Reproductive Biology and Rearing Improvements of Apanteles opuntiarum, Potential Biocontrol Agent of the Argentine Cactus Moth, Cactoblastis cactorum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Insect Colonies for Morphology, Polyembryony, and Superparasitism Studies
2.3. Morphology of Preimaginal Stages
2.4. Polyembryony and Superparasitism
2.5. Insect Colonies for Larval Exposure and Host Density Studies
2.6. Host Exposure and Host Density Studies
2.7. Data Analysis
2.7.1. Morphology, Polyembryony, and Superparasitism
2.7.2. Preference Testing
2.8. Larval Exposure and Host Density Studies
3. Results
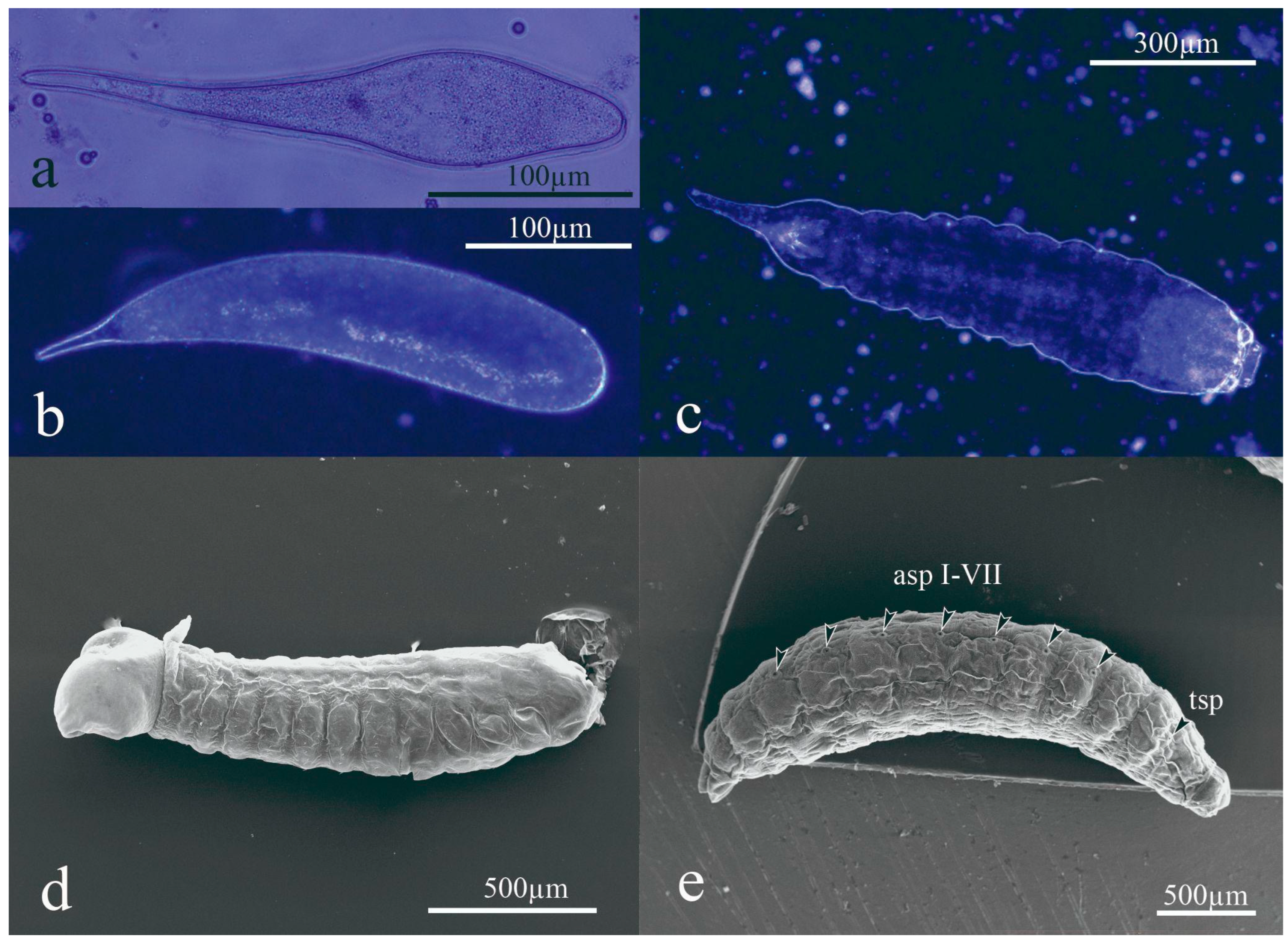
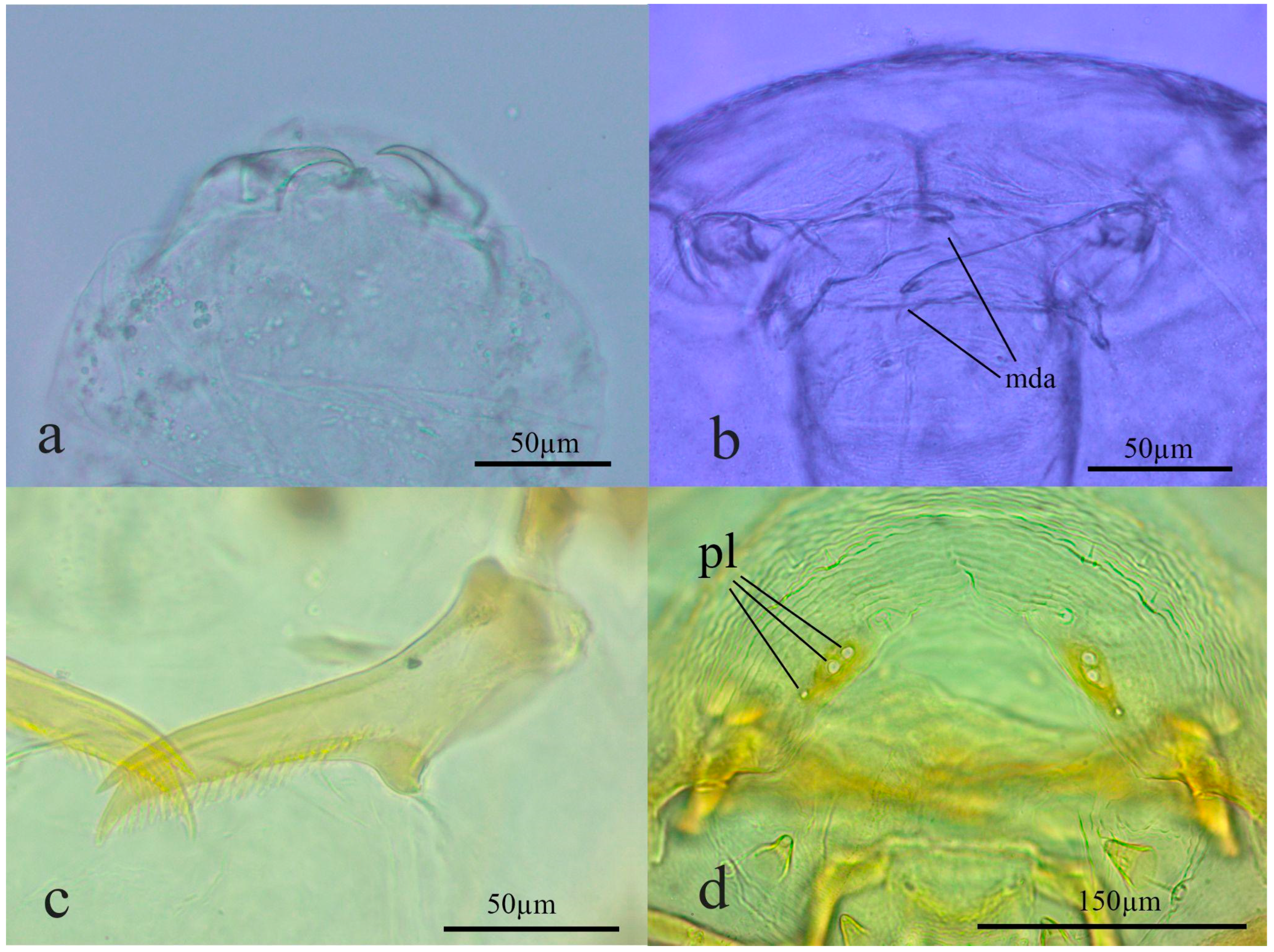
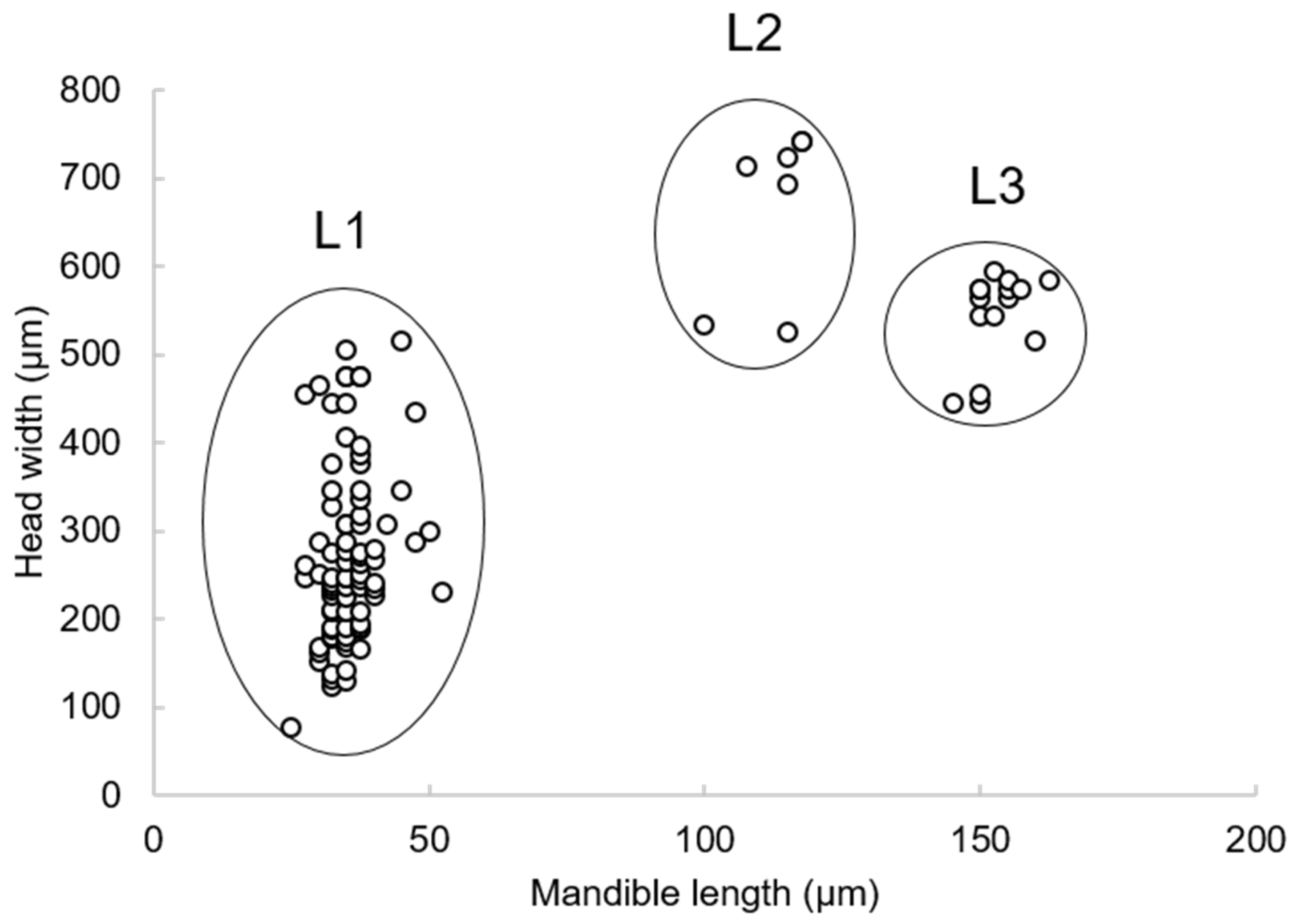
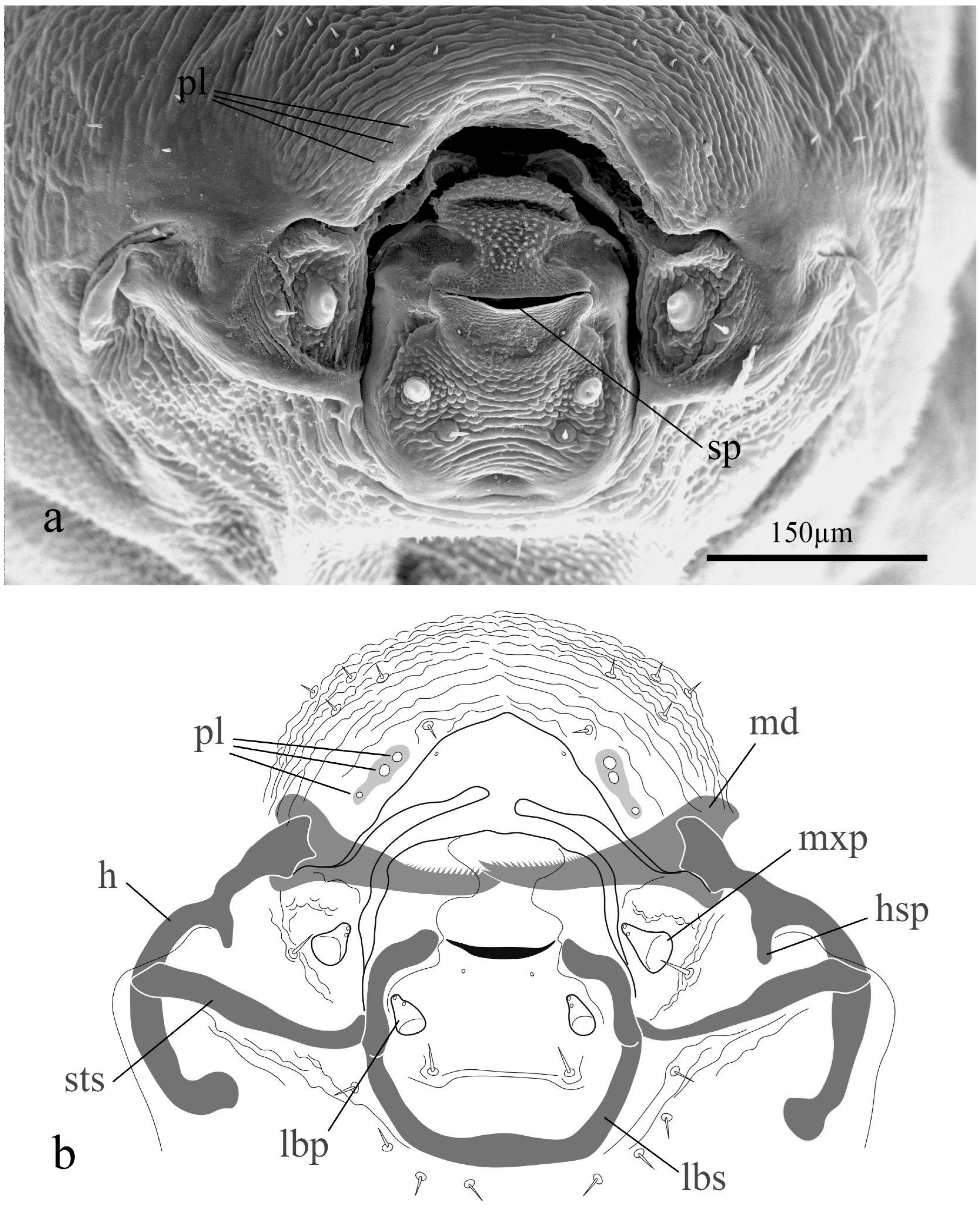
3.1. Preimaginal Stages
3.2. Polyembryony and Superparasitism
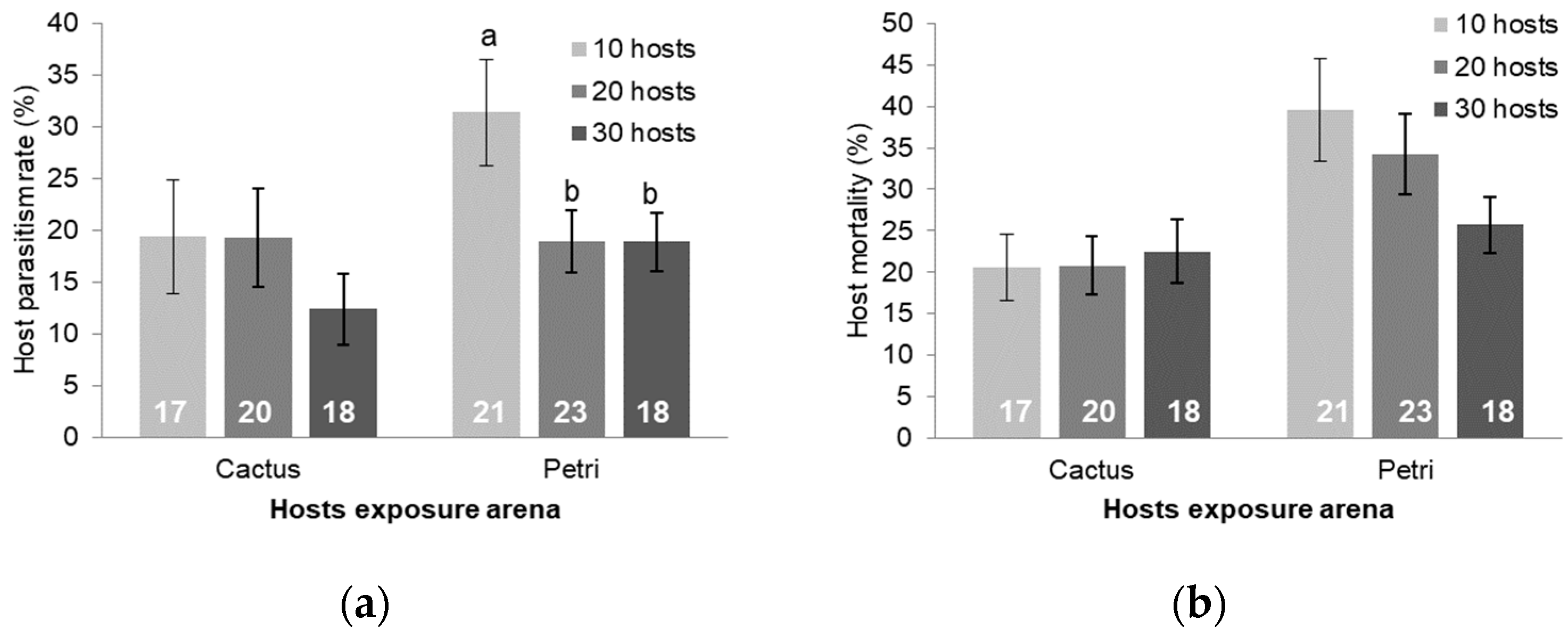
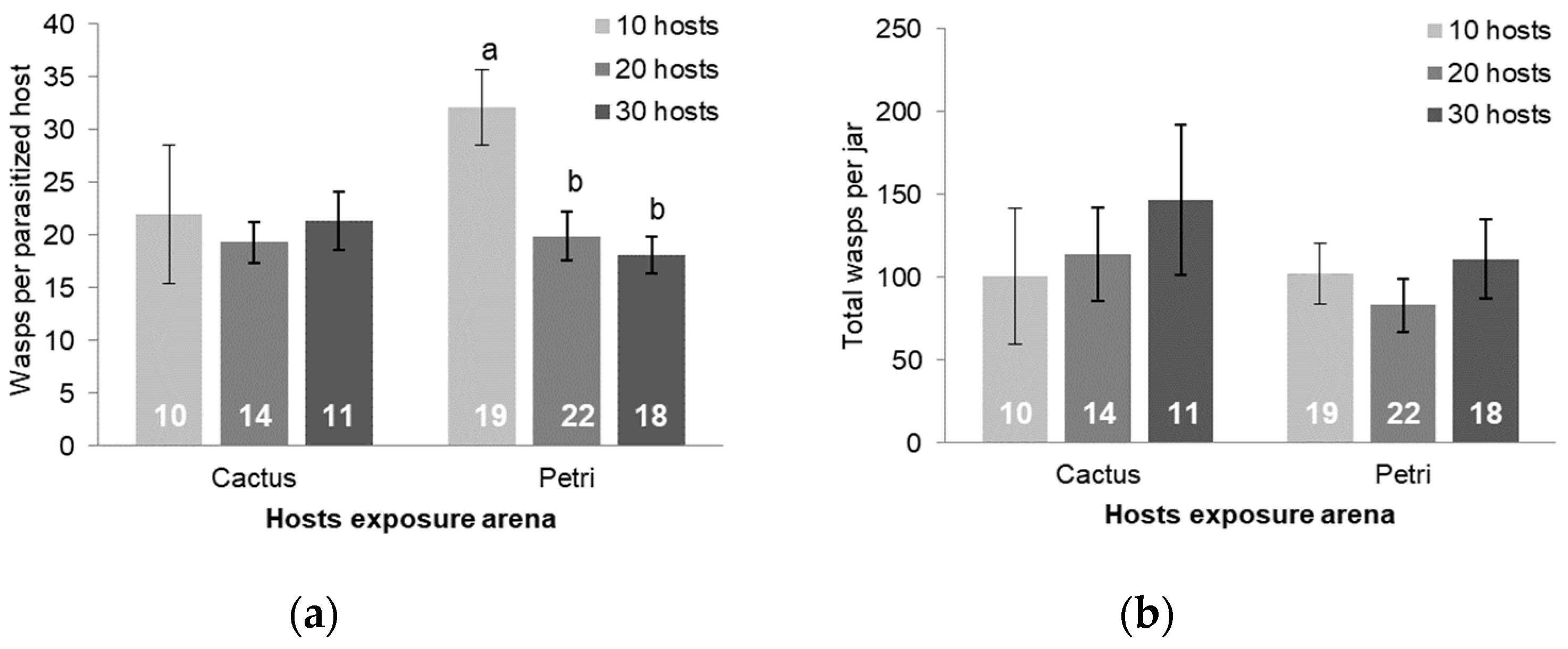
