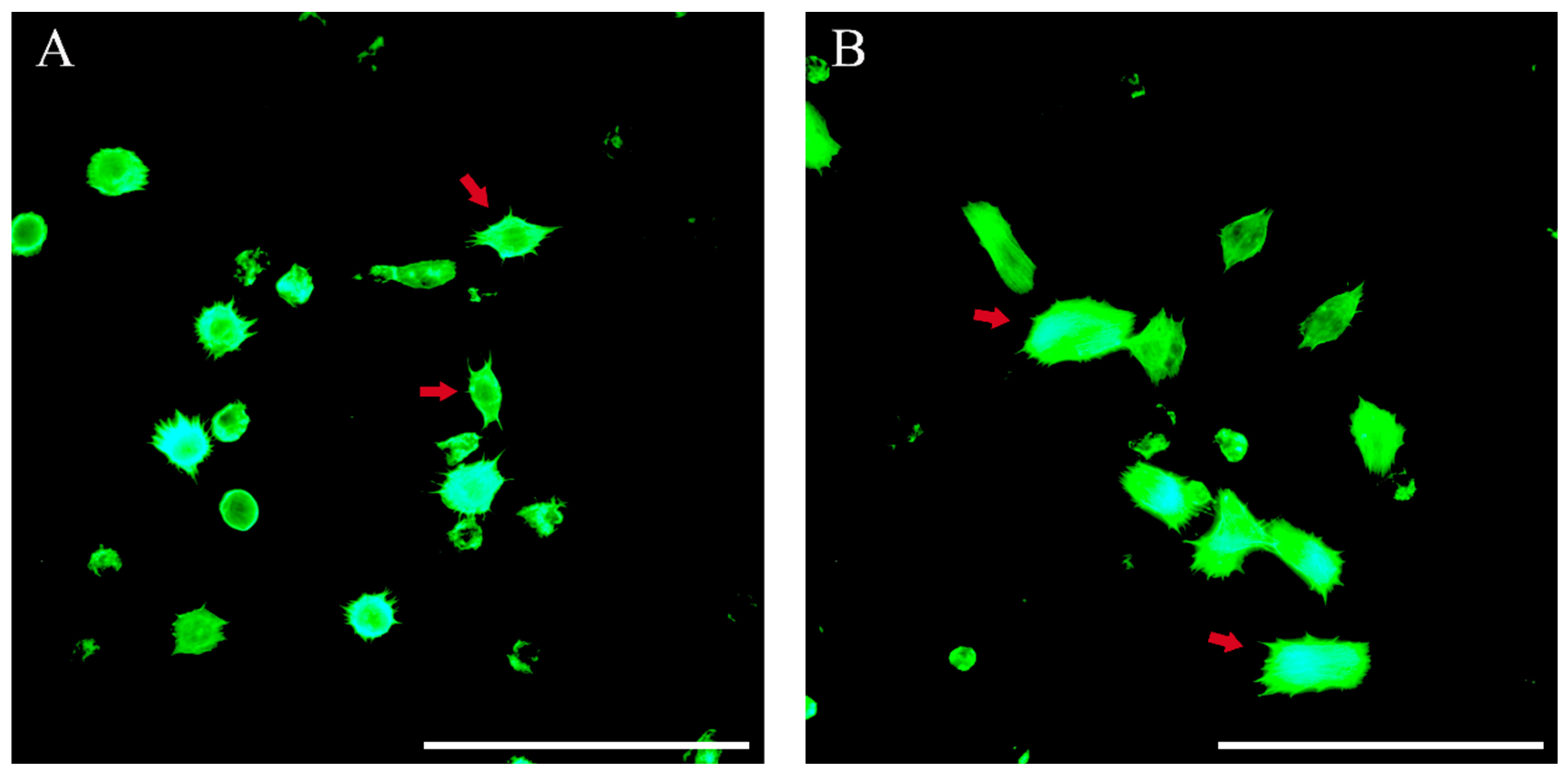
3.8. Plasmatocyte-Spreading Assay
3.9. Hemocyte Cytoskeleton Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, R.C.M.C.; Ramos, I.B.; Travassos, L.H.; Mendez, A.P.G.; Gomes, F.M. Evolution of Innate Immunity: Lessons from Mammalian Models Shaping Our Current View of Insect Immunity. J. Comp. Physiol. B 2024, 194, 105–119. [Google Scholar] [CrossRef]
- Andoh, M.; Ueno, T.; Kawasaki, K. Tissue-Dependent Induction of Antimicrobial Peptide Genes after Body Wall Injury in House Fly (Musca domestica) Larvae. Drug Discov. Ther. 2018, 12, 355–362. [Google Scholar] [CrossRef]
- Siva-Jothy, M.T.; Moret, Y.; Rolff, J. Insect Immunity: An Evolutionary Ecology Perspective. Adv. Insect Physiol. 2005, 32, 1–48. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Strand, M.R. Mosquito Hemocyte-Mediated Immune Responses. Curr. Opin. Insect. Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- Majumder, J.; Ghosh, D.; Agarwala, B.K. Haemocyte Morphology and Differential Haemocyte Counts of Giant Ladybird Beetle, Anisolemnia dilatata (F.) (Coleoptera: Coccinellidae): A Unique Predator of Bamboo Woolly Aphids. Curr. Sci. India 2017, 112, 160. [Google Scholar] [CrossRef]
- Ratcliffe, N.A. Cellular Defense Responses of Insects: Unresolved Problems. In Parasites and Pathogens of Insects; Beckage, N.E., Thompson, S.N., Federici, B.A., Eds.; Elsevier: New York, NY, USA, 1993; Volume 2, pp. 267–304. [Google Scholar] [CrossRef]
- Strand, M.R.; Pech, L.L. Immunological Basis for Compatibility in Parasitoid-Host Relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef]
- Li, S.; Liu, F.; Kang, Z.; Li, X.; Lu, Y.; Li, Q.; Pang, Y.; Zheng, F.; Yin, X. Cellular Immune Responses of the Yellow Peach Moth, Conogethes punctiferalis (Lepidoptera: Crambidae), to the Entomopathogenic Fungus, Beauveria bassiana (Hypocreales: Cordycipitaceae). J. Invertebr. Pathol. 2022, 194, 107826. [Google Scholar] [CrossRef]
- Arteaga Blanco, L.A.; Crispim, J.S.; Fernandes, K.M.; De Oliveira, L.L.; Pereira, M.F.; Bazzolli, D.M.S.; Martins, G.F. Differential Cellular Immune Response of Galleria mellonella to Actinobacillus pleuropneumoniae. Cell Tissue Res. 2017, 370, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Theopold, U.; Strand, M. Innate Immunity and Its Evasion and Suppression by Hymenopteran Endoparasitoids. BioEssays 2001, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Krendel, M.; Gauthier, N.C. Building the Phagocytic Cup on an Actin Scaffold. Curr. Opin. Cell Biol. 2022, 77, 102112. [Google Scholar] [CrossRef]
