Evaluation and Validation of Reference Genes for Gene Expression Analysis Using qRT-PCR in the Sugarcane Stem Borer Chilo sacchariphagus (Lepidoptera: Pyralidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Experimental Conditions
2.2. Selection of Reference Genes and Primer Design
2.3. Total RNA Isolation and First Strand cDNA Synthesis
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Evaluation of Reference Gene Expression Stability
2.6. Validation of Recommended Reference Genes
3. Results
3.1. Specificity and Efficiency of Primers
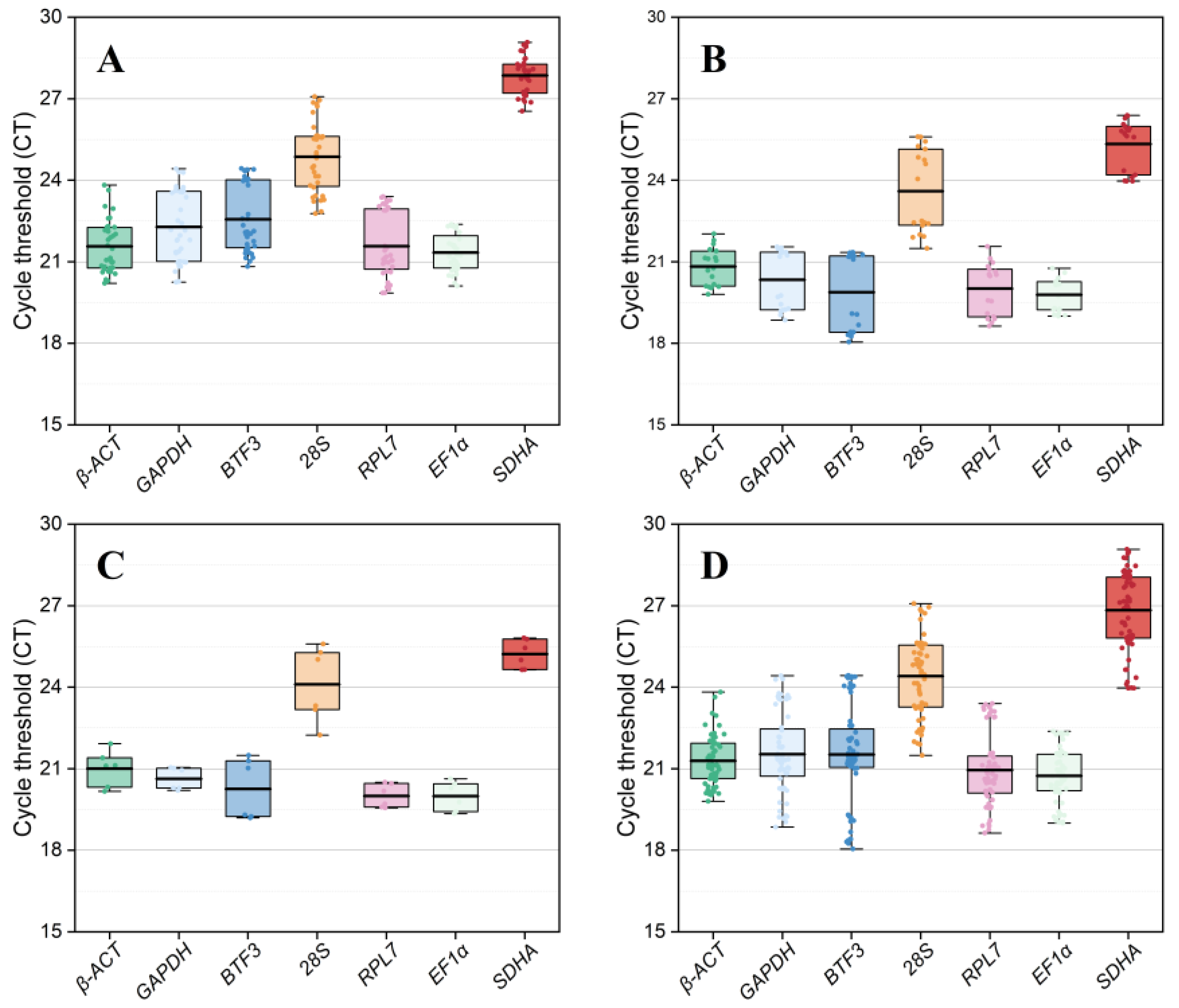
3.2. Expression Levels of Candidate Reference Genes
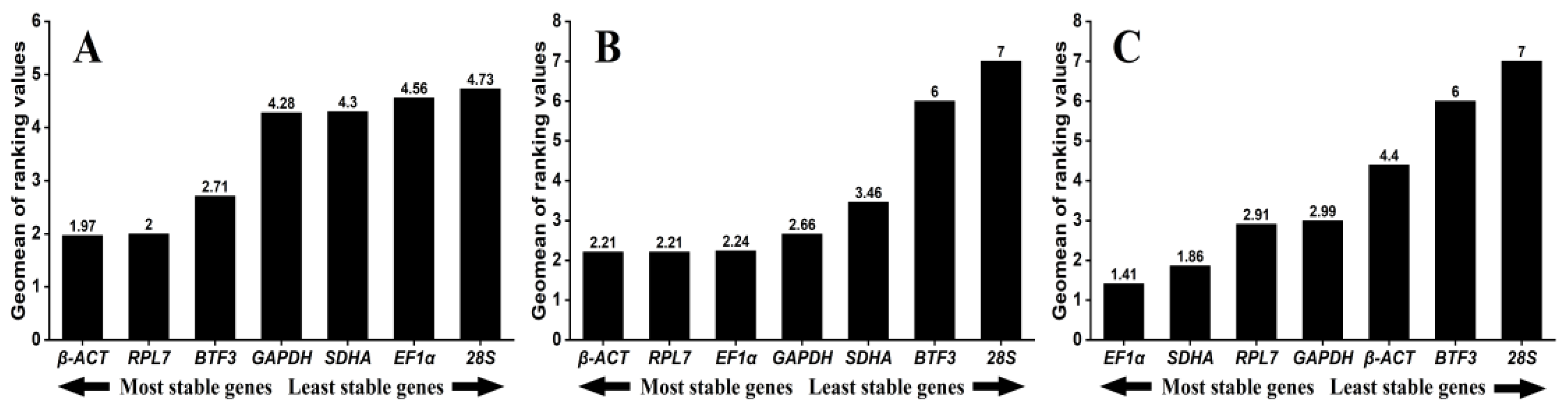
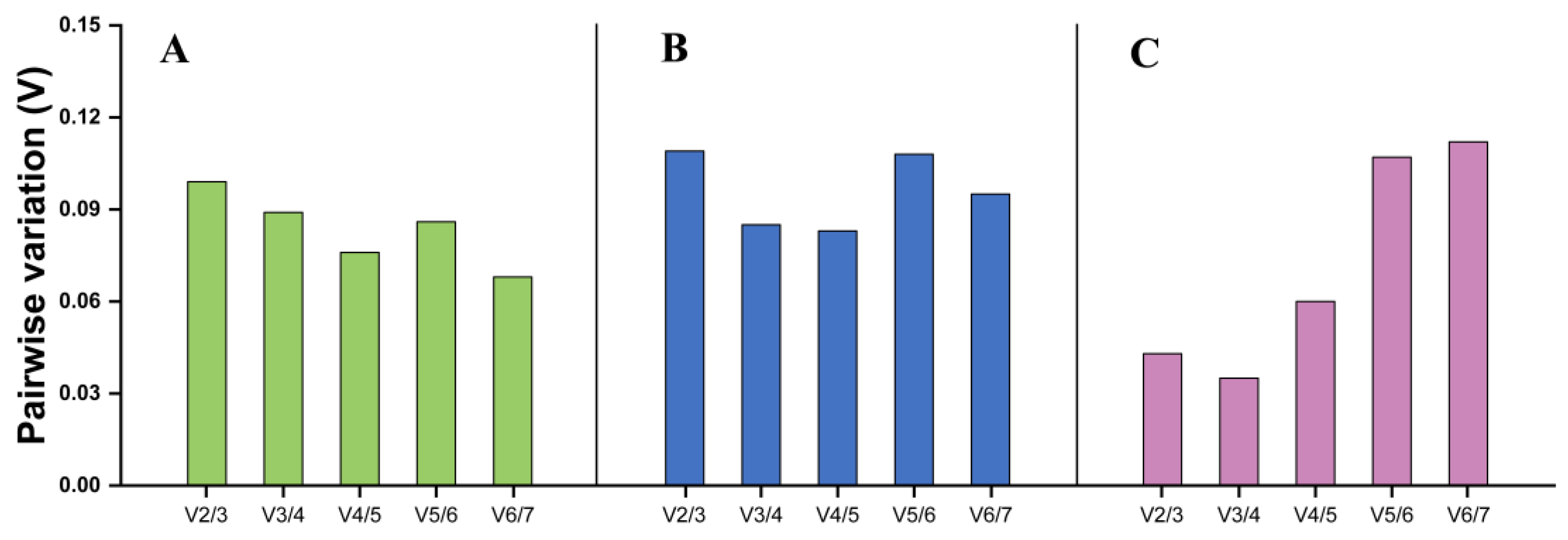
3.3. Expression Stability of Seven Reference Genes Across Different Tissues
3.4. Expression Stability of Seven Reference Genes Across Different Temperature Treatments
3.5. Expression Stability of Seven Reference Genes Across Different Sexes
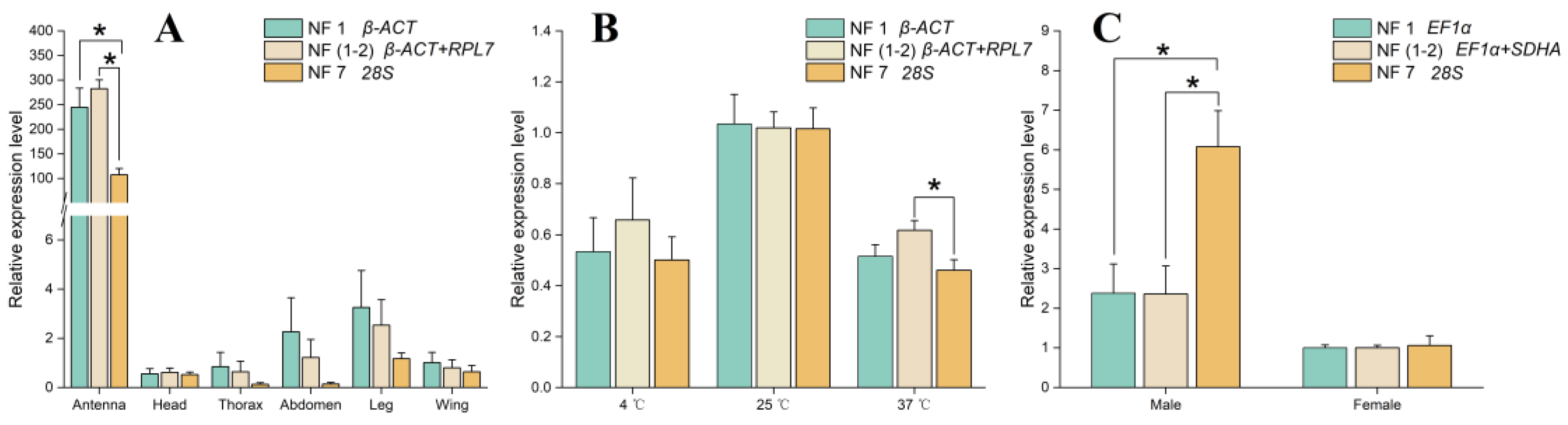
3.6. Validation of Recommended Reference Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nibouche, S.; Tibère, R.; Costet, L. The use of Erianthus arundinaceus as a trap crop for the stem borer Chilo sacchariphagus reduces yield losses in sugarcane: Preliminary results. Crop Prot. 2012, 42, 10–15. [Google Scholar] [CrossRef]
- Nibouche, S.; Tibère, R.; Costet, L. Erianthus arundinaceus as a trap crop for the sugarcane stem borer Chilo sacchariphagus: Field validation and disease risk assessment. Crop Prot. 2019, 124, 104877. [Google Scholar] [CrossRef]
- Li, A.; Chen, Z.; Liao, F.; Zhao, Y.; Qin, C.; Wang, M.; Pan, Y.; Wei, S.; Huang, D. Sugarcane borers: Species, distribution, damage and management options. J. Pest Sci. 2024, 1–31. [Google Scholar] [CrossRef]
- Way, M.J.; Conlong, D.E.; Rutherford, R.S. Biosecurity against invasive alien insect pests: A case study of Chilo sacchariphagus (Lepidoptera: Crambidae) in the southern African region. Proc. S. Afr. Sugar Technol. Assoc. 2011, 84, 84–91. [Google Scholar]