3.3. Larval Exposure and Host Density Studies
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodd, A.P. The Biological Campaign against Prickly Pear. Ann. Entomol. Soc. Am. 1940, 34, 273–274. [Google Scholar] [CrossRef]
- Pettey, F.W. The biological control of prickly pear in South Africa. Sci. Bull. Dep. Agric. Union S. Afr. 1948, 271, 1–163. [Google Scholar]
- Hoffmann, J.H.; Moran, V.C.; Zeller, D.A. Evaluation of Cactoblastis cactorum (Lepidoptera: Phycitidae) as a Biological Control Agent of Opuntia stricta (Cactaceae) in the Kruger National Park, South Africa. Biol. Control 1998, 12, 20–24. [Google Scholar] [CrossRef]
- Hoffmann, J.H.; Moran, V.C.; Zimmermann, H.G.; Impson, F.A.C. Biocontrol of a prickly pear cactus in South Africa: Reinterpreting the analogous, renowned case in Australia. J. Appl. Ecol. 2020, 57, 2475–2484. [Google Scholar] [CrossRef]
- Zimmermann, H.G.; Bloem, S.; Klein, H. Biology, History, Threat, Surveillance and Control of the Cactus Moth, Cactoblastis cactorum; FAO: Vienna, Austria, 2004. [Google Scholar]
- NAPPO. Detection and Eradication of a Cactus Moth (Cactoblastis cactorum Berg) Outbreak in Isla Contoy, Municipality of Isla Mujeres, Quintana Roo, Mexico. North American Plant Protection Organization. 2009. Available online: https://www.pestalerts.org/nappo/official-pest-reports/266/ (accessed on 9 January 2024).
