Impacts of Combining Steinernema carpocapsae and Bracon hebetor Parasitism on Galleria mellonella Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Nematode Cultures
2.2. Sample Processing and Analysis
2.3. Biochemical Analyses
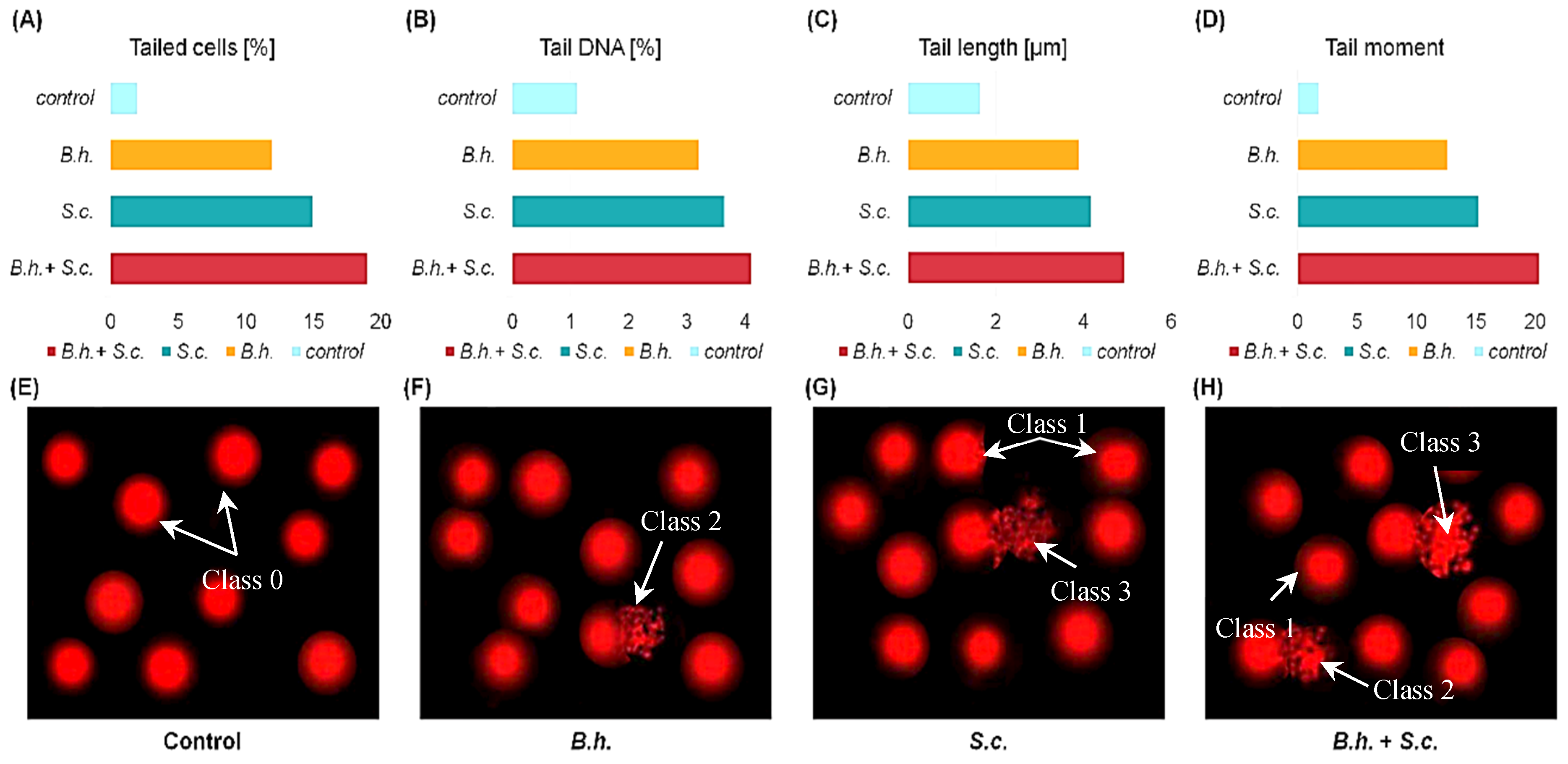
2.4. DNA Damage Assessment
2.5. Cell Viability Evaluation
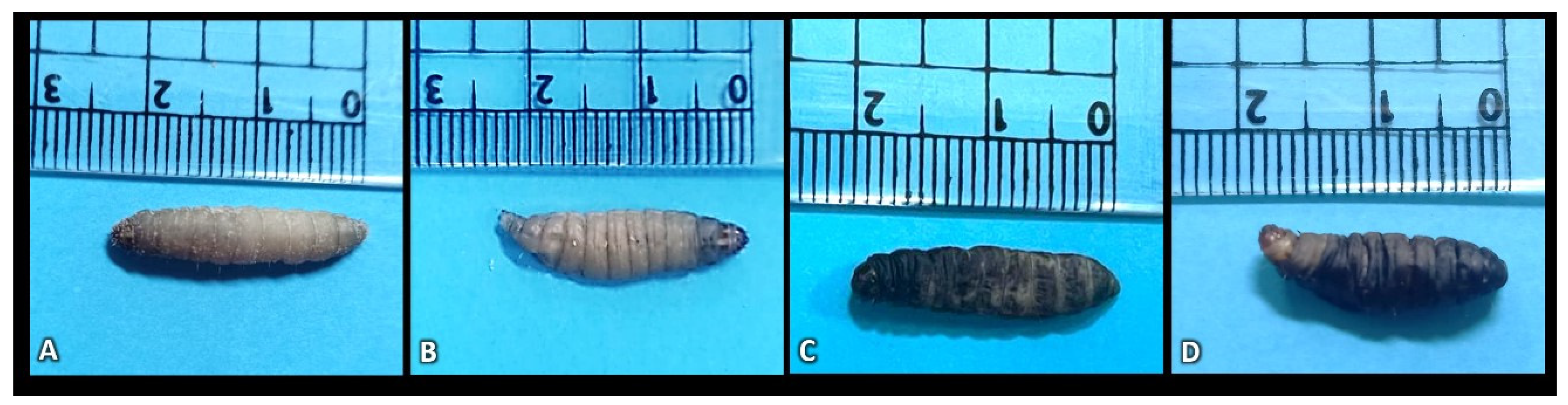
2.6. Morphological Analysis
2.7. Statistical Analysis
3. Results
3.1. Stress Biomarkers
3.2. Cytochrome P450 and Principal Component Analysis (PCA)
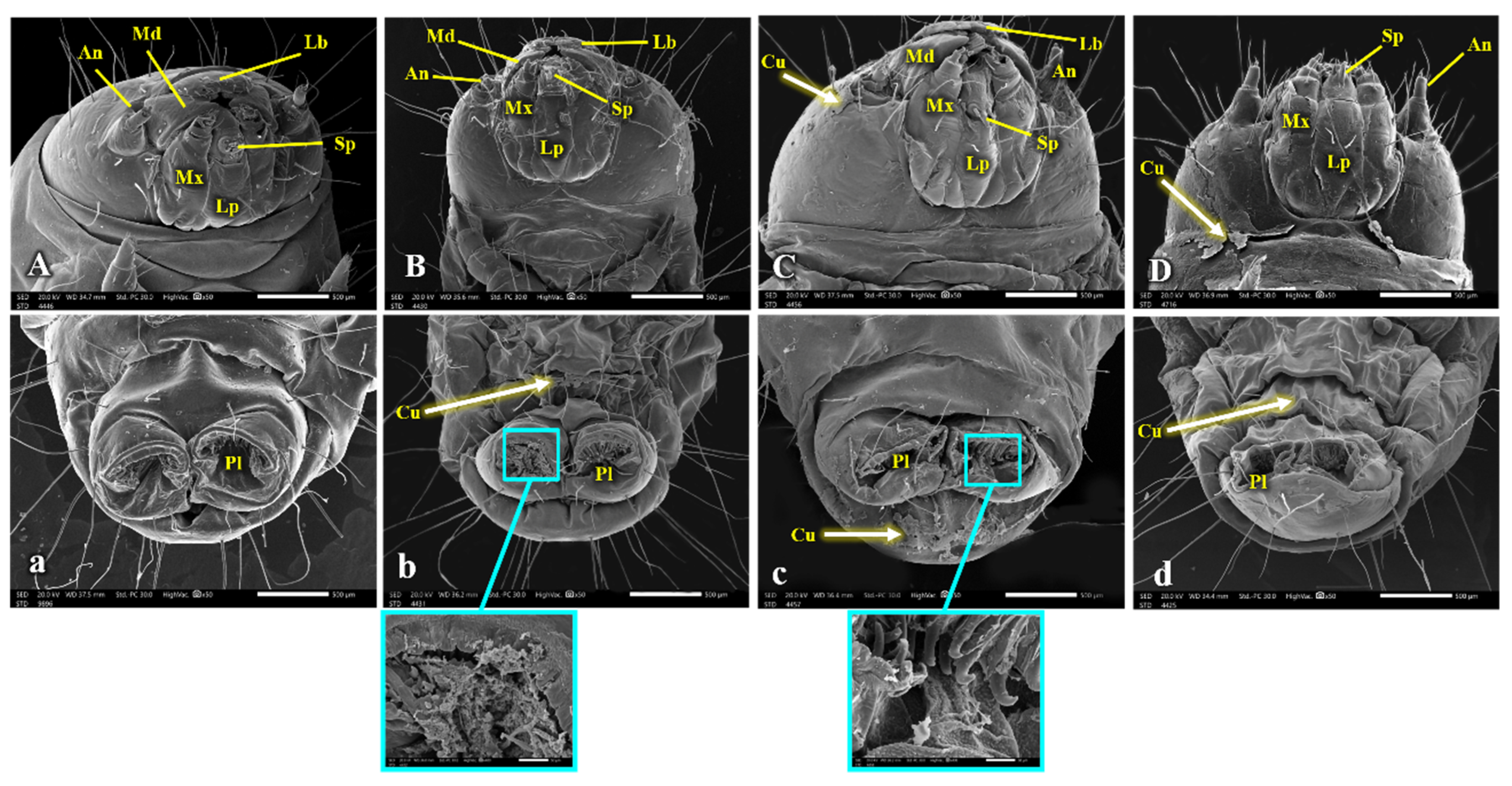
3.3. SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Hickin, M.; Nadel, H.; Schal, C.; Cohen, A.C. Optimization of a diet for the greater wax moth (Lepidoptera: Pyralidae) using full factorial and mixture design. J. Econ. Entomol. 2021, 114, 1091–1103. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The virtuous Galleria mellonella model for scientific experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria mellonella as a model host for microbiological and toxin research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef]
- Wright, C.L.; Kavanagh, O. Galleria mellonella as a novel in vivo model to screen natural product-derived modulators of innate immunity. Appl. Sci. 2022, 12, 6587. [Google Scholar] [CrossRef]
- Natarajan, V.; Anusha, N.; Govindarajan, S.; Priya, S. Chapter: 10 insect resistance to pesticides. In Entomology Chronicles: A Collaborative Insight Volume 1; Stella Publications: Kurukshetra, India, 2024. [Google Scholar]
- Zhang, Y.; Xu, H.; Tu, C.; Han, R.; Luo, J.; Xu, L. Enhanced capacity of a leaf beetle to combat dual stress from entomopathogens and herbicides mediated by associated microbiota. Integr. Zool. 2024, 27, 63. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological Control of Tephritid Fruit Flies in the Americas and Hawaii: A Review of the Use of Parasitoids and Predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The biology and control of the greater wax moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.L.; Ye, W.T.; Jiang, X.J.; Feng, P.; Zhu, Q.Y.; Sun, H.N.; Li, F.C.; Wei, J.; Li, B. Effect of tachinid parasitoid Exorista japonica on the larval development and pupation of the host silkworm Bombyx mori. Front. Physiol. Invertebr. Physiol. 2022, 13, 824203. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, L. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. NPJ Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef]
