Air-Drying Time Affects Mortality of Pyrethroid-Susceptible Aedes aegypti Exposed to Transfluthrin-Treated Filter Papers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Chemicals
2.3. Transfluthrin-Impregnated Filter Papers
2.4. High-Throughput Screening System
2.5. Data Analysis
3. Results
3.1. Dose-Dependent Toxicity Bioassay
3.2. Toxicity of Transfluthrin-Impregnated Filter Papers at Different Air-Drying Times
3.3. Mosquito Recovery Rates of Transfluthrin-Exposed Aedes aegypti
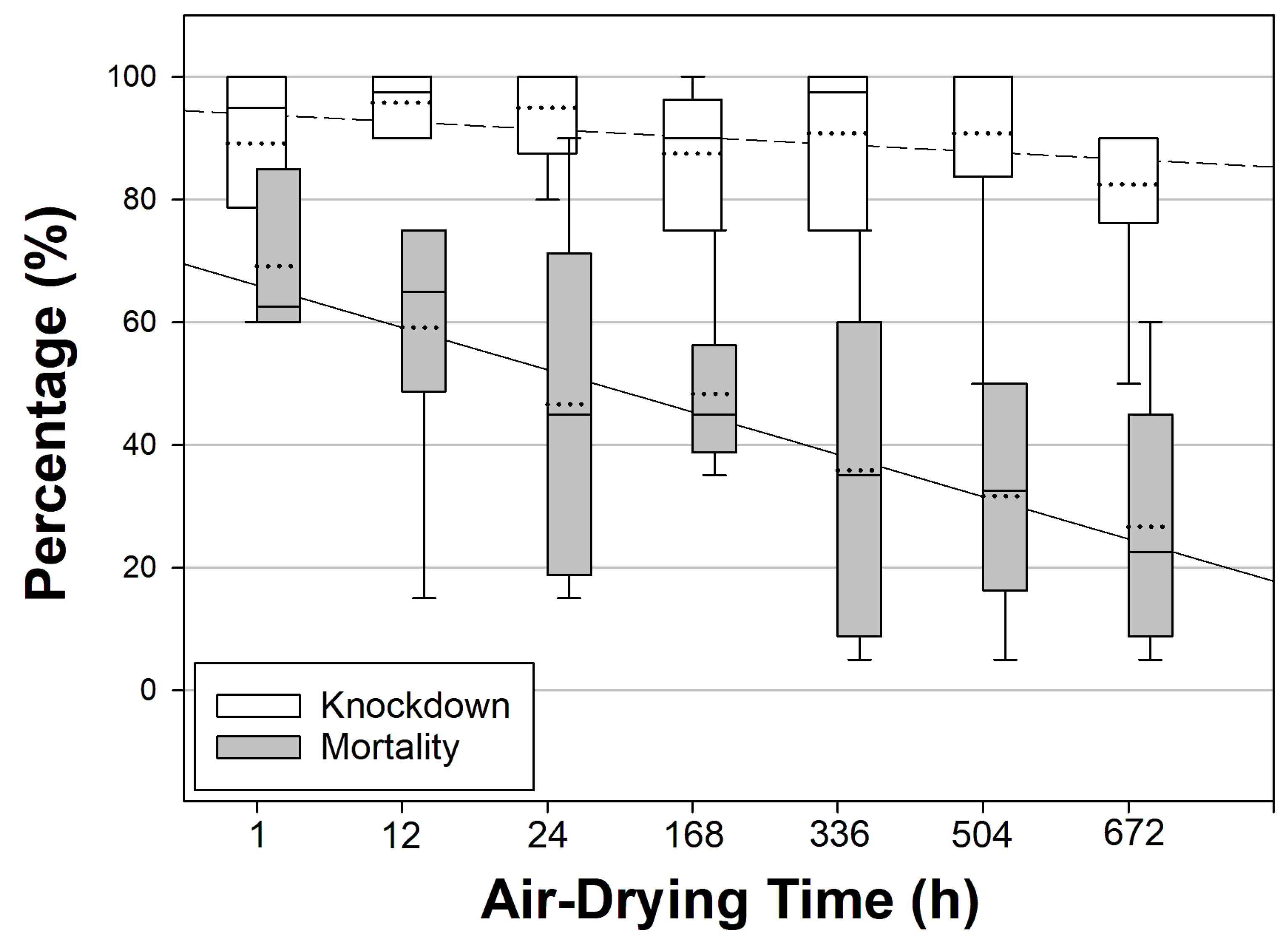
3.4. Effect of Air-Drying Time on Aedes aegypti Mortality
3.5. Effect of Air-Drying Time on Discriminating Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Gyawali, N.; Bradbury, R.S.; Taylor-Robinson, A.W. The global spread of Zika virus: Is public and media concern justified in regions currently unaffected? Infect. Dis. Poverty 2016, 5, 37. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, urbanization and globalization: The unholy trinity of the 21st century. Trop. Med. Health 2011, 39 (Suppl. 4), S3–S11. [Google Scholar] [CrossRef]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is Dengue vector control deficient in effectiveness or evidence? systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.J.; Leong, Y.Q.; bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: A review. Parasit. Vectors 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Black, W.C.; Eisen, L.; Flores, A.E.; García-Rejón, J.E.; Loroño-Pino, M.; Saavedra-Rodriguez, K. The intensifying storm: Domestication of Aedes aegypti, urbanization of arboviruses, and emerging insecticide resistance. In Global Health Impacts of Vector-Borne Diseases: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Ritchie, S. Rear and release: A new paradigm for dengue control. Austral Entomol. 2014, 53, 363–367. [Google Scholar] [CrossRef]
- Nordin, O.; Ney, T.G.; Ahmad, N.W.; Benjamin, S.; Lim, L.H. Identification of Aedes aegypti (L.) and Aedes albopictus (Skuse) breeding habitats in dengue endemic sites in Kuala Lumpur Federal Territory and Selangor State, Malaysia. Southeast Asian J. Trop. Med. Public Health 2014, 48, 786–798. [Google Scholar]
- World Health Organization and UNICEF. In Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.who.int/publications/i/item/9789241512978 (accessed on 14 August 2024).
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Brown, D.J.; An, M.; Xue, R.D.; Liu, N. Insecticide resistance: Status and potential mechanisms in Aedes aegypti. Pestic. Biochem. Physiol. 2023, 195, 105577. [Google Scholar] [CrossRef]