- Carpenter, J.E.; Bloem, S.; Bloem, K.A. Inherited Sterility in Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2001, 84, 537–542. [Google Scholar] [CrossRef]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective overflooding ratios and release-recapture field studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Hight, S.D.; Bellom, A. Eradication and containment of Cactoblastis cactorum in Mexico and the United States. In Proceedings of the XXIII International Congress of Entomology, Durban, South Africa, 6–12 July 2008. [Google Scholar]
- Martínez, J.J.; Berta, C.; Varone, L.; Logarzo, G.A.; Zamudio, P.; Zaldívar-Riverón, A.; Aguilar-Velasco, R.G. DNA barcodes and morphological identification of the southern South American species of Apanteles (Hymenoptera: Braconidae) parasitoids of cactus feeding moths (Lepidoptera: Pyralidae: Phycitinae), with a description of a new species. Invertebr. Syst. 2012, 26, 435–444. [Google Scholar] [CrossRef]
- Mengoni Goñalons, C.; Varone, L.; Logarzo, G.A.; Guala, M.E.; Rodriguero, M.; Hight, S.D.; Carpenter, J.E. Geographical range and laboratory studies on Apanteles opuntiarum (Hymenoptera: Braconidae) in Argentina, a candidate for biological control of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2014, 97, 1458–1468. [Google Scholar] [CrossRef]
- Awad, J.; Hodges, A.; Hight, S.; Srivastava, M.; Howe, A.; Rohrig, E. Laboratory rearing and sex ratio of Apanteles opuntiarum (Hymenoptera: Braconidae), a potential biocontrol agent of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2019, 102, 216–221. [Google Scholar] [CrossRef]
- Varone, L.; Mengoni Goñalons, C.; Faltlhauser, A.C.; Guala, M.E.; Wolaver, D.; Srivastava, M.; Hight, S.D. Effect of rearing Cactoblastis cactorum on an artificial diet on the behaviour of Apanteles opuntiarum. J. Appl. Entomol. 2020, 144, 278–286. [Google Scholar] [CrossRef]
- Varone, L.; Logarzo, G.A.; Martínez, J.J.; Navarro, F.; Carpenter, J.E.; Hight, S.D. Field host range of Apanteles opuntiarum (Hymenoptera: Braconidae) in Argentina, a potential biocontrol agent of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2015, 98, 803–806. [Google Scholar] [CrossRef]
- Varone, L.; Aguirre, M.B.; Lobos, E.; Ruiz Pérez, D.; Hight, S.D.; Palottini, F.; Guala, M.; Logarzo, G.A. Causes of mortality at different stages of Cactoblastis cactorum in the native range. BioControl 2019, 64, 249–261. [Google Scholar] [CrossRef]
- Pérez-De la O, N.B.; Espinosa-Zaragoza, S.; López-Martínez, V.D.; Hight, S.; Varone, L. Ecological Niche Modeling to calculate ideal sites to introduce a natural enemy: The case of Apanteles opuntiarum (Hymenoptera: Braconidae) to control Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Insects 2020, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, D.A.; Greenberg, S.M. Facilities and automation for the mass production of arthropod predators and parasitoids. Biocon. News Inform. 1994, 15, 45–55. [Google Scholar]
- Leppla, N.C. Concepts and methods of quality assurance for mass-reared parasitoids and predators. In Mass Production of Beneficial Organisms, 2nd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 261–290. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Hongkham, D. Clutch size in an obligately siblicidal parasitoid wasp. Anim. Behav. 1996, 51, 841–852. [Google Scholar] [CrossRef]
- Pexton, J.J.; Mayhew, P.J. Clutch size adjustment, information use and the evolution of gregarious development in parasitoid wasps. Behav. Ecol. Sociobiol. 2005, 58, 99–110. Available online: https://www.jstor.org/stable/i25063576 (accessed on 22 December 2023). [CrossRef]
- Boivin, G.; Brodeur, J. Intra- and Interspecific Interactions among Parasitoids: Mechanisms, Outcomes and Biological Control. In Trophic and Guild in Biological Interactions Control; Progress in Biological, Control; Brodeur, J., Boivin, G., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 3. [Google Scholar] [CrossRef]
- Fischer, M.T.; Pardo, X.; Asís, J.D. Description of adults, preimaginal phases, and the venom apparatus of a new species of Aspilota Förster from Spain (Hymenoptera: Braconidae) Zool. Studies 2008, 47, 247–257. [Google Scholar]
- Čapek, M. A new classification of the Braconidae (Hymenoptera) based on the cephalic structures of the final instar larva and biological evidence. Can. Entomol. 1970, 102, 846–875. [Google Scholar] [CrossRef]
- Mason, W.R.M. The polyphyletic nature of Apanteles Foerster (Hymenoptera: Braconidae): A phylogeny and reclassification of Microgastrinae. Mem. Ent. Soc. Can. 1981, 115, 1–147. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Version 2008. Grupo InfoStat, FCA. Universidad Nacional de Córdoba: Cordoba, Argentina, 2008.