- Dieckhoff, C.; Tatman, K.M.; Hoelmer, K.A. Natural biological control of Halyomorpha halys by native egg parasitoids: A multi-year survey in northern Delaware. J. Pest Sci. 2017, 90, 1143–1158. [Google Scholar] [CrossRef]
- Resh, V.H.; Cardé, R.T. Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Cuny, M.A.; Poelman, E.H. Evolution of koinobiont parasitoid host regulation and consequences for indirect plant defence. Evol. Ecol. 2022, 36, 299–319. [Google Scholar] [CrossRef]
- Arbuckle, K. Evolutionary context of venom in animals. In Evolution of Venomous Animals and Their Toxins; Springer: Dordrecht, The Netherlands, 2017; Volume 24, pp. 3–31. [Google Scholar] [CrossRef]
- Battaglia, D.; Colella, T.; Laurino, S.; Grossi, G.; Salvia, R.; Riviello, L.; Falabella, P. The effect of Leptomastix dactylopii parasitism and venom injection on host Planococcus citri. Invertebr. Surviv. J. 2014, 11, 273–285. [Google Scholar]
- Laurino, S.; Grossi, G.; Pucci, P.; Flagiello, A.; Bufo, S.A.; Bianco, G.; Falabella, P. Identification of major Toxoneuron nigriceps venom proteins using an integrated transcriptomic/proteomic approach. Insect Biochem. Mol. Biol. 2016, 76, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.O. Biochemistry of insect venoms. Ann. Rev. Entomol. 1982, 27, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Salvia, R.; Franco, A.; Pezzi, M.; Cozzolino, F.; Chicca, M.; Ferracini, C. An integrated transcriptomic and proteomic approach to identify the main Torymus sinensis venom components. Sci. Rep. 2021, 11, 5032. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, F.; Hussain, F.; Arshad, M.; Abbas, S.K.; Shabbir, M.Z. Effects of envenomation on survival and development of the host-parasitoid system of Galleria mellonella (Lepidoptera: Pyralidae) and Bracon hebetor (Hymenoptera: Braconidae). Pak. J. Zool. 2023, 55, 1073. [Google Scholar] [CrossRef]
- Cusumano, A.; Duvic, B.; Jouan, V.; Ravallec, M.; Legeai, F.; Peri, E.; Volkoff, A.-N. First extensive characterization of the venom gland from an egg parasitoid: Structure, transcriptome and functional role. J. Insect Physiol. 2018, 107, 68–80. [Google Scholar] [CrossRef]
- Falabella, P. The mechanism utilized by Toxoneuron nigriceps in inhibiting the host immune system. Invertebr. Surviv. J. 2018, 15, 240–255. [Google Scholar] [CrossRef]
