Gut Bacterial Communities in the Ground Beetle Carabus convexus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Species and Sampling Design
2.2. Gut Dissection, Microbial DNA Extraction, Amplification, and Sequencing
2.3. Bioinformatic and Statistical Analyses
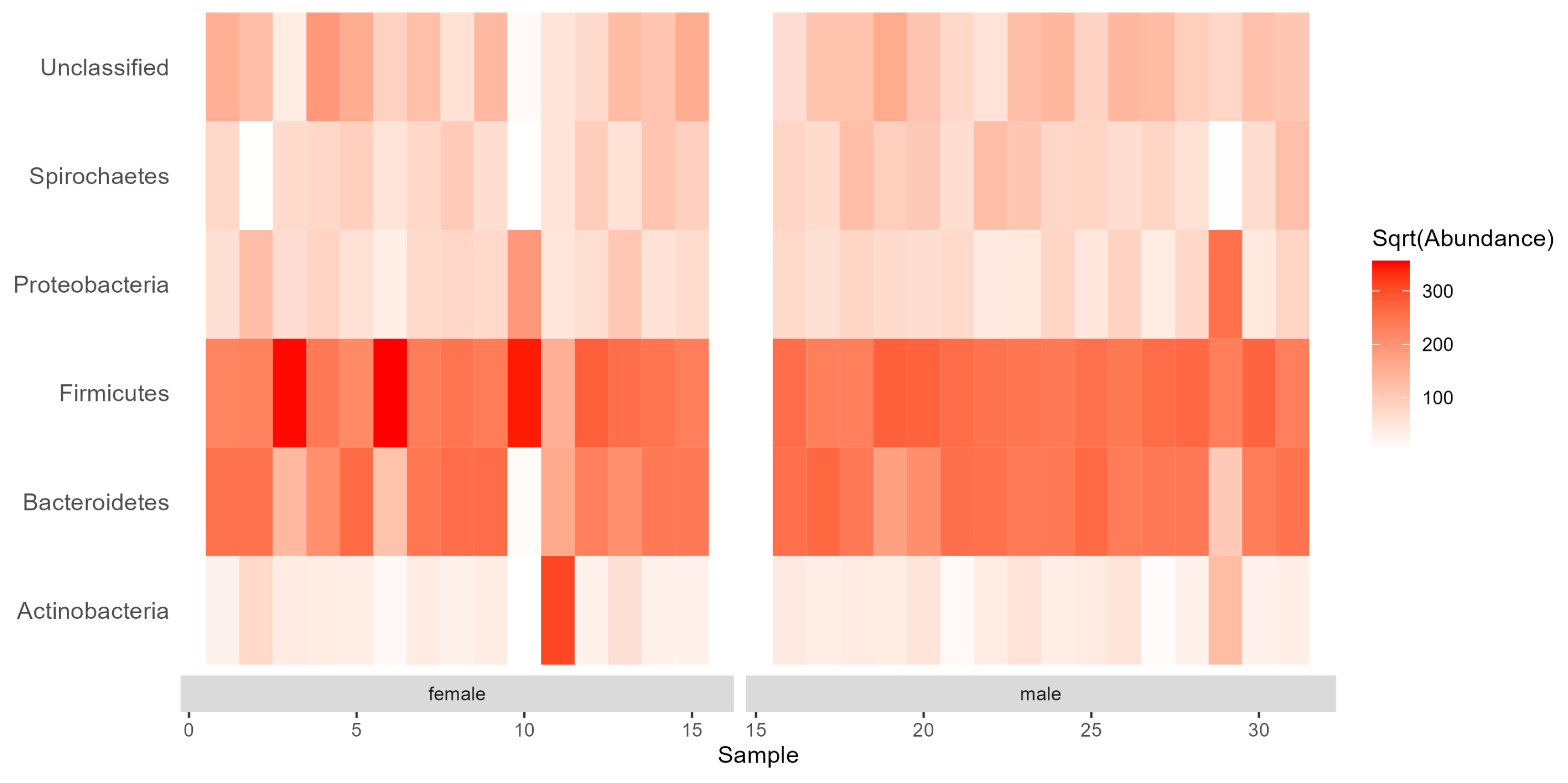
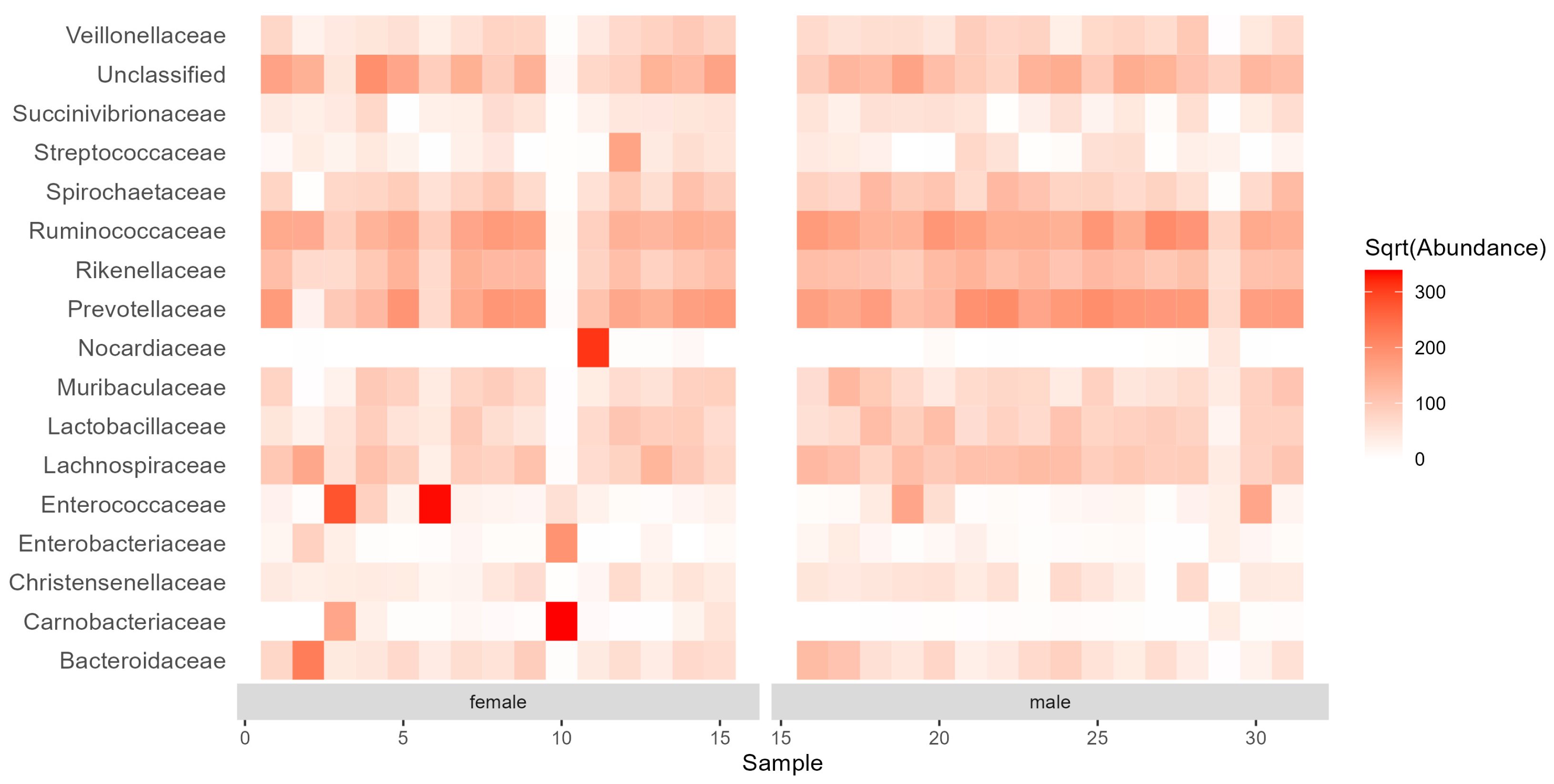
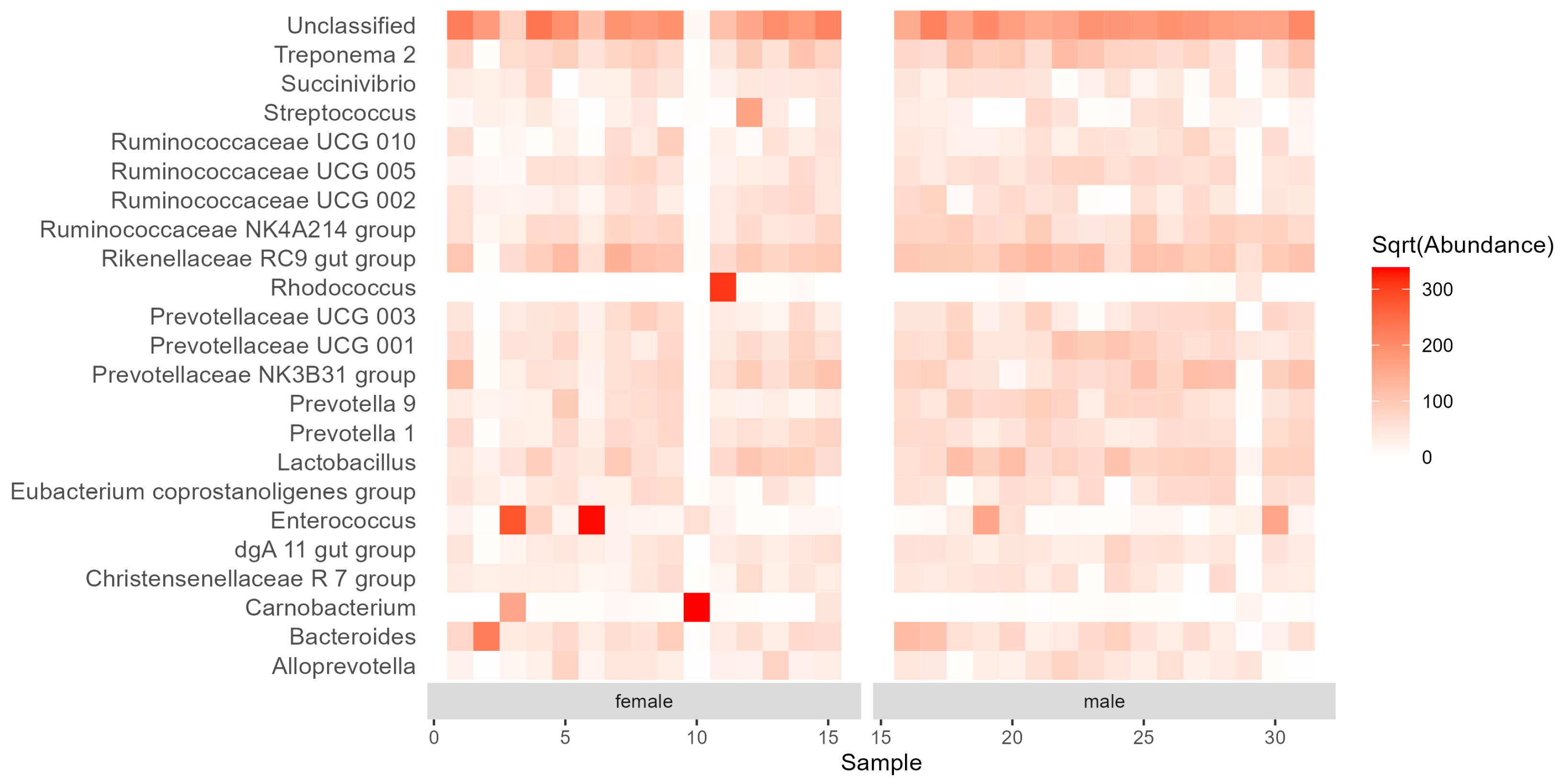
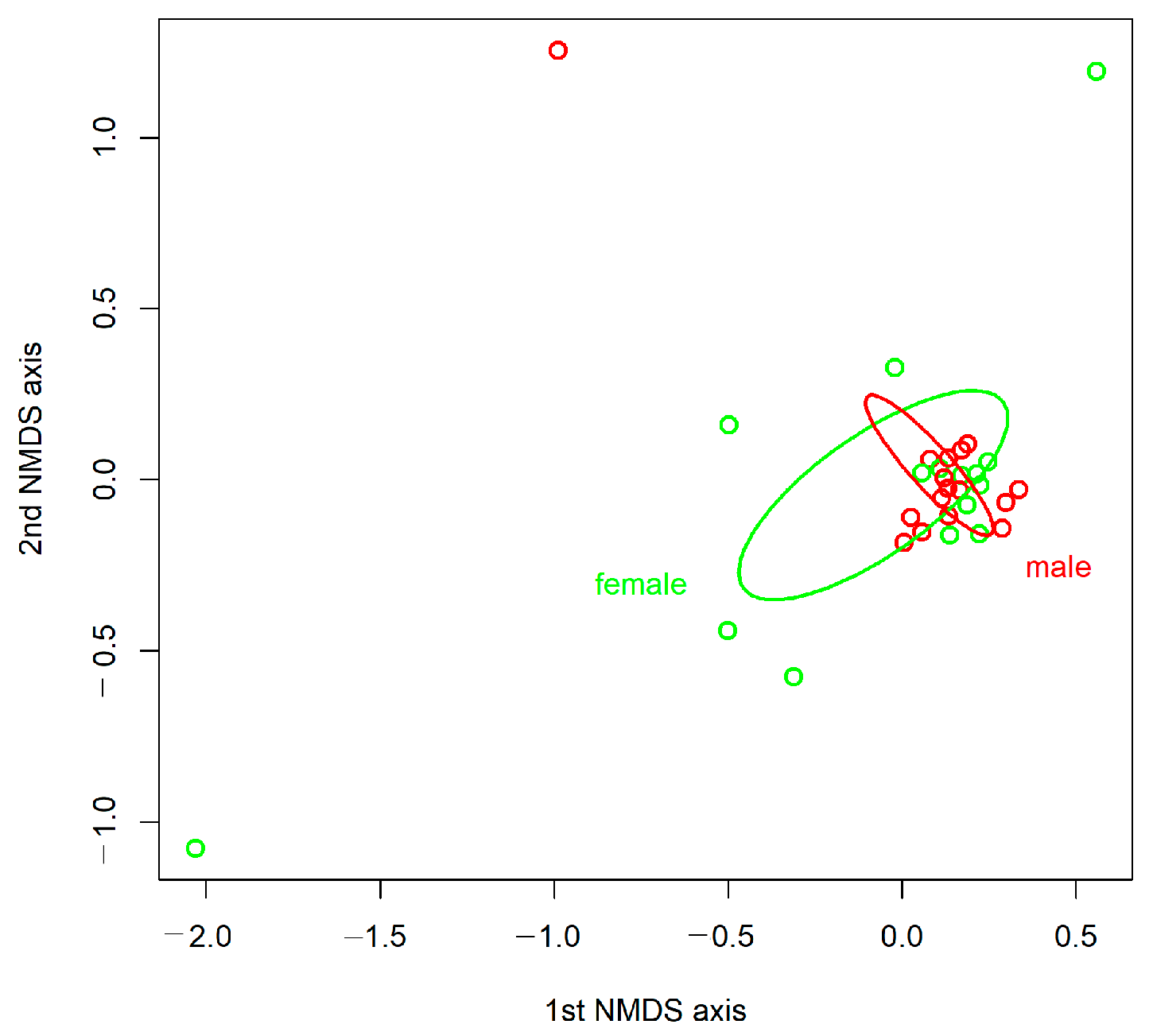
3. Results
3.1. Sequencing Data
3.2. Diversity and Composition of the Gut Bacterial Communities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Taggart, P.L.; Liddicoat, C.; Tong, W.H.; Breed, M.F.; Weinstein, P.; Wheeler, D.; Vyas, A. Gut microbiota composition does not associate with toxoplasma infection in rats. Mol. Ecol. 2022, 31, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Deng, J.; Pan, X.; Meng, D.; Zhu, Y.; Bai, Y.; Shi, C.; Duan, Y.; Wang, T.; Li, X.; et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome 2022, 10, 82. [Google Scholar] [CrossRef]
- Brown, B.R.P.; Goheen, J.R.; Newsome, S.D.; Pringle, R.M.; Palmer, T.M.; Khasoha, L.M.; Kartzinel, T.R. Host phylogeny and functional traits differentiate gut microbiomes in a diverse natural community of small mammals. Mol. Ecol. 2023, 32, 2320–2334. [Google Scholar] [CrossRef] [PubMed]
- Solomon, G.; Love, A.C.; Vaziri, G.J.; Harvey, J.; Verrett, T.; Chernicky, K.; Simons, S.; Albert, L.; Chaves, J.A.; Knutie, S.A. Effect of urbanization and parasitism on the gut microbiota of Darwin’s finch nestlings. Mol. Ecol. 2023, 32, 6059–6069. [Google Scholar] [CrossRef]
- Härer, A.; Mauro, A.A.; Laurentino, T.G.; Rosenblum, E.B.; Rennison, D.J. Gut microbiota parallelism and divergence associated with colonisation of novel habitats. Mol. Ecol. 2023, 32, 5661–5672. [Google Scholar] [CrossRef]
