Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Literature Review
3.1. Regulations on the Use of Insects in Ruminant Feeding
3.2. Fly Larvae
3.2.1. Chemical Constituents
Housefly Larvae Meal and Housefly Pupae Meal
Quantitative and Qualitative Aspects of Animal Performance
3.2.2. Chemical Composition and Nutritional Value of H. illucens Larvae
H. illucens Larvae and Their Impacts on Ruminant Digestibility
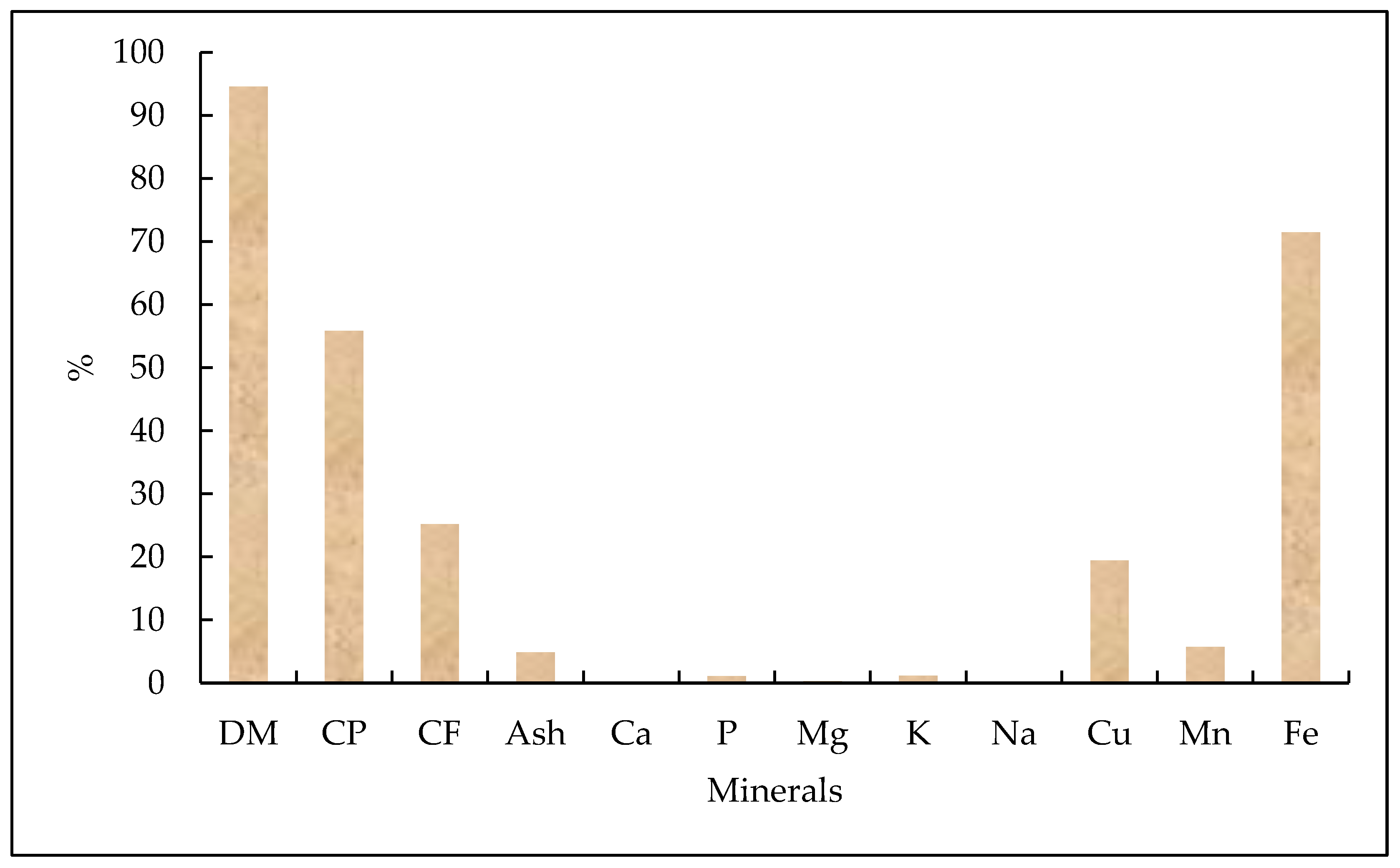
3.2.3. Chemical Composition and Nutritional Quality of M. domestic Larvae
3.3. Effect of Housefly Larvae on Nutrient Digestibility in Farm Animals
4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviation | Definition |
| CP | Crude Protein |
| PAPs | Processed animal protein |
| EE | Ethereal extract |
| SBM | Soybean meal |
| VFA’s | Volatile Fatty Acids |
| pH | Hydrogen potential |
| mg | Milligram |
| mm | Millimeters |
| BSFL | BSF larvae |
| CF | Crude fiber |
| CE | Crude energy |
| Ca | Calcium |
| Fe | Iron |
| Mg | Magnesium |
| Na | Sodium |
| Zn | Zinc |
| EAA | Essential amino acids |
| Ala | Alanine |
| Glu | Glutamic acid |
| Leu | Leucine |
| Val | Valine |
| Arg | Arginine |
| Hist | Histidine |
| Lys | Lysine |
| Meth | Methionine |
| Isoleu | Isoleucine |
| Phy | Phenylanine |
| Thar | Threonine |
| DM | Dry matter |
| NDF | Neutral Detergent Fiber |
| ADF | Acid detergent fiber |
| AG | Fatty acids |
| NH3 | Ammonia |
| HFL | House fly |
| Phy | Phosphorus |
| OM | Organic matter |
| HF | Hermetia illucens |
| AA | Amino acids |
References
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, K.; Leury, B.J. Insect meal: A future source of protein feed for pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Marnila, P.; Mooney, P.; Mäki, M.; Siljander-Rasi, H.; et al. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Dabbou, S.; Trocino, A.; Xiccato, G.; Capucchio, M.T.; Biasato, I.; Dezzutto, D.; Birolo, M.; Meneguz, M.; Schiavone, A.; et al. Effect of dietary supplementation with insect fats on growth performance, digestive efficiency and health of rabbits. J. Anim. Sci. Biotechnol. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Prospects of insects as food and feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Grmelová, N.; Hénault-Ethier, L.; Deschamps, M.H.; Vandenberg, G.W.; Zhao, A.; Zhang, Y.; Yang, B.; Nemane, V. Insects as food and feed: Laws of the European union, United States, Canada, Mexico, Australia, and China. Eur. Food Feed Law Rev. 2017, 12, 22–36. [Google Scholar]
- DiGiacomo, K.; Akit, H.; Leury, B.J. Insects: A novel animal-feed protein source for the Australian market. Anim. Prod. Sci. 2019, 59, 2037–2045. [Google Scholar] [CrossRef]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Apri, A.D.; Komalasari, K. Feed and animal nutrition: Insect as animal feed. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 465, p. 012002. [Google Scholar]
- Jayanegara, A.; Novandri, B.; Yantina, N.; Ridla, M. Use of BSF larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: An in vitro rumen fermentation study. Vet. World 2017, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Yantina, N.; Novandri, B.; Laconi, E.B.; Ridla, M. Evaluation of some insects as potential feed ingredients for ruminants: Chemical composition, in vitro rumen fermentation and methane emissions. J. Indones. Trop. Anim. Agric. 2017, 42, 247–254. [Google Scholar] [CrossRef]