- Thurstone, L.L. A law of comparative judgment. Psyc. Rev. 1927, 34, 273–286. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Rubin, D.B. Bayesian Data Analysis, Texts in Statistical Science; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Geweke, J. Evaluating the Accuracy of Sampling-Based Approaches to the Calculation of Posterior Moments MN: Federal Reserve Bank of Minneapolis; Research Department: Minneapolis, MN, USA, 1991. [Google Scholar]
- Patil, A.; Huard, D.; Fonnesbeck, C.J. PyMC: Bayesian stochastic modelling in Python. J. Stat. Soft. 2010, 35, 181. [Google Scholar] [CrossRef]
- Narayanan, E.S.; Subba Rao, B.R.; Gangrade, G.A. The biology and rate of reproduction and the morphology of the immature stages of Apanteles angaleti Muesebeck (Hymenoptera: Braconidae). Beiträge Entomol. Contrib. Entomol. 1956, 6, 296–320. [Google Scholar] [CrossRef]
- Arthur, A.P.; Mason, P.G. Life history and immature stages of the parasitoid Microplitis mediator (Hymenoptera: Braconidae), reared on the bertha armyworm Mamestra configurata (Lepidoptera: Noctuidae). Can. Entomol. 1986, 118, 487–491. [Google Scholar] [CrossRef]
- Lewis, W.J. Life history and anatomy of Microplitis croceipes (Hymenoptera: Braconidae), a parasite of Heliothis spp. (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1970, 63, 67–70. [Google Scholar] [CrossRef]
- Peter, C.; David, B.V. Biology of Apanteles machaeralis Wilkinson (Hymenoptera: Braconidae) a parasite of Diaphania indica (Saunders) (Lepidoptera: Pyralidae). Proc. Anim. Sci. 1990, 99, 353–362. [Google Scholar] [CrossRef]
- Yu, R.; Shi, M.; Huang, F.; Chen, X. Immature Development of Cotesia vestalis (Hymenoptera: Braconidae), an Endoparasitoid of Plutella xylostella (Lepidoptera: Plutellidae). Ann. Entomol. Soc. Am. 2008, 101, 189–196. [Google Scholar] [CrossRef]
- Pinheiro, D.O.; Rossi, G.D.; Cônsoli, F.L. External morphology of Cotesia flavipes (Hymenoptera: Braconidae) during larval development. Zoologia 2010, 27, 986–992. [Google Scholar] [CrossRef]
- Thorpe, W.H. Experiments upon respiration in the larvae of certain parasitic Hymenoptera. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1932, 109, 450–471. [Google Scholar] [CrossRef]
- Edson, K.M.; Vinson, S.B. The function of the anal vesicle in respiration and excretion in the braconid wasp, Microplitis croceipes. J. Insect Physiol. 1976, 22, 1037–1043. [Google Scholar] [CrossRef]
- Edson, K.M.; Vinson, S.B. Nutrient absorption by the anal vesicle of the braconid wasp, Microplitis croceipes. J. Insect Physiol. 1977, 23, 5–8. [Google Scholar] [CrossRef]
- Kaeslin, M.; Wyler, T.; Grossniklaus-Bürgin, C.; Lanzrein, B. Development of the anal vesicle, salivary glands and gut in the egg-larval parasitoid Chelonus inanitus: Tools to take up nutrients and to manipulate the host? J. Insect Physiol. 2006, 52, 269–281. [Google Scholar] [CrossRef]
- Strand, M.R. Polyembryony. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 821–825. [Google Scholar]
- Bakker, K.; van Alphen, J.J.M.; Batenburg, F.H.D.v.; Hoeven, N.v.d.; Nell, H.W.; van Strien-van Liempt, W.T.F.H.; Turlings, T.C.J. The function of host discrimination and superparasitization in parasitoids. Oecologia 1985, 67, 572–576. [Google Scholar] [CrossRef]
- Godfray, C.J.H. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar] [CrossRef]
- Hanan, A.; Shakeel, M.; He, X.Z.; Razzaq, A.; Wang, Q. Superparasitism and host discrimination behavior of Eretmocerus warrae Naumann & Schmidt (Hymenoptera: Aphelinidae). Turk. J. Agric. For. 2016, 40, 1. [Google Scholar] [CrossRef]
- Gauthier, N.; Monge, J.P.; Huignard, J. Superparasitism and host discrimination in the solitary ectoparasitoid Dinarmus basalis. Entomol. Exp. Appl. 1996, 79, 91–99. [Google Scholar] [CrossRef]