- Hallett, M.B. Localisation of Intracellular Signals and Responses during Phagocytosis. Int. J. Mol. Sci. 2023, 24, 2825. [Google Scholar] [CrossRef]
- Satyavathi, V.V.; Minz, A.; Nagaraju, J. Nodulation: An Unexplored Cellular Defense Mechanism in Insects. Cell. Signal. 2014, 26, 1753–1763. [Google Scholar] [CrossRef]
- Pech, L.L.; Strand, M.R. Granular Cells Are Required for Encapsulation of Foreign Targets by Insect Haemocytes. J. Cell Sci. 1996, 109, 2053–2060. [Google Scholar] [CrossRef]
- Strand, M.R. Insect Hemocytes and Their Role in Immunity. Insect Biochem. Molec. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Wang, S.; Li, X.; Liu, J.; Yu, Y.; Liu, X. Infection of the Entomopathogenic Fungus Metarhizium rileyi Suppresses Cellular Immunity and Activates Humoral Antibacterial Immunity of the Host Spodoptera frugiperda. Pest. Manga. Sci. 2022, 78, 2828–2837. [Google Scholar] [CrossRef]
- Vorselen, D.; Labitigan, R.L.D.; Theriot, J.A. A Mechanical Perspective on Phagocytic Cup Formation. Curr. Opin. Cell Biol. 2020, 66, 112–122. [Google Scholar] [CrossRef]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Csh. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef]
- Bashirzadeh, Y.; Liu, A.P. Encapsulation of the Cytoskeleton: Towards Mimicking the Mechanics of a Cell. Soft Matter 2019, 15, 8425–8436. [Google Scholar] [CrossRef]
- Yu, G.; Zheng, L.; Quan, Y.; Wei, H. Sublethal Pesticide Exposure Improves Resistance to Infection in the a Sian Corn Borer. Ecol. Entomol. 2018, 43, 326–331. [Google Scholar] [CrossRef]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune Priming: The Secret Weapon of the Insect World. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef]
- Opare, L.O.; Meister, H.; Holm, S.; Kaasik, A.; Esperk, T. High Larval Densities and High Temperatures Lead to a Stronger Immune Response in the Black Soldier Fly. J. Insects Food Feed. 2023, 9, 1177–1186. [Google Scholar] [CrossRef]
- James, R.R.; Xu, J. Mechanisms by Which Pesticides Affect Insect Immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Delpuech, J.; Frey, F.; Carton, Y. Action of Insecticides on the Cellular Immune Reaction of Drosophila melanogaster against the Parasitoid Leptopilina boulardi. Environ. Toxicol. Chem. 1996, 15, 2267–2271. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Annoscia, D.; Di Prisco, G.; Becchimanzi, A.; Caprio, E.; Frizzera, D.; Linguadoca, A.; Nazzi, F.; Pennacchio, F. Neonicotinoid Clothianidin Reduces Honey Bee Immune Response and Contributes to Varroa Mite Proliferation. Nat. Commun. 2020, 11, 5887. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef]