- Conlong, D.E.; Goebel, R. Biological control of Chilo sacchariphagus (Lepidoptera: Crambidae) in Moçambique: The firsts steps. Proc. S. Afr. Sugar Technol. Assoc. 2002, 76, 310–320. [Google Scholar]
- Sallam, M. A review of sugarcane stem borers and their natural enemies in Asia and Indian Ocean Islands: An Australian perspective. Ann. soc. entomol. Fr. 2006, 42, 263–283. [Google Scholar] [CrossRef]
- Goebel, F.; Way, M.J. Losses due to two sugarcane stem borers in Reunion and South Africa. Proc. S. Afr. Sugar Technol. Assoc. 2007, 26, 805–814. [Google Scholar]
- Wei, J.; Pan, X.; Shang, X.; Huang, C.; Ma, Y. Screening of Artificial Diet Formulations for Sugarcane Stalk Borer Chilo sacchariphagus Bojer. Sugar Tech 2023, 25, 727–734. [Google Scholar] [CrossRef]
- Goebel, F.; Roux, E.; Marquier, M.; Frandon, J.; Khanh, H.N.; Tabone, E. Biocontrol of Chilo sacchariphagus (Lepidoptera: Cramidae) a key pest of sugarcane: Lessons from the past and future prospects. Sugar Cane Int. 2010, 28, 128–132. [Google Scholar]
- Xiong, Y.; Wang, H.; Zhou, X.; Wang, L.; Wang, J.; Wang, J.; Wu, S. Comprehensive prevention and treatment measures for sugarcane borer: A review. J. South. Agric. 2021, 52, 2776–2785. [Google Scholar]
- Geetha, N.; Shekinah, E.D.; Rakkiyappan, P. Comparative Impact of Release Frequency of Trichogramma chilonis Ishii against Chilo sacchariphagus indicus (Kapur) in Sugarcane. J. Biol. Control 2010, 23, 343–351. [Google Scholar]
- Qin, Z.; Goebel, F.R.; Li, D.; Wei, J.; Song, X.; Luo, Y.; Liu, L.; Deng, Z. Occurrence of Telenomus dignus (Gahan) on the Sugarcane Borers, Scirpophaga intacta Snellen and Chilo sacchariphagus Bojer in Guangxi Province, China. Sugar Tech 2018, 20, 725–729. [Google Scholar] [CrossRef]
- Negm, A.A.; Hensley, S.D. Evaluation of certain biological control agents of the sugarcane borer in Louisiana. J. Econ. Entomol. 1969, 62, 1008–1013. [Google Scholar] [CrossRef]
- Wu, S.; Yang, N.; Yang, B.; Xiong, G.; Feng, C.; Wang, W.; Zhang, S. Research Progress on IPM of Sugarcane Borer. J. Trop. Biol. 2013, 4, 289–295. [Google Scholar]
- Way, M.J.; Goebel, F.; Conlong, D.E. Trapping Chilo sacchariphagus (Lepidoptera: Crambidae) in sugarcane using synthetic pheromones. Proc. S. Afr. Sugar Technol. Assoc. 2004, 78, 291–296. [Google Scholar]