- Rashmi, K.M.; Chandrasekharaia, M.; Soren, N.M.; Prasad, K.S.; David, C.G.; Thirupathaiah, Y.; Shivaprasad, V. Effect of dietary incorporation of silkworm pupae meal on in vitro rumen fermentation and digestibility. Indian J. Anim. Sci. 2018, 88, 731–735. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; González-Rosales, M.G.; Mendoza, A.G.; Robles-Jiménez, L.E.; Frutos, P. Insects as alternative feed for ruminants: Comparison of protein evaluation methods. J. Anim. Sci. Biotechnol. 2022, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Vale-Hagan, W.; Singhal, S.; Grigoletto, I.; Totaro-Fila, C.; Theodoridou, K.; Koidis, A. Edible insects in mixed-sourced protein meals for animal feed and food: An EU focus. Food Humanit. 2023, 1, 1180–1187. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Raab, L.; Cafantaris, B.; Jilg, T.; Menke, K.H. Rumen protein degradation and biosynthesis: 1. A new method for determination of protein degradation in rumen fluid in vitro. Br. J. Nutr. 1983, 50, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.; Bayat, A.; Yáñez-Ruiz, D.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.U.; Banday, M.T.; Baba, I.A.; Adil, S.; Nissa, S.S.; Zaffer, B.; Bulbul, K.H. Utilization of silkworm pupae meal as an alternative source of protein in the diet of livestock and poultry: A review. J. Entomol. Zool. Stud. 2018, 6, 1010–1016. [Google Scholar]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. Insects as novel ruminant feed and a potential mitigation strategy for methane emissions. Animals 2021, 11, 2648. [Google Scholar] [CrossRef] [PubMed]
- Sukmak, R.; Suttinun, C.; Kovitvadhi, U.; Kovitvadhi, A.; Vongsangnak, W. Uncovering nutrients and energy related gene functions of black soldier fly Hermetia illucens strain KUP. Gene 2024, 896, 148045. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Matsakidou, A.; Sarivasiliou, S.I.; Pissia, M.A.; Rumbos, C.I.; Athanassiou, C.G.; Paraskevopoulou, A. Compositional, volatile, and structural features of Hermetia illucens (black soldier fly) flours: The effect of population and life stages. Future Foods 2024, 9, 100320. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)–Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valoriz. 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Mannaa, M.; Mansour, A.; Park, I.; Lee, D.W.; Seo, Y.S. Insect-based agri-food waste valorization: Agricultural applications and roles of insect gut microbiota. Environ. Sci. Ecotechnol. 2023, 17, 100287. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Hernández, J. Insects in ruminant nutrition as an urgent measure in the light of the scarcity of raw feedstock. Res. Vet. Sci. 2023, 155, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Józefiak, A.; Kozłowski, K.; Jankowski, J.; Józefiak, D. Tenebrio molitor and Zophobas morio full-fat meals in broiler chicken diets: Effects on nutrients digestibility, digestive enzyme activities, and cecal microbiome. Animals 2019, 9, 1128. [Google Scholar] [CrossRef]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bergagna, S.; Sardi, L.; et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Cullere, M.; Woods, M.J.; Van Emmenes, L.; Pieterse, E.; Hoffman, L.C.; Dalle Zotte, A. Hermetia illucens larvae reared on different substrates in broiler quail diets: Effect on physicochemical and sensory quality of the quail meat. Animals 2019, 9, 525. [Google Scholar] [CrossRef]