- Horstick, O.; Runge-Ranzinger, S.; Nathan, M.B.; Kroeger, A. Dengue vector-control services: How do they work? A systematic literature review and country case studies. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Esu, E.; Lenhart, A.; Smith, L.; Horstick, O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop. Med. Int. Health 2010, 15, 619–663. [Google Scholar] [CrossRef]
- Achee, N.L.; Sardelis, M.R.; Dusfour, I.; Chauhan, K.R.; Grieco, J.P. Characterization of spatial repellent, contact irritant, and toxicant chemical actions of standard vector control compounds. J. Am. Mosq. Control Assoc. 2009, 25, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Bangs, M.J.; Farlow, R.; Killeen, G.F.; Lindsay, S.; Logan, J.G.; Moore, S.J.; Rowland, M.; Sweeney, K.; Torr, S.J.; et al. Spatial repellents: From discovery and development to evidence-based validation. Malar. J. 2012, 11, 164. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Hii, J.; Chareonviriyaphap, T. Transfluthrin and metofluhtin as effective repellents against pyrethroid-susceptible and pyrethroid-resistant Aedes aegypti L. (Diptera: Culicidae). Insects 2023, 14, 767. [Google Scholar] [CrossRef]
- Sukkanon, C.; Bangs, M.J.; Nararak, J.; Hii, J.; Chareonviriyaphap, T. Discriminating lethal concentrations for transfluthrin, a volatile pyrethroid compound for mosquito control in Thailand. J. Am. Mosq. Control Assoc. 2019, 35, 258–266. [Google Scholar] [CrossRef]
- Grieco, J.P.; Achee, N.L.; Sardelis, M.R.; Chauhan, K.R.; Roberts, D.R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Control Assoc. 2005, 21, 404–411. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Roberts, D.R.; Andre, R.G.; Harlan, H.; Bangs, M.J. Pesticide avoidance behaviorin Anopheles albimanus Wiedemann. J. Am. Mosq. Control Assoc. 1997, 13, 171–183. [Google Scholar] [PubMed]
- Grieco, J.P.; Achee, N.L.; Chareonviriyaphap, T.; Suwonkerd, W.; Chauhan, K.; Sardelis, M.R.; Roberts, D.R. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE 2007, 2, e716. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Leepasert, T.; Bangs, M.J.; Chareonviriyaphap, T. Dose–response assay for synthetic mosquito (Diptera: Culicidae) attractant using a high-throughput screening system. Insects 2021, 12, 355. [Google Scholar] [CrossRef]
- Sukkanon, C.; Nararak, J.; Bangs, M.J.; Hii, J.; Chareonviriyaphap, T. Behavioral responses to transfluthrin by Aedes aegypti, Anopheles minimus, Anopheles harrisoni, and Anopheles dirus (Diptera: Culicidae). PLoS ONE 2020, 15, e0237353. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Determining Discriminating Concentrations of Insecticides for Monitoring Resistance in Mosquitoes: Report of a Multi-Center Laboratory Study and WHO Expert Consultations; World Health Organization: Geneva, Switzerland, 2022. Available online: https://iris.who.int/handle/10665/352616 (accessed on 14 August 2024).