- Gu, Q.; Wu, Z.; Zhou, Y.; Wang, Z.; Shi, M.; Huang, J.; Chen, X. A teratocyte-specific serpin from the endoparasitoid wasp Cotesia vestalis inhibits the prophenoloxidase-activating system of its host Plutella xylostella. Insect Mol. Biol. 2022, 31, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, F.; Caccia, S.; Digilio, M.C. Host regulation and nutritional exploitation by parasitic wasps. Curr. Opin. Insect Sci. 2014, 6, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.J.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, P.; Falabella, P.; Sordetti, R.; Graziani, F.; Malva, C.; Pennacchio, F. Cardiochiles nigriceps polydnavirus: Molecular characterization and gene expression in parasitized Heliothis virescens larvae. Insect Biochem. Mol. Biol. 1999, 29, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Fathy, R.; Zohdy, N.; Abd-El-Samie, E.M.; Abou-Elfadl, H.; Moamen, S.; Younes, A. Effects of parasitism by the braconid wasp, Bracon hebetor (Hymenoptera: Braconidae), on the host hemolymph and phenoloxidase activation of the rice moth, Corcyra cephalonica larvae (Lepidoptera: Pyralidae). Egypt. J. Biol. Pest Control 2023, 33, 30. [Google Scholar] [CrossRef]
- Adams, B.J.; Fodor, A.; Koppenhöfer, H.S.; Stackebrandt, E.; Stock, S.P.; Klein, M.G. Reprint of “Biodiversity and systematics of nematode–bacterium entomopathogens” [Biol. Control 37 (2006) 32–49]. Biol. Control 2006, 38, 4–21. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Advances in use of entomopathogenic nematodes in integrated pest management. In Integrated Management of Insect Pests; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 649–678. [Google Scholar]
- Le Vieux, P.; Malan, A.P. The potential use of entomopathogenic nematodes to control Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). S. Afr. J. Ecol. Vitic. 2013, 34, 269–306. [Google Scholar] [CrossRef][Green Version]
- Lewis, E.E.; Ricci, M.; Gaugler, R. Host recognition behavior reflects host suitability for the entomopathogenic nematode, Steinernema carpocapsae. Parasitology 1996, 113, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.I.; Hiltpold, I.; Lewis, E.E. Ecology of invertebrate pathogens: Nematodes. In Ecology of Invertebrate Diseases; Hajek, A.E., Shapiro-Ilan, D.I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 415–440. [Google Scholar]
- Koppenhöfer, A.M.; Grewal, P.S. Compatibility and interactions with agrochemicals and other biocontrol agents. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.-U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 363–381. [Google Scholar]