- Nibouche, S.; Tibère, R. Mechanism of resistance to the spotted stalk borer, Chilo sacchariphagus, in the sugarcane cultivar R570. Entomol. Exp. Appl. 2010, 135, 308–314. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y. Advances in reference gene for real-time quantitative reverse transcription PCR (qRT-PCR) of insects research. Chin. J. Appl. Entomol. 2016, 53, 237–246. [Google Scholar]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Ginzinger, D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002, 30, 503–512. [Google Scholar] [CrossRef]
- Shen, C.; Tang, M.; Li, X.; Zhu, L.; Li, W.; Deng, P.; Zhai, Q.; Wu, G.; Yan, X. Evaluation of reference genes for quantitative expression analysis in Mylabris sibirica (Coleoptera, Meloidae). Front. Physiol. 2024, 15, 1345836. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.0031. [Google Scholar] [CrossRef]
- Valasek, M.A.; Repa, J.J. The power of real-time PCR. Adv. Physiol. Educ. 2005, 29, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Sun, L.; Zhang, Q.; Cao, C. Screening and evaluation of the stability of expression of reference genes in Lymantria dispar (Lepidoptera: Erebidae) using qRT-PCR. Gene 2020, 749, 144712. [Google Scholar] [CrossRef] [PubMed]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef]
- Suzuki, T.; Higgins, P.J.; Crawford, D.R. Control selection for RNA quantitation. BioTechniques 2000, 29, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, F.; Liang, P.; Chen, X.; Liu, Y.; Gao, X. Identification and Validation of Reference Genes for the Normalization of Gene Expression Data in qRT-PCR Analysis in Aphis gossypii (Hemiptera: Aphididae). J. Insect Sci. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Majerowicz, D.; Alves-Bezerra, M.; Logullo, R.; Fonseca-de-Souza, A.L.; Meyer-Fernandes, J.R.; Braz, G.R.; Gondim, K.C. Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae). Insect Mol. Biol. 2011, 20, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef]