- Sułek, M.; Kordaczuk, J.; Wojda, I. Current Understanding of Immune Priming Phenomena in Insects. J. Invertebr. Pathol. 2021, 185, 107656. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Yaroslavtseva, O.N.; Kryukov, V.Y.; Benkovskaya, G.V.; Glupov, V.V. An Increase in the Immune System Activity of the Wax Moth Galleria mellonella and of the Colorado Potato Beetle Leptinotarsa decemlineata under Effect of Organophosphorus Insecticide. J. Evol. Biochem. Phys. 2013, 49, 592–596. [Google Scholar] [CrossRef]
- Grizanova, E.V.; Dubovskiy, I.M.; Whitten, M.M.A.; Glupov, V.V. Contributions of Cellular and Humoral Immunity of Galleria mellonella Larvae in Defence against Oral Infection by Bacillus thuringiensis. J. Invertebr. Pathol. 2014, 119, 40–46. [Google Scholar] [CrossRef]
- Lu, H.L.; St. Leger, R.J. Insect Immunity to Entomopathogenic Fungi. Adv. Genet. 2016, 94, 251–285. [Google Scholar] [CrossRef]
- Ashley, T.R.; Wiseman, B.R.; Davis, F.M.; Andrews, K.L. The Fall Armyworm: A Bibliography. Fla. Entomol. 1989, 72, 152–202. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, Y.; Wu, K. Analysis of migration routes of the fall armyworm Spodoptera frugiperda (J.E. Smith) from Myanmar to China. Plant Prot. 2019, 45, 18. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Guo, J.; He, K.; Wang, Z. Biological characteristics, trend of fall armyworm Spodoptera frugiperda, and the strategy for management of the pest. Chin. J. Appl. Entomol. 2019, 56, 361–369. [Google Scholar] [CrossRef]
- Su, X.; Li, C.; Xu, Y.; Huang, S.; Liu, W.; Liao, Z.; Zhang, Y. Feeding preference and adaptability of fall armyworm Spodoptera frugiperda on five species of host plants and six weeds. J. Environ. Entomol. 2022, 44, 263–272. [Google Scholar] [CrossRef]
- Li, Q.; Men, X.Y.; Jing, C.; Yu, Y.; Zhou, X.H.; Dai, X.Y.; Lv, S.H.; Li, L.L. Research progress in emergency prevention and control of Spodoptera frugiperda, in China. Plant Prot. 2021, 47, 21–27. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Wu, J.; Zheng, F.; Xu, K.; Lin, Y.; Zhang, Z.; Xu, H. Drip Application of Chlorantraniliprole Effectively Controls Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) and Its Distribution in Maize in China. Crop Prot. 2021, 143, 105474. [Google Scholar] [CrossRef]
- Notice of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China on the Issuance of “2020 National Plan for the Prevention and Control of the Fall Armyworm”. Available online: http://www.zzys.moa.gov.cn/gzdt/202002/t20200221_6337551.htm (accessed on 25 March 2024).