- Atwa, A.A.; Hegazi, E.M.; Khafagi, W.E.; Abd El-Aziz, G.M. Interaction of the koinobiont parasitoid Microplitis rufiventris of the cotton leafworm, Spodoptera littoralis, with two entomopathogenic rhabditids, Heterorhabditis bacteriophora and Steinernema carpocapsae. J. Insect Sci. 2013, 13, 84. [Google Scholar] [CrossRef][Green Version]
- Aamer, N.A. Effect of parasitism by Bracon hebetor (Say) on Nematode yields of Steinernema carpocapsae in wax moth larvae, Galleria mellonella (L.). Egypt. J. Biol. Pest Control 2017, 27, 129–134. [Google Scholar]
- Farooqui, T.; Farooqui, A.A. Oxidative Stress in Vertebrates and Invertebrates: Molecular Aspects of Cell Signaling; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 377–384. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Özcan, O.; Erdal, H.; Çakırca, G.; Yönden, Z. Oksidatif stres ve hücre içi lipit, protein ve DNA yapıları üzerine etkileri. J. Clin. Exp. Investig. 2015, 6, 331–336. [Google Scholar] [CrossRef]
- Ahmad, S. Biochemical defence of pro-oxidant plant allelochemicals by herbivorous insects. Biochem. Syst. Ecol. 1992, 20, 269–296. [Google Scholar] [CrossRef]
- Altuntaş, H.; Duman, E.; Demirci, S.N.Ş.; Ergin, E. Toxicological and physiological effects of ethephon on the model organism, Galleria mellonella L. 1758 (Lepidoptera: Pyralidae). Turk. J. Entomol. 2016, 40, 413–423. [Google Scholar] [CrossRef][Green Version]
- Büyükgüzel, E.; Kalender, Y. Exposure to streptomycin alters oxidative and antioxidative response in larval midgut tissues of Galleria mellonella. Pest. Biochem. Physiol. 2009, 94, 112–118. [Google Scholar] [CrossRef]
- Güneş, E.; Büyükgüzel, E. Oxidative effects of boric acid on different developmental stages of Drosophila melanogaster Meigen, 1830 (Diptera: Drosophilidae). Turk. J. Entomol. 2017, 41, 3–15. [Google Scholar] [CrossRef]
- Kodrík, D.; Bednářová, A.; Zemanová, M.; Krishnan, N. Hormonal regulation of response to oxidative stress in insects—An update. Int. J. Mol. Sci. 2015, 16, 25788–25816. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Qian, X. Experimental study on the control effect of 24% chlorfenapyr suspending agent on the mulberry pest, Glyphodes pyloalis (Walker). China Seric 2012, 33, 48–49. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 236–316. [Google Scholar]