- Meng, Q.; Shu, B.; Sun, S.; Wang, Y.; Yang, M.; Zhu, E.; Liu, A.; Gao, S.; Gou, Y.; Wang, Z. Selection of reference genes for quantitative real-time PCR normalization in the coffee white stem borer, Xylotrechus quadripes Chevrolat (Coleoptera: Cerambycidae). Bull. Entomol. Res. 2022, 112, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Glare, E.M.; Divjak, M.; Bailey, M.J.; Walters, E.H. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax 2002, 57, 765–770. [Google Scholar] [CrossRef]
- Zhu, L.J.; Altmann, S.W. mRNA and 18S-RNA coapplication-reverse transcription for quantitative gene expression analysis. Anal. Biochem. 2005, 345, 102–109. [Google Scholar] [CrossRef]
- Shen, C.; Peng, L.; Zhang, Y.; Zeng, H.; Yu, H.; Jin, L.; Li, G. Reference Genes for Expression Analyses by qRT-PCR in Phthorimaea operculella (Lepidoptera: Gelechiidae). Insects 2022, 13, 140. [Google Scholar] [CrossRef]
- Fu, H.; Huang, T.; Yin, C.; Xu, Z.; Li, C.; Liu, C.; Wu, T.; Song, F.; Feng, F.; Yang, F. Selection and Validation of Reference Genes for RT-qPCR Normalization in Bradysia odoriphaga (Diptera: Sciaridae) Under Insecticides Stress. Front. Physiol. 2022, 12, 818210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Lu, Y.; Zhu, X.; Wan, H.; Shakeel, M.; Zhan, S.; Jin, B.R.; Li, J. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS ONE 2014, 9, e86503. [Google Scholar] [CrossRef]
- Xu, J.; Lu, M.; Cui, Y.; Du, Y. Selection and Evaluation of Reference Genes for Expression Analysis Using qRT-PCR in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2017, 110, 683–691. [Google Scholar]
- Zhao, X.; Geng, Y.; Hu, T.; Zhao, Y.; Yang, S.; Hao, D. Evaluation of Optimal Reference Genes for qRT-PCR Analysis in Hyphantria cunea (Drury). Insects 2022, 13, 97. [Google Scholar] [CrossRef]
- Liu, X.; Gu, H.; Xu, Q.; Jiang, Z.; Li, B.; Wei, J. Determination of suitable reference genes for RT-qPCR normalisation in Bombyx mori (Lepidoptera: Bombycidae) infected by the parasitoid Exorista sorbillans (Diptera, Tachinidae). Bull. Entomol. Res. 2023, 113, 845–857. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Liu, Q.; Zhang, X.; Mei, X.; Zhang, T.; Ning, J. Validation and Evaluation of Reference Genes for Quantitative Real-Time PCR Analysis in Mythimna loreyi (Lepidoptera: Noctuidae). Insects 2024, 15, 185. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, X.; Xie, X.; Liu, C.; Zhang, H.; Yuan, J.; Lin, J.; Shu, B.; Zhang, J. Evaluation of the expression stability of potential reference genes for RT-qPCR in Spodoptera frugipreda larvae exposed to camptothecin. J. Asia-Pac. Entomol. 2024, 27, 102271. [Google Scholar] [CrossRef]
- Wu, S.; Luo, Y.; Zeng, Z.; Yu, Y.; Zhang, S.; Hu, Y.; Chen, L. Determination of internal controls for quantitative gene expression of Spodoptera litura under microbial pesticide stress. Sci. Rep. 2024, 14, 6143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Qiu, Z.; Hu, P.; Chen, X.; Yang, Z. Identification and Validation of Reference Genes for Expression Analysis Using RT-qPCR in Leptocybe invasa Fisher and La Salle (Hymenoptera: Eulophidae). Insects 2023, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 1986, 55, 987–1035. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, J.; Xia, Y.; Wu, Q.; Zhao, C.; Li, D. Selection and validation of reference genes for RT-qPCR-based analyses of Anastatus japonicus Ashmead (Hymenoptera: Helicopteridae). Front. Physiol. 2022, 13, 1046204. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Deng, W.; Zhu, F. Reference gene selection for quantitative gene expression analysis in black soldier fly (Hermetia illucens). PLoS ONE 2019, 14, e0221420. [Google Scholar] [CrossRef]
- Pan, H.; Yang, X.; Siegfried, B.D.; Zhou, X. A Comprehensive Selection of Reference Genes for RT-qPCR Analysis in a Predatory Lady Beetle, Hippodamia convergens (Coleoptera: Coccinellidae). PLoS ONE 2015, 10, e0125868. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Shi, D.; Wang, C.; Zhai, R.; Lyu, L.; He, Y.; Wang, D. Identification and Validation of Reference Genes for Expression Analysis Using qRT-PCR in Cimex hemipterus (Hemiptera: Cimicidae). Insects 2022, 13, 784. [Google Scholar] [CrossRef]