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and Selective Ryanodine Receptor Activators for Insect Control. Bioorgan. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to Sublethal Doses of Insecticide and Their Effects on Insects at Cellular and Physiological Levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef]
- Vryzas, Z. Pesticide Fate in Soil-Sediment-Water Environment in Relation to Contamination Preventing Actions. Curr. Opin. Environ. Sci. Health 2018, 4, 5–9. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guan, D.; Wei, J.; Ge, H.; Cao, X.; Lv, S.; Zhou, X.; Zheng, Y.; Meng, X.; Wang, J.; et al. Mechanisms Underlying the Effects of Low Concentrations of Chlorantraniliprole on Development and Reproduction of the Fall Armyworm, Spodoptera frugiperda. Pestic. Biochem. Phys. 2023, 191, 105362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Wu, L.; Chen, W. Transgenerational Sublethal Effects of Chlorantraniliprole and Emamectin Benzoate on the Development and Reproduction of Spodoptera frugiperda. Insects 2023, 14, 537. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, Y.; Gu, S.; Liang, P.; Zhang, L.; Gao, X. Comparison of bioassay methods for the toxicities of chemical insecticides against Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2020, 63, 590–596. [Google Scholar] [CrossRef]
- Mao, M.; Jiang, C.; Liu, M.; Li, Q.; Yang, Q.; Wang, H. Study on hemocytes classification of Hermetia illucens (L.) larve. J. Environ. Entomol. 2017, 39, 1342–1349. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Wang, G.; Li, Y.; Yu, H.; Yan, D.; Guo, Y.; Zhang, T.; Chen, P. Reasons for Changes of Hemocyte Densities and the Relationship between Hemocyte Density and High Temperature Resistance of Bombyx mori Larvae. Acta Entomol. Sin. 2022, 65, 130–143. [Google Scholar] [CrossRef]
- Walkowiak-Nowicka, K.; Nowicki, G.; Kuczer, M.; Rosiński, G. New Activity of Yamamarin, an Insect Pentapeptide, on Immune System of Mealworm, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Tezuka, M.; Tang, S.; Shu, M.; Yamagishi, T.; Sato, R. A Humoral Factor, Hemolymph Proteinase 8, Elicits a Cellular Defense Response of Nodule Formation in Bombyx mori Larvae in Association with Recognition by C-Type Lectins. J. Insect Physiol. 2021, 132, 104252. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Stanley, D.; Kim, Y. Nitric Oxide Mediates Insect Cellular Immunity via Phospholipase A2 Activation. J. Innate Immun. 2018, 10, 70–81. [Google Scholar] [CrossRef]
- Zhang, L.; Goodman, C.L.; Ringbauer, J.A.; Jiang, X.; Lv, W.; Xie, D.; Reall, T.; Stanley, D. Trade-Offs among Immune Mechanisms: Bacterial-Challenged Spodoptera frugiperda Larvae Reduce Nodulation Reactions during Behavioral Fever. Insects 2023, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Meng, Q.; Zhang, H.; Zhang, J.; Qin, Q. Types, morphology and cellular immune functions of hemocytes in larvae of Thitarodes xiaojinensis (Lepidoptera: Hepialidae). Acta Entomol. Sin. 2018, 60, 432–438. [Google Scholar] [CrossRef]
- Hu, Q.; Wei, X.; Li, Y.; Wang, J.; Liu, X. Identification and Characterization of a Gene Involved in the Encapsulation Response of Helicoverpa armigera Haemocytes. Insect Mol. Biol. 2017, 26, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Shi, M.; Chen, X.; Zhang, J. Effect of parasitism by Diadegma semiclausum (Hymenoptera: Ichneumonidae) and its venom on the phagocytic ability of hemocytes from Plutella xylostella (Lepidoptera: Plutellidae) larvae. Acta Entomol. Sin. 2011, 54, 989–996. [Google Scholar] [CrossRef]
- Liu, Y.; Mollaeian, K.; Shamim, M.H.; Ren, J. Effect of F-Actin and Microtubules on Cellular Mechanical Behavior Studied Using Atomic Force Microscope and an Image Recognition-Based Cytoskeleton Quantification Approach. Int. J. Mol. Sci. 2020, 21, 392. [Google Scholar] [CrossRef] [PubMed]
- Ali Mohammadie Kojour, M.; Han, Y.S.; Jo, Y.H. An Overview of Insect Innate Immunity. Entomol. Res. 2020, 50, 282–291. [Google Scholar] [CrossRef]
- Yan, R.; Liu, H.; Wan, Q. Research Progress on Morphological Classification and Immunization of Insect Hemocytes. J. Anhui Agric. Sci. 2010, 38, 9542–9544. [Google Scholar] [CrossRef]