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochem. Pharmacol. 2011, 81, 703–712. [Google Scholar] [CrossRef]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Kheirallah, D.A.M.; El-Samad, L.M.; Toto, N.A. Relative expression of microRNAs, apoptosis, and ultrastructure anomalies induced by gold nanoparticles in Trachyderma hispida (Coleoptera: Tenebrionidae). PLoS ONE 2020, 15, e0241837. [Google Scholar] [CrossRef]
- Mishra, N.; Srivastava, R.; Agrawal, U.R.; Tewari, R.R. An insight into the genotoxicity assessment studies in dipterans. Mutat. Res./Rev. Mutat. Res. 2017, 773, 220–229. [Google Scholar] [CrossRef]
- Augustyniak, M.; Gladysz, M.; Dziewięcka, M. The Comet assay in insects—Status, prospects and benefits for science. Mutat. Res./Rev. Mutat. Res. 2016, 767, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Chadha, P.; Kaur, S.; Kaur, A.; Kaur, R.; Yadav, A.K.; Kaur, R. Schizophyllum commune induced genotoxic and cytotoxic effects in Spodoptera litura. Sci. Rep. 2018, 8, 4693. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Technology for Production of Natural Enemies; Project Directorate of Biological Control: Bangalore, India, 1994. [Google Scholar]
- Woodring, J.L. Steinernematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques; Southern Cooperative Series Bulletin; Arkansas Agricultural Experiment Station: Fayetteville, AR, USA, 1988; Volume 331, 30p. [Google Scholar]
- White, G. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Yu, S. Host plant induction of glutathione S-transferase in the fall armyworm. Pest. Biochem. Physiol. 1982, 18, 101–106. [Google Scholar] [CrossRef]
- Yu, S.J.; Hsu, E.L. Induction of detoxification enzymes in phytophagous insects: Role of insecticide synergists, larval age, and species. Arch. Insect Biochem. Physiol. 1993, 24, 21–32. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Brogdon, W.; Chan, A. Guidelines for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay/Methods in Anopheles Research; CDC Technical Report; CDC: Atlanta, GA, USA, 2010. Available online: https://stacks.cdc.gov/view/cdc/21777/cdc_21777_DS1.pdf (accessed on 24 July 2024).
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Dmochowska-Ślęzak, K.; Giejdasz, K.; Fliszkiewicz, M.; Żółtowska, K. Variations in antioxidant defense during the development of the solitary bee Osmia bicornis. Apidologie 2015, 46, 432–444. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Salem, H.H.; Taha, M. Dual application of entomopathogenic nematodes and fungi on immune and antioxidant enzymes of the greater wax moth, Galleria mellonella L. Egypt. J. Biol. Pest Control 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Karthi, S.; Vengateswari, G.; Karthikraja, R.; Perumal, P.; Shivakumar, M. Effect of entomopathogenic nematode of Heterorhabditis indica infection on immune and antioxidant system in lepidopteran pest Spodoptera litura (Lepidoptera: Noctuidae). J. Parasit. Dis. 2018, 42, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zóltowska, K.; Grochla, P.; Lopienska-Biernat, E. Activity of superoxide dismutase in Galleria mellonella larvae infected with entomopathogenic nematodes Steinernema affinis and S. feltiae. Ann. Parasitol. 2006, 52, 283–286. [Google Scholar]
- Zhu, J.; Ze, S.Z.; Stanley, D.W.; Yang, B. Parasitization by Scleroderma guani influences expression of superoxide dismutase genes in Tenebrio molitor. Arch. Insect Biochem. Physiol. 2014, 87, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Boudreault, S.; Michaud, D.; Cloutier, C. Proteomes of the aphid Macrosiphum euphorbiae in its resistance and susceptibility responses to differently compatible parasitoids. Insect Biochem. Mol. Biol. 2008, 38, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, P.; Zhang, Z.; Wu, G.-X.; Yang, B. Transcriptomic immune response of Tenebrio molitor pupae to parasitization by Scleroderma guani. PLoS ONE 2013, 8, e54411. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, T.; Ulabdin, Z.; Lee, K.Y. Induction of stress and immune associated genes in the Indian meal moth Plodia interpunctella against envenomation by the ectoparasitoid Bracon hebetor. Arch. Insect Biochem. Physiol. 2017, 96, e21405. [Google Scholar] [CrossRef]
- Çim, S.; Altuntaş, H. Anti-oxidative, genotoxic and mutagenic effects of idiobiont, endoparasitoid, Pimpla turionellae L. (Hymenoptera: Ichneumonidae) venom on its host Galleria mellonella L. (Lepidoptera: Pyralidae). Biol. Control 2021, 158, 104595. [Google Scholar] [CrossRef]
- Duman Erbas, E.; Altuntas, H. Effects of Juglone on the Antioxidant Metabolism in the Larval Hemolymph of the Greater Wax Moth Galleria mellonella L. (Lepidoptera: Pyralidae). Karadeniz Bilim. Derg. 2021, 11, 18–28. [Google Scholar] [CrossRef]