- Landry-Voyer, A.M.; Mir Hassani, Z.; Avino, M.; Bachand, F. Ribosomal Protein uS5 and Friends: Protein-Protein Interactions Involved in Ribosome Assembly and Beyond. Biomolecules 2023, 13, 853. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Guo, P.; Gao, Z.; Chen, D.; Zhang, T.; Ning, J. Evaluation of Reference Genes for Quantitative Real-Time PCR Analysis in the Bean Bug, Riptortus pedestris (Hemiptera: Alydidae). Insects 2023, 14, 960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Q.; Shen, C.; Wu, J.; Wu, Y.; Li, W.; Jin, L.; Li, G. Reference gene selection for transcriptional profiling by RT-qPCR in the 28-spotted larger potato ladybird. J. Asia-Pac. Entomol. 2022, 25, 101900. [Google Scholar] [CrossRef]
- Han, S.; Qin, Q.; Wang, D.; Zhou, Y.; He, Y. Selection and Evaluation of Reference Genes for qRT-PCR in Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2021, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y.; Xu, K.; Wang, Y.; Yang, W. Selection and Validation of Reference Genes for Gene Expression Analysis in Tuta absoluta Meyrick (Lepidoptera: Gelechiidae). Insects 2021, 12, 589. [Google Scholar] [CrossRef]
- Tang, J.; Liang, G.; Dong, S.; Shan, S.; Zhao, M.; Guo, X. Selection and Validation of Reference Genes for Quantitative Real-Time PCR Normalization in Athetis dissimilis (Lepidoptera: Noctuidae) Under Different Conditions. Front. Physiol. 2022, 13, 842195. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, G.; Zhao, Y.; Zhu, X.; Yu, X.; Yang, M.; Zhang, F. Selection and validation of optimal reference genes for RT-qPCR analyses in Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae). Front. Physiol. 2023, 14, 1277942. [Google Scholar] [CrossRef]
- Zhong, H.; Simons, J.W. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem. Biophys. Res. Commun. 1999, 259, 523–526. [Google Scholar] [CrossRef]
- Zhang, S.; An, S.; Li, Z.; Wu, F.; Yang, Q.; Liu, Y.; Cao, J.; Zhang, H.; Zhang, Q.; Liu, X. Identification and validation of reference genes for normalization of gene expression analysis using qRT-PCR in Helicoverpa armigera (Lepidoptera: Noctuidae). Gene 2015, 555, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Yan, W.; Coates, B.; Zhou, X.; Wang, C.; Gao, H.; Zhang, Y.; Zhu, X. Selection of Reference Genes for RT-qPCR Analysis of Wing Dimorphism in English Grain Aphid, Sitobion avenae (Hemiptera: Aphididae). J. Econ. Entomol. 