- Brunetti, A.E.; Lyra, M.L.; Monteiro, J.P.C.; Zurano, J.P.; Baldo, D.; Haddad, C.F.B.; Moeller, A.H. Convergence of gut microbiota in myrmecophagous amphibians. Proc. R. Soc. B Biol. Sci. 2023, 290, 20232223. [Google Scholar] [CrossRef]
- Sadeghi, J.; Chaganti, S.R.; Johnson, T.B.; Heath, D.D. Host species and habitat shape fish-associated bacterial communities: Phylosymbiosis between fish and their microbiome. Microbiome 2023, 11, 258. [Google Scholar] [CrossRef]
- Bai, J.; Ling, Y.; Li, W.-J.; Wang, L.; Xue, X.-B.; Gao, Y.-Y.; Li, F.-F.; Li, X.-J. Analysis of intestinal microbial diversity of four species of grasshoppers and determination of cellulose digestibility. Insects 2022, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Hyun, S. Role of gut commensal bacteria in juvenile developmental growth of the host: Insights from Drosophila studies. Anim. Cells Syst. 2023, 27, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76. [Google Scholar] [CrossRef]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of Ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Silver, A.; Perez, S.; Gee, M.; Xu, B.; Garg, S.; Will, K.; Gill, A. Persistence of the Ground beetle (Coleoptera: Carabidae) microbiome to diet manipulation. PLoS ONE 2021, 16, e0241529. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.; Ravenscraft, A.; Moore, W. Bacterial associates of a gregarious riparian beetle with explosive defensive chemistry. Front. Microbiol. 2018, 9, 2361. [Google Scholar] [CrossRef]