- Corbel, V.; Kont, M.D.; Ahumada, M.L.; Andréo, L.; Bayili, B.; Bayili, K.; Brooke, B.; Pinto Caballero, J.A.; Lambert, B.; Churcher, T.S.; et al. A new WHO bottle bioassay method to assess the susceptibility of mosquito vectors to public health insecticides: Results from a WHO coordinated multicentre study. Parasit. Vectors 2023, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2016. Available online: https://iris.who.int/handle/10665/250677 (accessed on 14 August 2024).
- World Health Organization. Guidelines for Efficacy Testing of Spatial Repellents; World Health Organization: Geneva, Switzerland, 2013. Available online: https://www.who.int/publications/i/item/9789241505024 (accessed on 14 August 2024).
- Althoff, R.A.; Huijben, S. Comparison of the variability in mortality data generated by CDC bottle bioassay, WHO tube test, and topical application bioassay using Aedes aegypti mosquitoes. Parasit. Vectors 2022, 15, 476. [Google Scholar] [CrossRef] [PubMed]
- Waits, C.M.; Fulcher, A.; Louton, J.E.; Richardson, A.G.; Becnel, J.J.; Estep, A.S. A comparative analysis of resistance testing methods in Aedes albopictus (Diptera: Culicidae) from St. Johns County, Florida. Fla. Entomol. 2017, 100, 571–577. [Google Scholar] [CrossRef]
- Owusu, H.F.; Jančáryová, D.; Malone, D.; Müller, P. Comparability between insecticide resistance bioassays for mosquito vectors: Time to review current methodology? Parasit. Vectors 2015, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, P.R. The activity of some pyrethroids, DDT and lindane in smoke from coils for biting inhibition, knockdown, and kill of mosquitoes (Diptera, Culicidae). Bull. Entomol. Res. 1975, 65, 97–107. [Google Scholar] [CrossRef]
- Barnes, J.M.; Verschoyle, R.D. Toxicity of new pyrethroid insecticide. Nature 1974, 248, 5450. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J. Degradation, metabolism, and toxicity of synthetic pyrethroids. Environ. Health Perspect. 1976, 14, 15–28. [Google Scholar] [CrossRef]
- Harrison, C.M. Inheritance of resistance of DDT in the housefly, Musca domestica L. Nature 1951, 167, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Achee, N.; Masuoka, P.; Smith, P.; Martin, N.; Chareonviryiphap, T.; Polsomboon, S.; Hendarto, J.; Grieco, J.P. Identifying the effective concentration for spatial repellency of the dengue vector Aedes aegypti. Parasit. Vectors 2012, 5, 300. [Google Scholar] [CrossRef]
- World Health Organization. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Bottle Bioassays; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.who.int/publications/i/item/9789240043770 (accessed on 14 August 2024).