2022, 115, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Z.; Li, Z.; Chen, Z.; Liu, F.; Kang, Z.; Xu, Y. Evaluation of reference genes for real-time quantitative PCR in the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Acta Entomol. Sin. 2023, 66, 663–675. [Google Scholar]

| Gene Symbol | Gene Name | Primer Sequence (5′-3′) | Length (bp) | Efficiency (%) | R2 |
|---|---|---|---|---|---|
| β-ACT | Beta-Actin | F: CAATCCTAAAGCCAACAGA | 180 | 95.06 | 0.9948 |
| R: GCGTAGCCCTCGTAGAT | |||||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | F: CATGCCACTACTGCTACCC | 131 | 101.47 | 0.9922 |
| R: GGAATGACTTTGCCTACGG | |||||
| BTF3 | Basic transcription factor 3 | F: AAGAAGGTTGTTCACGCTAC | 155 | 91.75 | 0.9994 |
| R: GCTTGTGCTTTCGGATTA | |||||
| 28S | 28S ribosomal | F: TCGCAGAATGTAGCAGGTT | 129 | 90.02 | 0.9990 |
| R: AGCATTGATTCGGGTCCTC | |||||
| RPL7 | Ribosomal protein L7 | F: TTTTGTTATCCGTATTCGTG | 131 | 95.61 | 0.9989 |
| R: ACAGTCGCCTTGTTGAGA | |||||
| EF1α | Elongation factor 1 alpha | F: GCTCTGCTCGCTTTCACC | 90 | 107.87 | 0.9994 |
| R: TCGGGATTCACTGTATGG | |||||
| SDHA | Succinate dehydrogenase complex subunit A | F: AGAGGTGATAACGCACTACAA | 86 | 105.35 | 0.9993 |
| R: CGTGAACAGAGGCACAAGA | |||||
| PBP1 | Pheromone binding protein 1 | F: CGCTGATTCGGACAC | 158 | 99.59 | 0.9995 |
| R: TCACCTCTACACTGGGAT |
| Conditions | Reference Gens | geNorm | Normfinder | BestKeeper | ΔCT | ||||
|---|---|---|---|---|---|---|---|---|---|
| Stability | Rank | Stability | Rank | Stability | Rank | Stability | Rank | ||
| Tissues | β-ACT | 0.363 | 5 | 0.198 | 1 | 0.82 | 3 | 0.41 | 1 |
| GAPDH | 0.254 | 3 | 0.325 | 4 | 1.14 | 7 | 0.44 | 4 | |
| BTF3 | 0.151 | 1 | 0.315 | 3 | 1.10 | 6 | 0.43 | 3 | |
| 28S | 0.318 | 4 | 0.335 | 5 | 1.06 | 5 | 0.46 | 5 | |
| RPL7 | 0.151 | 1 | 0.286 | 2 | 1.03 | 4 | 0.42 | 2 | |
| EF1α | 0.428 | 6 | 0.406 | 6 | 0.59 | 2 | 0.49 | 6 | |
| SDHA | 0.456 | 7 | 0.460 | 7 | 0.54 | 1 | 0.53 | 7 | |
| Temperature | β-ACT | 0.161 | 1 | 0.390 | 4 | 0.59 | 2 | 0.51 | 3 |