- Li, E.; Lu, Q.; Zhang, D.; Kong, W.; An, C. Effects of infection of the entomopathogenic nematode Steinernema carpocapsae All on the innate immune response in Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Acta Entomol. Sin. 2022, 65, 1623–1635. [Google Scholar] [CrossRef]
- Ribeiro, C.; Brehélin, M. Insect Haemocytes: What Type of Cell Is That? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar] [CrossRef]
- Perveen, N.; Ahmad, M. Toxicity of Some Insecticides to the Haemocytes of Giant Honeybee, Apis dorsata F. under Laboratory Conditions. Saudi J. Biol. Sci. 2017, 24, 1016–1022. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L.; Yang, C.; Yun, X.; He, Y. The Competence of Hemocyte Immunity in the Armyworm Mythimna separata Larvae to Sublethal Hexaflumuron Exposure. Pesti. Biochem. Phys. 2016, 130, 31–38. [Google Scholar] [CrossRef]
- Çakıcı, Ö.; Uysal, M.; Demirözer, O.; Gösterit, A. Sublethal Effects of Thiamethoxam on Immune System Cells in the Workers of Bombus terrestris (Hymenoptera: Apidae). Environ. Sci. Pollut. R. 2023, 30, 87424–87432. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, X.; Liu, Q.; Zhang, X. Effect of azadirachtin on the hemocytes of Oxya chinensis. Chin. J. Appl. Entomol. 2019, 56, 546–556. [Google Scholar] [CrossRef]
- Cho, Y.; Cho, S. Hemocyte-Hemocyte Adhesion by Granulocytes Is Associated with Cellular Immunity in the Cricket, Gryllus bimaculatus. Sci. Rep. 2019, 9, 18066. [Google Scholar] [CrossRef] [PubMed]
- Yucel, M.S.; Kayis, T. Imidacloprid Induced Alterations in Oxidative Stress, Biochemical, Genotoxic, and Immunotoxic Biomarkers in Non-Mammalian Model Organism Galleria mellonella L. (Lepidoptera: Pyralidae). J. Environ. Sci. Heal. B 2019, 54, 27–34. [Google Scholar] [CrossRef]
- Ravaiano, S.V.; Barbosa, W.F.; Tomé, H.V.V.; Campos, L.A.D.O.; Martins, G.F. Acute and Oral Exposure to Imidacloprid Does Not Affect the Number of Circulating Hemocytes in the Stingless Bee Melipona quadrifasciata Post Immune Challenge. Pesti. Biochem. Phys. 2018, 152, 24–28. [Google Scholar] [CrossRef]
- Kwon, H.; Bang, K.; Cho, S. Characterization of the Hemocytes in Larvae of Protaetia brevitarsis seulensis: Involvement of Granulocyte-Mediated Phagocytosis. PLoS ONE 2014, 9, e103620. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kim, Y. PGE2 Mediates Hemocyte-Spreading Behavior by Activating Aquaporin via cAMP and Rearranging Actin Cytoskeleton via Ca2+. Dev. Comp. Immunol. 2021, 125, 104230. [Google Scholar] [CrossRef]
- Richards, E.H.; Edwards, J.P. Parasitism of Lacanobia oleracea (Lepidoptera) by the Ectoparasitic Wasp, Eulophus pennicornis, Disrupts the Cytoskeleton of Host Haemocytes and Suppresses Encapsulation in Vivo. Arch. Insect Biochem. Physiol. 2002, 49, 108–124. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, X.; Liu, J.; Zhao, H.; Wang, P.; Hu, S.; Chen, J.; Zhang, W.; Hu, J. Ostrinia furnacalis Integrin β1 May Be Involved in Polymerization of Actin to Modulate Spreading and Encapsulation of Plasmatocytes. Dev. Comp. Immunol. 2012, 37, 438–445. [Google Scholar] [CrossRef]







| Regression Equation | LD10 (μg/g) (95% CL) | LD20 (μg/g) (95% CL) | LD30 (μg/g) (95% CL) | LD50 (μg/g) (95% CL) | χ2 (df) | p |
|---|---|---|---|---|---|---|
| y = −0.832 + 1.906x | 0.581 (0.306~0.834) | 0.988 (0.648~1.330) | 1.449 (1.051~1.971) | 2.731 (2.004~4.408) | 5.963 (13) | 0.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Deng, X.; Wang, L.; Xie, W.; Zhang, H.; Li, Q.; Yang, Q.; Jiang, C. Chlorantraniliprole Enhances Cellular Immunity in Larvae of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Insects 2024, 15, 586. https://doi.org/10.3390/insects15080586
Liu Q, Deng X, Wang L, Xie W, Zhang H, Li Q, Yang Q, Jiang C. Chlorantraniliprole Enhances Cellular Immunity in Larvae of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Insects. 2024; 15(8):586. https://doi.org/10.3390/insects15080586
Chicago/Turabian StyleLiu, Qingyan, Xiaoyue Deng, Liuhong Wang, Wenqi Xie, Huilai Zhang, Qing Li, Qunfang Yang, and Chunxian Jiang. 2024. "Chlorantraniliprole Enhances Cellular Immunity in Larvae of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)" Insects 15, no. 8: 586. https://doi.org/10.3390/insects15080586