- Rezaei, M.; Karimzadeh, J.; Shakarami, J. Mass rearing optimization of Cotesia vestalis (Hymenoptera: Braconidae) based on the host and parasitoid densities. Neotrop. Entomol. 2020, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, M.N.; Phillips, T.W. Mass rearing of Harbobracon hebetor Say (Hymenoptera: Braconidae) on larvae of the Indian meal moth, Plodia interpunctella (Lepidoptera: Pyralidae): Effects of host density, parasitoid density, and rearing containers. J. Stored Prod. Res. 2010, 46, 214–220. [Google Scholar] [CrossRef]
- van Lenteren, J.C. Host discrimination in parasitoids. In Semiochemicals: Their Role in Pest Control; Nordlund, D.A., Jones, R.L., Lewis, W.J., Eds.; Wiley and Sons: Hoboken, NJ, USA, 1981; pp. 153–179. [Google Scholar]
- van Alphen, J.M.M.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Ann. Rev. Entomol. 1990, 35, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Speirs, D.C.; Hubbard, S.F. Parasitoid diets: Does superparasitism pay? Trends Ecol. Evol. 1991, 6, 22–25. [Google Scholar] [CrossRef]
- Hubbard, S.F.; Harvey, I.F.; Fletcher, J.P. Avoidance of superparasitism: A matter of learning? Anim. Behav. 1999, 57, 1193–1197. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Aluja, M. Functional Response and Superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a Parasitoid of Fruit Flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2000, i93, 47–54. [Google Scholar] [CrossRef]
- Gross, P. Insect behavioral and morphological defenses against parasitoids. Annu. Rev. Entomol. 1993, 38, 251–273. [Google Scholar] [CrossRef]
- Allen, G.R. Influence of host behavior and host size on the success of oviposition of Cotesia urabae and Dolichogenidea eucalypti (Hymenoptera: Braconidae). J. Insect Behav. 1990, 3, 733–749. [Google Scholar] [CrossRef]
- Vinson, S.B. The general host selection behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, H.; Dorn, S. Selection on olfactory response to semiochemicals from a plant-host complex in a parasitic wasp. Heredity 2003, 91, 430–435. [Google Scholar] [CrossRef]

| Parameter of Time Since Parasitization | Median | 2.5% CI | 97.5% CI |
|---|---|---|---|
| μ0 | 0 | 0 | 0 |
| μ1 | 0.410 | 0.198 | 0.615 |
| μ2 | 0.423 | 0.200 | 0.663 |
| μ5 | −0.315 | −0.575 | −0.065 |
| Parasitized Hosts Produced | Female Offspring Produced | |||||
|---|---|---|---|---|---|---|
| Exposure Arena | Yes | No | Total | Yes | No | Total |
| Cactus | 24 | 24 | 48 | 5 | 17 | 22 |
| Petri dish | 0 | 40 | 40 | 6 | 29 | 35 |
| Total | 24 | 64 | 88 | 11 | 46 | 57 |
| Larval Exposure Type | No. Exposure Jars | Parasitized (%) | No. Exposure Jars w/Parasitized Hosts | Total Wasp Offspring/20 Larvae | Wasp Offspring/Parasitized Host |
|---|---|---|---|---|---|
| Cactus | 48 | 13.1 ± 2.4 | 22 | 88.3 ± 14.6 | 19.2 ± 2.3 |
| Petri dish | 40 | 33.6 ± 2.6 | 35 | 135 ± 14.4 | 21.2 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varone, L.; Benda, N.; Guala, M.E.; Martínez, J.J.; Bruzzone, O.A. Reproductive Biology and Rearing Improvements of Apanteles opuntiarum, Potential Biocontrol Agent of the Argentine Cactus Moth, Cactoblastis cactorum. Insects 2024, 15, 604. https://doi.org/10.3390/insects15080604
Varone L, Benda N, Guala ME, Martínez JJ, Bruzzone OA. Reproductive Biology and Rearing Improvements of Apanteles opuntiarum, Potential Biocontrol Agent of the Argentine Cactus Moth, Cactoblastis cactorum. Insects. 2024; 15(8):604. https://doi.org/10.3390/insects15080604
Chicago/Turabian StyleVarone, Laura, Nicole Benda, Mariel Eugenia Guala, Juan José Martínez, and Octavio Augusto Bruzzone. 2024. "Reproductive Biology and Rearing Improvements of Apanteles opuntiarum, Potential Biocontrol Agent of the Argentine Cactus Moth, Cactoblastis cactorum" Insects 15, no. 8: 604. https://doi.org/10.3390/insects15080604