| GAPDH | 0.380 | 5 | 0.069 | 1 | 1.04 | 5 | 0.45 | 1 | |
| BTF3 | 0.477 | 6 | 0.513 | 6 | 1.36 | 6 | 0.62 | 6 | |
| 28S | 0.544 | 7 | 0.650 | 7 | 1.43 | 7 | 0.71 | 7 | |
| RPL7 | 0.278 | 3 | 0.179 | 2 | 0.86 | 4 | 0.45 | 1 | |
| EF1α | 0.161 | 1 | 0.437 | 5 | 0.57 | 1 | 0.54 | 4 | |
| SDHA | 0.326 | 4 | 0.386 | 3 | 0.83 | 3 | 0.54 | 4 | |
| Sex | β-ACT | 0.199 | 5 | 0.274 | 3 | 0.51 | 5 | 0.45 | 5 |
| GAPDH | 0.129 | 4 | 0.326 | 5 | 0.37 | 1 | 0.42 | 4 | |
| BTF3 | 0.352 | 6 | 0.489 | 6 | 1.01 | 6 | 0.62 | 6 | |
| 28S | 0.483 | 7 | 0.777 | 7 | 1.19 | 7 | 0.81 | 7 | |
| RPL7 | 0.109 | 3 | 0.287 | 4 | 0.38 | 2 | 0.39 | 3 | |
| EF1α | 0.063 | 1 | 0.100 | 1 | 0.47 | 4 | 0.34 | 1 | |
| SDHA | 0.063 | 1 | 0.145 | 2 | 0.46 | 3 | 0.35 | 2 | |
| Conditions | Recommended Reference Genes | |
|---|---|---|
| Tissues | β-ACT | RPL7 |
| Temperature | β-ACT | RPL7 |
| Sex | EF1α | SDHA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shang, X.; Wei, J.; Tian, X.; Liu, Y.; Zhang, G. Evaluation and Validation of Reference Genes for Gene Expression Analysis Using qRT-PCR in the Sugarcane Stem Borer Chilo sacchariphagus (Lepidoptera: Pyralidae). Insects 2024, 15, 594. https://doi.org/10.3390/insects15080594
Wang Z, Shang X, Wei J, Tian X, Liu Y, Zhang G. Evaluation and Validation of Reference Genes for Gene Expression Analysis Using qRT-PCR in the Sugarcane Stem Borer Chilo sacchariphagus (Lepidoptera: Pyralidae). Insects. 2024; 15(8):594. https://doi.org/10.3390/insects15080594
Chicago/Turabian StyleWang, Zhixiong, Xiankun Shang, Jili Wei, Xiaoli Tian, Yi Liu, and Guohui Zhang. 2024. "Evaluation and Validation of Reference Genes for Gene Expression Analysis Using qRT-PCR in the Sugarcane Stem Borer Chilo sacchariphagus (Lepidoptera: Pyralidae)" Insects 15, no. 8: 594. https://doi.org/10.3390/insects15080594
APA StyleWang, Z., Shang, X., Wei, J., Tian, X., Liu, Y., & Zhang, G. (2024). Evaluation and Validation of Reference Genes for Gene Expression Analysis Using qRT-PCR in the Sugarcane Stem Borer Chilo sacchariphagus (Lepidoptera: Pyralidae). Insects, 15(8), 594. https://doi.org/10.3390/insects15080594