- Do, Y.; Park, W.-B.; Park, J.-K.; Park, S.; Kwon, O.; Choi, M.B. Host and environmental influences on the gut bacterial community of carabid beetles in distinct paddy fields. Entomol. Res. 2023, 53, 509–517. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Lehman, R.M.; Chee-sanford, J. Bacterial communities within digestive tracts of Ground beetles (Coleoptera: Carabidae). Ann. Entomol. Soc. Am. 2007, 100, 275–282. [Google Scholar] [CrossRef]
- Kudo, R.; Masuya, H.; Endoh, R.; Kikuchi, T.; Ikeda, H. Gut bacterial and fungal communities in ground-dwelling beetles are associated with host food habit and habitat. ISME J. 2019, 13, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; Park, J.-K.; Park, W.-B.; Kim, M.-S. The gut bacterial community of Chlaenius pallipes (Coleoptera: Carabidae) associates with their habitat and morphology. Insects 2022, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.G.; Lehman, R.M. Bacterial gut symbionts contribute to seed digestion in an omnivorous beetle. PLoS ONE 2010, 5, e10831. [Google Scholar] [CrossRef] [PubMed]
- Magagnoli, S.; Alberoni, D.; Baffoni, L.; Martini, A.; Marini, F.; Di Gioia, D.; Mazzon, M.; Marzadori, C.; Campanelli, G.; Burgio, G. The ground beetle Pseudoophonus rufipes gut microbiome is influenced by the farm management system. Sci. Rep. 2022, 12, 22638. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.M.; Lundgren, J.G.; Petzke, L.M. Bacterial communities associated with the digestive tract of the predatory ground beetle, Poecilus chalcites, and their modification by laboratory rearing and antibiotic treatment. Microb. Ecol. 2009, 57, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.; Vommaro, M.L.; Gionechetti, F.; Pallavicini, A. Gut microbial community response to herbicide exposure in a ground beetle. J. Appl. Entomol. 2021, 145, 986–1000. [Google Scholar] [CrossRef]