- Fontes, T.V.; de Oliveira, K.R.B.; Gomes Almeida, I.L.; Orlando, T.M.; Rodrigues, P.B.; da Costa, D.V.; Rosa, P.V.E. Digestibility of insect meals for Nile tilapia fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Chemello, G.; Renna, M.; Caimi, C.; Guerreiro, I.; Oliva-Teles, A.; Enes, P.; Biasato, I.; Schiavone, A.; Gai, F.; Gasco, L. Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): Effects on growth performance, diet digestibility and metabolic responses. Animals 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gasco, L.; Lussiana, C.; Brugiapaglia, A.; Biasato, I.; Renna, M.; Cavallarin, L.; Gai, F.; Schiavone, A. Yellow mealworm (Tenebrio molitor L.) larvae inclusion in diets for free-range chickens: Effects on meat quality and fatty acid profile. Renew. Agric. Food Syst. 2020, 35, 571–578. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional composition of black soldier fly larvae (Hermetia illucens L.) and its potential uses as alternative protein sources in animal diets: A review. Insects 2022, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, T.; Gasco, L. Insects as global opportunity. Anim. Front. 2023, 13, 3–5. [Google Scholar] [CrossRef]
- Camargo-Júnior, R.N.C.; Silva, W.C.D.; Silva, É.B.R.D.; Sá, P.R.D.; Friaes, E.P.P.; Costa, B.O.D.; Rocha, C.B.R.; Silva, L.C.M.S.D.; Borges, D.C.; Cruz, S.L.F.D.; et al. Revisão integrativa, sistemática e narrativa-aspectos importantes na elaboração de uma revisão de literatura. Rev. ACB Bibliotecon. Santa Catarina 2023, 28, 4. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar] [PubMed]
- Pieterse, E.; Pretorius, Q. Nutritional evaluation of dried larvae and pupae meal of the housefly (Musca domestica) using chemical-and broiler-based biological assays. Anim. Prod. Sci. 2013, 54, 347–355. [Google Scholar] [CrossRef]
- Schiavone, A.; DE MARCO, M.; Rotolo, L.; Belforti, M.; Martinez Mirò, S.; Madrid Sanchez, J.; Gasco, L. Nutrient digestibility of Hermetia illucens and Tenebrio molitor meal in broiler chickens. In Proceedings of the 1st International Conference “Insects to Feed the World”, Wageningen, The Netherlands, 14–17 May 2014; p. 73. [Google Scholar]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed. Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Marono, S.; Piccolo, G.; Parisi, G.; Iaconisi, V.; Gasco, L.; Nizza, A. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 2016, 94, 639–647. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Heo, P.S.; Hong, J.S.; Kim, N.J.; Kim, Y.Y. Supplementation of dried mealworm (Tenebrio molitor larva) on growth performance, nutrient digestibility and blood profiles in weaning pigs. Asian-Australas. J. Anim. Sci. 2016, 29, 979. [Google Scholar] [CrossRef] [PubMed]