- Jansma, J.W.; Linders, J.B. Volatilization of Pesticides from Soil and Plants after Spraying; National Institute of Public Health and Environmental Protection: Utrecht, The Netherlands, 1995. [Google Scholar]
- Argueta, T.B.O.; Kawada, H.; Takagi, M. Spatial repellency of metofluthrin-impregnated multilayer paper strip against Aedes albopictus under outdoor conditions, Nagasaki, Japan. Med. Entomol. Zool. 2004, 55, 211–216. [Google Scholar] [CrossRef]
- Pettebone, M.S. Characterization of Transfluthrin Emissions over Time in an Enclosed Space over a Range of Discreet Temperatures. Master’s Thesis, Uniformed Services University, School of Medicine Graduate Programs, Bethesda, MD, USA, 2014. [Google Scholar]
- Wagman, J.M.; Achee, N.L.; Grieco, J.P. Insensitivity to the spatial repellent action of transfluthrin in Aedes aegypti: A heritable trait associated with decreased insecticide susceptibility. PLoS Negl. Trop. Dis. 2015, 9, e0003726. [Google Scholar] [CrossRef] [PubMed]

| TFT Conc. (%) | Mean % (SE) Ae. aegypti (USDA) at Air-Drying Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Knockdown | p-Value † | Mortality | p-Value † | Recovery | p-Value † | ||||
| 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | ||||
| 0.01706 | 89.2 (6.2) a | 95.0 (3.4) a | 0.392 | 69.2 (5.1) a | 46.7 (11.7) a | 0.169 | 20.0 (7.1) a | 48.3 (10.0) a | 0.041 |
| 0.00853 | 63.3 (8.4) ab | 69.2 (9.9) a | 0.809 | 45.8 (12.7) ab | 23.3 (7.1) a | 0.153 | 17.5 (8.3) a | 45.8 (8.3) a | 0.037 |
| 0.00427 | 17.5 (3.6) b | 23.3 (3.3) b | 0.262 | 14.2 (3.5) b | 5.8 (2.4) ab | 0.078 | 3.3 (4.8) a | 17.5 (5.3) ab | 0.075 |
| 0.00213 | 6.7 (2.5) bc | 1.7 (1.7) c | 0.105 | 1.7 (1.1) b | 3.3 (2.1) b | 0.702 | 5.0 (2.9) a | −1.7 (1.7) b | 0.073 |
| 0.00107 | 1.7 (1.1) c | 0.0 (0.0) c | 0.138 | 0.8 (0.8) b | 0.0 (0.0) b | 0.317 | 0.8 (0.8) a | 0.0 (0.0) b | 0.317 |
| Air-Drying Time (h) | Mean % (SE) Knockdown [95% CI] | Mean % (SE) Mortality [95% CI] | Mortality OR [95% CI] | Z-Value | p-Value |
|---|---|---|---|---|---|
| 1 | 89.17 (6.25) [73.11–105.23] | 69.17 (5.07) [56.14–82.20] | - | 63.94 | - |
| 12 | 95.83 (2.01) [90.67–100.99] | 59.17 (9.26) [35.37–82.96] | 0.646 [0.380–1.099] | 2.60 | 0.107 |
| 24 | 95.00 (3.42) [86.22–103.78] | 46.67 (11.74) [16.49–76.84] | 0.390 [0.230–0.661] | 12.22 | <0.001 |
| 168 | 87.50 (4.43) [76.12–98.88] | 48.33 (5.73) [33.62–63.05] | 0.417 [0.246–0.707] | 10.56 | 0.001 |