- Kolasa, M.; Ścibior, R.; Mazur, M.A.; Kubisz, D.; Dudek, K.; Kajtoch, Ł. How hosts taxonomy, trophy, and endosymbionts shape microbiome diversity in beetles. Microb. Ecol. 2019, 78, 995–1013. [Google Scholar] [CrossRef]
- Magura, T.; Mizser, S.; Horváth, R.; Tóth, M.; Likó, I.; Lövei, G.L. Urbanization reduces gut bacterial microbiome diversity in a specialist ground beetle, Carabus convexus. Mol. Ecol. 2024, 33, e17265. [Google Scholar] [CrossRef] [PubMed]
- Turin, H.; Penev, L.; Casale, A.; Arndt, E.; Assmann, T.; Makarov, K.V.; Mossakowski, D.; Szél, G.; Weber, F. Species accounts. In The Genus Carabus in Europe: A Synthesis; Turin, H., Penev, L., Casale, A., Eds.; Pensoft Publishers: Sofia-Moscow, Bulgaria, 2003; pp. 151–284. ISBN 954-642-120-0. [Google Scholar]
- Hůrka, K. Carabidae of the Czech and Slovak Republics; Kabourek: Zlin, Czech Republic, 1996. [Google Scholar]
- Digweed, S.C.; Currie, C.R.; Carcamo, H.A.; Spence, J.R. Digging out the digging-in effect of pitfall traps: Influences of depletion and disturbance on catches of ground beetles (Coleoptera: Carabidae). Pedobiologia 1995, 39, 561–576. [Google Scholar] [CrossRef]
- Molnár, T.; Magura, T.; Tóthmérész, B.; Elek, Z. Ground beetles (Carabidae) and edge effect in oak-hornbeam forest and grassland transects. Eur. J. Soil Biol. 2001, 37, 297–300. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in low microbial biomass microbiome studies: Issues and recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 May 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Dillies, M.-A.; Rau, A.; Aubert, J.; Hennequet-Antier, C.; Jeanmougin, M.; Servant, N.; Keime, C.; Marot, G.; Castel, D.; Estelle, J.; et al. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief. Bioinform. 2012, 14, 671–683. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package, R Package Version 2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 15 May 2024).
- Venables, W.; Ripley, B. Modern Applied Statistics with S.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Tinker, K.A.; Ottesen, E.A. The core gut microbiome of the american cockroach, Periplaneta americana, is stable and resilient to dietary shifts. Appl. Environ. Microbiol. 2016, 82, 6603–6610. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Bozorov, T.A.; Rasulov, B.A.; Zhang, D. Characterization of the gut microbiota of invasive Agrilus mali Matsumara (Coleoptera: Buprestidae) using high-throughput sequencing: Uncovering plant cell-wall degrading bacteria. Sci. Rep. 2019, 9, 4923. [Google Scholar] [CrossRef] [PubMed]
- Briones-Roblero, C.I.; Hernández-García, J.A.; Gonzalez-Escobedo, R.; Soto-Robles, L.V.; Rivera-Orduña, F.N.; Zúñiga, G. Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PLoS ONE 2017, 12, e0175470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Wang, T.-Z.; Zhu, H.-F.; Pan, H.-B.; Yu, X.-P. Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. 2020, 27, 883–894. [Google Scholar] [CrossRef]
- Larochelle, A. The food of the Carabid beetles (Coleoptera: Carabidae, including Cicindelinae). Fabreries Suppl. 1990, 5, 1–132. [Google Scholar]
- Schmid, R.B.; Lehman, R.M.; Brözel, V.S.; Lundgren, J.G. An indigenous gut bacterium, Enterococcus faecalis (Lactobacillales: Enterococcaceae), increases seed consumption by Harpalus pensylvanicus (Coleoptera: Carabidae). Florida Entomol. 2014, 97, 575–584. [Google Scholar] [CrossRef]