- Valle, T.A.D.; de Paiva, P.G.; de Jesus, E.F.; de Almeida, G.F.; Zanferari, F.; Costa, A.G.; Bueno, I.C.; Rennó, F.P. Dietary chitosan improves nitrogen use and feed conversion in diets for mid-lactation dairy cows. Livest. Sci. 2017, 201, 22–29. [Google Scholar] [CrossRef]
- Dias, A.; Goes, R.; Gandra, J.; Takiya, C.; Branco, A.; Jacaúna, A.; Oliveira, R.; Souza, C.; Vaz, M. Increasing doses of chitosan to grazing beef steers: Nutrient intake and digestibility, ruminal fermentation, and nitrogen utilization. Anim. Feed Sci. Technol. 2017, 225, 73–80. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Cho, K.H.; Hong, J.S.; Jang, H.S.; Chung, Y.H.; Kwon, G.T.; Shin, D.G.; Kim, Y.Y. Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 387. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Gustanti, R.; Ridwan, R.; Widyastuti, Y. Fatty acid profiles of some insect oils and their effects on in vitro bovine rumen fermentation and methanogenesis. Ital. J. Anim. Sci. 2020, 19, 1310–1317. [Google Scholar] [CrossRef]
- Matin, N.; Utterback, P.; Parsons, C.M. True metabolizable energy and amino acid digestibility in black soldier fly larvae meals, cricket meal, and mealworms using a precision-fed rooster assay. Poult. Sci. 2021, 100, 101146. [Google Scholar] [CrossRef]
- Kar, S.K.; Schokker, D.; Harms, A.C.; Kruijt, L.; Smits, M.A.; Jansman, A.J.M. Local intestinal microbiota response and systemic effects of feeding black soldier fly larvae to replace soybean meal in growing pigs. Sci. Rep. 2021, 11, 15088. [Google Scholar] [CrossRef]
- Hervás, G.; Boussalia, Y.; Labbouz, Y.; Della Badia, A.; Toral, P.G.; Frutos, P. Insect oils and chitosan in sheep feeding: Effects on in vitro ruminal biohydrogenation and fermentation. Anim. Feed Sci. Technol. 2022, 285, 115222. [Google Scholar] [CrossRef]
- Tansil, F.; Pezzali, J.G.; Cargo-Froom, C.; Huber, L.-A.; Kiarie, E.G.; Courtney-Martin, G.; Levesque, C.L.; Shoveller, A.K. Evaluation of standardized ileal digestibility of amino acids and metabolic availability of methionine, using the indicator amino acid oxidation method, in black soldier fly larvae (Hermetia illucens) meal fed to growing pigs. J. Anim. Sci. 2023, 101, skac420. [Google Scholar] [CrossRef]
- AbdelHakeam, M.A.; Mohamed, M.Y.; AbdEl-Salam, O.M.; Ali, S.M. Effect of insect meal as substitute for Soybean meal on performance of Ossimi lambs. J. Livest. Sci. 2024, 14, 27–41. [Google Scholar] [CrossRef]
- Renna, M.; Rastello, L.; Gasco, L. Can insects be used in the nutrition of ruminants? J. Insects Food Feed 2022, 8, 1041–1045. [Google Scholar] [CrossRef]
- Renna, M.; Rastello, L.; Veldkamp, T.; Toral, P.G.; Gonzalez-Ronquillo, M.; Jimenez, L.E.R.; Gasco, L. Are insects a solution for feeding ruminants? Legislation, scientific evidence, and future challenges. Anim. Front. 2023, 13, 102–111. [Google Scholar] [CrossRef]
- Anselmo, A.; Veys, P.; Fumière, O.; Lecrenier, M.C.; Cordonnier, A.; Michez, D.; Baeten, V. Challenges related to the application of analytical methods to control insect meals in the context of European legislation. Food Addit. Contam. Part A 2023, 40, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Shurson, G.C.; Dierenfeld, E.S.; Dou, Z. Rules are meant to be broken–Rethinking the regulations on the use of food waste as animal feed. Resour. Conserv. Recycl. 2023, 199, 107273. [Google Scholar] [CrossRef]