| 336 | 90.83 (5.07) [77.80–103.86] | 35.83 (10.83) [7.99–63.68] | 0.249 [0.145–0.426] | 25.67 | <0.001 |
| 504 | 90.83 (8.21) [69.74–111.93] | 31.67 (7.15) [13.29–50.04] | 0.207 [0.120–0.357] | 32.06 | <0.001 |
| 672 | 82.50 (6.55) [65.66–99.34] | 26.67 (8.33) [5.25–48.09] | 0.162 [0.093–0.284] | 40.53 | <0.001 |
| Air-Drying Time (h) | % LC50 (95% FL) | % LC75 (95% FL) | % LC99 (95% FL) | % DCs | χ2 (df) * | p-Value |
|---|---|---|---|---|---|---|
| 1 | 0.01040 (0.00925–0.01189) a | 0.01852 (0.01576–0.02280) a | 0.07611 (0.05455–0.11971) a | 0.15222 | 3.217 (3) | 0.359 |
| 12 | 0.01515 (0.01039–0.03612) ab | 0.02666 (0.01628–0.12592) ab | 0.10636 (0.04148–3.15898) ab | 0.21272 | 10.471 (3) | 0.015 |
| 24 | 0.01870 (0.01543–0.02434) b | 0.03784 (0.02831–0.05797) b | 0.21269 (0.12005–0.50602) b | 0.42538 | 2.468 (3) | 0.481 |
| 168 | 0.01790 (0.01480–0.02314) b | 0.03711 (0.02784–0.05621) b | 0.22121 (0.12524–0.51640) b | 0.44242 | 6.354 (3) | 0.096 |
| 336 | 0.02577 (0.01984–0.03820) bc | 0.05672 (0.03826–0.10560) b | 0.39147 (0.18471–1.31841) b | 0.78294 | 6.848 (3) | 0.077 |
| 504 | 0.02742 (0.02083–0.04220) bc | 0.05917 (0.03913–0.11677) b | 0.38927 (0.17758–1.45757) b | 0.77854 | 3.583 (3) | 0.310 |
| 672 | 0.03118 (0.02319–0.05157) c | 0.06318 (0.04072–0.13760) b | 0.35637 (0.15747–1.56318) b | 0.71273 | 1.301 (3) | 0.729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Hii, J.; Chareonviriyaphap, T. Air-Drying Time Affects Mortality of Pyrethroid-Susceptible Aedes aegypti Exposed to Transfluthrin-Treated Filter Papers. Insects 2024, 15, 616. https://doi.org/10.3390/insects15080616
Kim D-Y, Hii J, Chareonviriyaphap T. Air-Drying Time Affects Mortality of Pyrethroid-Susceptible Aedes aegypti Exposed to Transfluthrin-Treated Filter Papers. Insects. 2024; 15(8):616. https://doi.org/10.3390/insects15080616
Chicago/Turabian StyleKim, Dae-Yun, Jeffrey Hii, and Theeraphap Chareonviriyaphap. 2024. "Air-Drying Time Affects Mortality of Pyrethroid-Susceptible Aedes aegypti Exposed to Transfluthrin-Treated Filter Papers" Insects 15, no. 8: 616. https://doi.org/10.3390/insects15080616
APA StyleKim, D.-Y., Hii, J., & Chareonviriyaphap, T. (2024). Air-Drying Time Affects Mortality of Pyrethroid-Susceptible Aedes aegypti Exposed to Transfluthrin-Treated Filter Papers. Insects, 15(8), 616. https://doi.org/10.3390/insects15080616