- Martin, J.D.; Orvin, M.J. Enterococci in insects. Appl. Microbiol. 1972, 24, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, H.; He, J.; Muhammad, A.; Zhang, F.; Lu, X. Features and colonization strategies of Enterococcus faecalis in the gut of Bombyx mori. Front. Microbiol. 2022, 13, 921330. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A. Symbiotoxicity: The ability of environmental stressors to damage healthy microbiome structure and interactions with the host. Environ. Toxicol. Chem. 2023, 42, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Vommaro, M.L.; Zanchi, C.; Angelone, T.; Giglio, A.; Kurtz, J. Herbicide exposure alters the effect of the enthomopathogen Beauveria bassiana on immune gene expression in mealworm beetles. Environ. Pollut. 2023, 338, 122662. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Tu, C.; Han, R.; Luo, J.; Xu, L. Enhanced capacity of a leaf beetle to combat dual stress from entomopathogens and herbicides mediated by associated microbiota. Integr. Zool. 2024; in press. [Google Scholar] [CrossRef]
- Hom, E.F.Y.; Penn, A.S. Symbiosis and the Anthropocene. Symbiosis 2021, 84, 239–270. [Google Scholar] [CrossRef]
| Subfamily | Species | Feeding Habit | Location | No. of Individuals Analyzed | No. of OTUs/ASVs | Reference |
|---|---|---|---|---|---|---|
| Brachininae | Brachinus elongatulus Chaudoir, 1876 | Carnivore | USA | 11 | 37 ASVs/gut (mean) | [21] |
| Pheropsophus jessoensis A. Morawitz, 1862 | Carnivore | Republic of Korea | 10 | ~100–300 ASVs/ gut | [22] | |
| Harpalinae | Anisodactylus sanctaecrucis (Fabricius, 1798) | Omnivore | USA | 6 | 2 OTUs | [23] |
| Chlaenius pallipes (Gebler, 1823) | Carnivore | South Korea | 8 | ~110–190 ASVs/gut (mean) | [22] | |