- Diclaro, J.W.; Kaufman, P.E. Black soldier fly Hermetia illucens linnaeus (insecta: Diptera: Stratiomyidae). Eeny 2009, 461, 1–3. [Google Scholar] [CrossRef]
- Hardouin, J.; Mahoux, G. Zootechnie D’insectes-Elevage et Utilisation au Bénéfice deL’homme et de Certains Animaux; Bureau pour l’Echange et la Distribution de l’Information sur le Mini-élevage (BEDIM): Gembloux, Belgium, 2003; 164p. [Google Scholar]
- Eriksen, N.T. Metabolic performance and feed efficiency of black soldier fly larvae. Front. Bioeng. Biotechnol. 2024, 12, 1397108. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security (No. 171); Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Veldkamp, T.; Van Duinkerken, G.; van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, T. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets: A Feasibility Study = Insecten als Duurzame Diervoedergrondstof in Varkens-en Pluimveevoeders: Een Haalbaarheidsstudie (No. 638); Wageningen UR Livestock Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Arango Gutiérrez, G.P.; Vergara Ruiz, R.A.; Mejía Vélez, H. Compositional, microbiological and protein digestibility analysis of the larva meal of Hermetia illuscens L.(Diptera: Stratiomyiidae) at Angelópolis-Antioquia, Colombia. Rev. Fac. Nac. Agron. Medellín 2004, 57, 2491–2500. [Google Scholar]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiab, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C.; Fiaboe, K.K.M.; et al. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal as an alternative to fish meal and fish oil in Siberian sturgeon nutrition: The effects on physical properties of the feed, animal growth performance, and feed acceptance and utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Tyshko, N.V.; Zhminchenko, V.M.; Nikitin, N.S.; Trebukh, M.D.; Shestakova, S.I.; Pashorina, V.A.; Sadykova, E.O. The comprehensive studies of Hermetia illucens larvae protein’s biological value. Probl. Nutr. 2021, 90, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.; Alvarenga, T.I.; Andrew, N.R.; McPhee, M.; Kolakshyapati, M.; Hopkins, D.L.; Ruhnke, I. Technological quality, amino acid and fatty acid profile of broiler meat enhanced by dietary inclusion of black soldier fly larvae. Foods 2021, 10, 297. [Google Scholar] [CrossRef]
- Calvert, C.C.; Martin, R.D.; Morgan, N.O. House fly pupae as food for poultry. J. Econ. Èntomol. 1969, 62, 938–939. [Google Scholar] [CrossRef]
- Soifer, N.L. Housefly as a Protein Source for Shrimp and Mice. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 1979. [Google Scholar]
- Akpodiete, O.J.; Ologhobo, A.D.; Oluyemi, J.A. Production and nutritive value of housefly maggot meal on three substrates of poultry faeces. J. Appl. Anim. Res. 1997, 12, 101–106. [Google Scholar] [CrossRef]
- Dankwa, D.; Nelson, F.S.; Oddoye, E.O.K.; Duncan, J.L. Housefly larvae as a feed supplement for rural poultry-Research and Development Notes. Ghana J. Agric. Sci. 2002, 35, 185–187. [Google Scholar] [CrossRef]