- Rewitz, K.F.; Rybczynski, R.; Warren, J.T.; Gilbert, L.I. Developmental expression of Manduca shade, the P450 mediating the final step in molting hormone synthesis. Mol. Cell. Endocrinol. 2006, 247, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.F.; Rybczynski, R.; Warren, J.T.; Gilbert, L.I. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem. Soc. Trans. 2006, 34, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.F.; Rybczynski, R.; Warren, J.T.; Gilbert, L.I. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2006, 36, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Mansouri, R.; Ali-Hassanzadeh, M.; Muro, A.; Nguewa, P.; Manzano-Román, R. The most prominent modulated annexins during parasitic infections. Acta Trop. 2023, 243, 106942. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, S.; de Melo Costa, V.R.; Santos, F.M.; de Sousa, C.D.F.; Moreira, T.P.; Gonçalves, M.R.; Nogueira, M.L. Annexin A1-FPR2/ALX signaling Axis regulates acute inflammation during chikungunya virus infection. Cells 2022, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Tavares, L.P.; Riccardi, C.; Teixeira, M.M.; Sousa, L.P. Exploiting the pro-resolving actions of glucocorticoid-induced proteins Annexin A1 and GILZ in infectious diseases. Biomed. Pharmacother. 2021, 133, 111033. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218. [Google Scholar] [PubMed]
- Michalková, V.; Valigurová, A.; Dindo, M.L.; Vaňhara, J. Larval Morphology and Anatomy of the Parasitoid Exorista larvarum (Diptera: Tachinidae), with an Emphasis on Cephalopharyngeal Skeleton and Digestive Tract. J. Parasitol. 2009, 95, 544–554. [Google Scholar] [CrossRef]
- Wojda, I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017, 24, 342–357. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aamer, N.A.; El-Moaty, Z.A.; Augustyniak, M.; El-Samad, L.M.; Hussein, H.S. Impacts of Combining Steinernema carpocapsae and Bracon hebetor Parasitism on Galleria mellonella Larvae. Insects 2024, 15, 588. https://doi.org/10.3390/insects15080588
Aamer NA, El-Moaty ZA, Augustyniak M, El-Samad LM, Hussein HS. Impacts of Combining Steinernema carpocapsae and Bracon hebetor Parasitism on Galleria mellonella Larvae. Insects. 2024; 15(8):588. https://doi.org/10.3390/insects15080588
Chicago/Turabian StyleAamer, Neama A., Zeinab A. El-Moaty, Maria Augustyniak, Lamia M. El-Samad, and Hanaa S. Hussein. 2024. "Impacts of Combining Steinernema carpocapsae and Bracon hebetor Parasitism on Galleria mellonella Larvae" Insects 15, no. 8: 588. https://doi.org/10.3390/insects15080588
APA StyleAamer, N. A., El-Moaty, Z. A., Augustyniak, M., El-Samad, L. M., & Hussein, H. S. (2024). Impacts of Combining Steinernema carpocapsae and Bracon hebetor Parasitism on Galleria mellonella Larvae. Insects, 15(8), 588. https://doi.org/10.3390/insects15080588