| C. pallipes (Gebler, 1823) | Carnivore | South Korea | 32 | ~25–50 OTUs/ gut (mean) | [25] | |
| Harpalus pensylvanicus (DeGeer, 1774) | Omnivore | USA | 4 | 6 OTUs | [23] | |
| H. pensylvanicus (DeGeer, 1774) | Omnivore | USA | 80 | 35 OTUs (total) | [26] | |
| Pseudoophonus rufipes (DeGeer, 1774) | Omnivore | Italy | 29 | 798 OTUs (total) | [27] | |
| Pterostichinae | Poecilus chalcites (Say, 1823) | Carnivore | USA | 15 | 19 OTUs (total) | [28] |
| Pterostichus melas italicus (Dejean, 1828) | Carnivore | Italy | 30 | 2647 OTUs (total) | [29] | |
| Trechinae | Bembidion decorum (Panzer, 1799) | Carnivore | Poland | 9 | 195 OTUs/gut (mean) | [30] |
| B. modestum (Fabricius, 1801) | Carnivore | Poland | 10 | 108 OTUs/gut (mean) | [30] | |
| B. punctulatum Drapiez, 1820 | Carnivore | Poland | 10 | 164 OTUs/gut (mean) | [30] | |
| B. varicolor (Fabricius, 1803) | Carnivore | Poland | 10 | 165 OTUs/gut (mean) | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magura, T.; Mizser, S.; Horváth, R.; Tóth, M.; Kozma, F.S.; Kádas, J.; Lövei, G.L. Gut Bacterial Communities in the Ground Beetle Carabus convexus. Insects 2024, 15, 612. https://doi.org/10.3390/insects15080612
Magura T, Mizser S, Horváth R, Tóth M, Kozma FS, Kádas J, Lövei GL. Gut Bacterial Communities in the Ground Beetle Carabus convexus. Insects. 2024; 15(8):612. https://doi.org/10.3390/insects15080612
Chicago/Turabian StyleMagura, Tibor, Szabolcs Mizser, Roland Horváth, Mária Tóth, Ferenc Sándor Kozma, János Kádas, and Gábor L. Lövei. 2024. "Gut Bacterial Communities in the Ground Beetle Carabus convexus" Insects 15, no. 8: 612. https://doi.org/10.3390/insects15080612