- Ganda, H.; Zannou, E.T.; Kenis, M.; Abihona, H.A.; Houndonougbo, F.M.; Chrysostome, C.A.A.M.; Chougourou, D.C.; Mensah, G.A. Effect of four rearing substrates on the yield and the chemical composition of housefly larvae, Musca domestica L. 1758 (Diptera: Muscidae). Int. J. Trop. Insect Sci. 2022, 42, 1331–1339. [Google Scholar] [CrossRef]
- Viroje, W.; Malin, S. Preliminary study on producing of fly larva meal from pig faeces as protein source in animal diets. King Mongkut’s Agric. J. 1988, 6, 25–31. [Google Scholar]
- Zhao, Y.; Wang, W.; Zhu, F.; Wang, X.; Wang, X.; Lei, C. The gut microbiota in larvae of the housefly Musca domestica and their horizontal transfer through feeding. AMB Express 2017, 7, 147. [Google Scholar] [CrossRef]
- Van der Poel, A.F.B.; Abdollahi, M.R.; Cheng, H.; Colovic, R.; Den Hartog, L.A.; Miladinovic, D.; Page, G.; Sijsens, K.; Smillie, J.F.; Thomas, M.; et al. Future directions of animal feed technology research to meet the challenges of a changing world. Anim. Feed Sci. Technol. 2020, 270, 114692. [Google Scholar] [CrossRef]
- Renna, M.; Coppa, M.; Lussiana, C.; Le Morvan, A.; Gasco, L.; Maxin, G. Full-fat insect meals in ruminant nutrition: In vitro rumen fermentation characteristics and lipid biohydrogenation. J. Anim. Sci. Biotechnol. 2022, 13, 138. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The effect of substrate on the nutrient content and fatty acid composition of edible insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Owens, F.N.; Qi, S.; Sapienza, D.A. Invited Review: Applied protein nutrition of ruminants—Current status and future directions. Prof. Anim. Sci. 2014, 30, 150–179. [Google Scholar] [CrossRef]
- Jayanegara, A.; Dewi, S.P.; Ridla, M. Nutrient content, protein fractionation, and utilization of some beans as potential alternatives to soybean for ruminant feeding. Media Peternak. 2016, 39, 195–202. [Google Scholar] [CrossRef]
- Moreki, J.C.; Tiroesele, B.; Chiripasi, S.C. Prospects of utilizing insects as alternative sources of protein in poultry diets in Botswana: A review. J. Anim. Sci. Adv. 2012, 2, 649–658. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Stratakos, A.C.; Linton, M.; Corcionivoschi, N.; Elliott, T.; Koidis, A.; Theodoridou, K. Impact of thermal and high-pressure treatments on the microbiological quality and in vitro digestibility of black soldier fly (Hermetia illucens) larvae. Animals 2020, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, M.; Moon, C.; Seo, D.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.; Kim, S.; Kim, Y.W.; et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- El Boushy, A.R. House-fly pupae as poultry manure converters for animal feed: A review. Bioresour. Technol. 1991, 38, 45–49. [Google Scholar] [CrossRef]
- Khan, S.H. Recent advances in role of insects as alternative protein source in poultry nutrition. J. Appl. Anim. Res. 2018, 46, 1144–1157. [Google Scholar] [CrossRef]
- Bosch, G.; Zhang, S.; Oonincx, D.G.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef]
- Ukanwoko, A.I.; Olalekan, O.A. Effects of source and time of harvest on the proximate composition of maggot (Musca domestica) larva meal. Int. J. Livest. Res. 2015, 5, 84. [Google Scholar] [CrossRef]
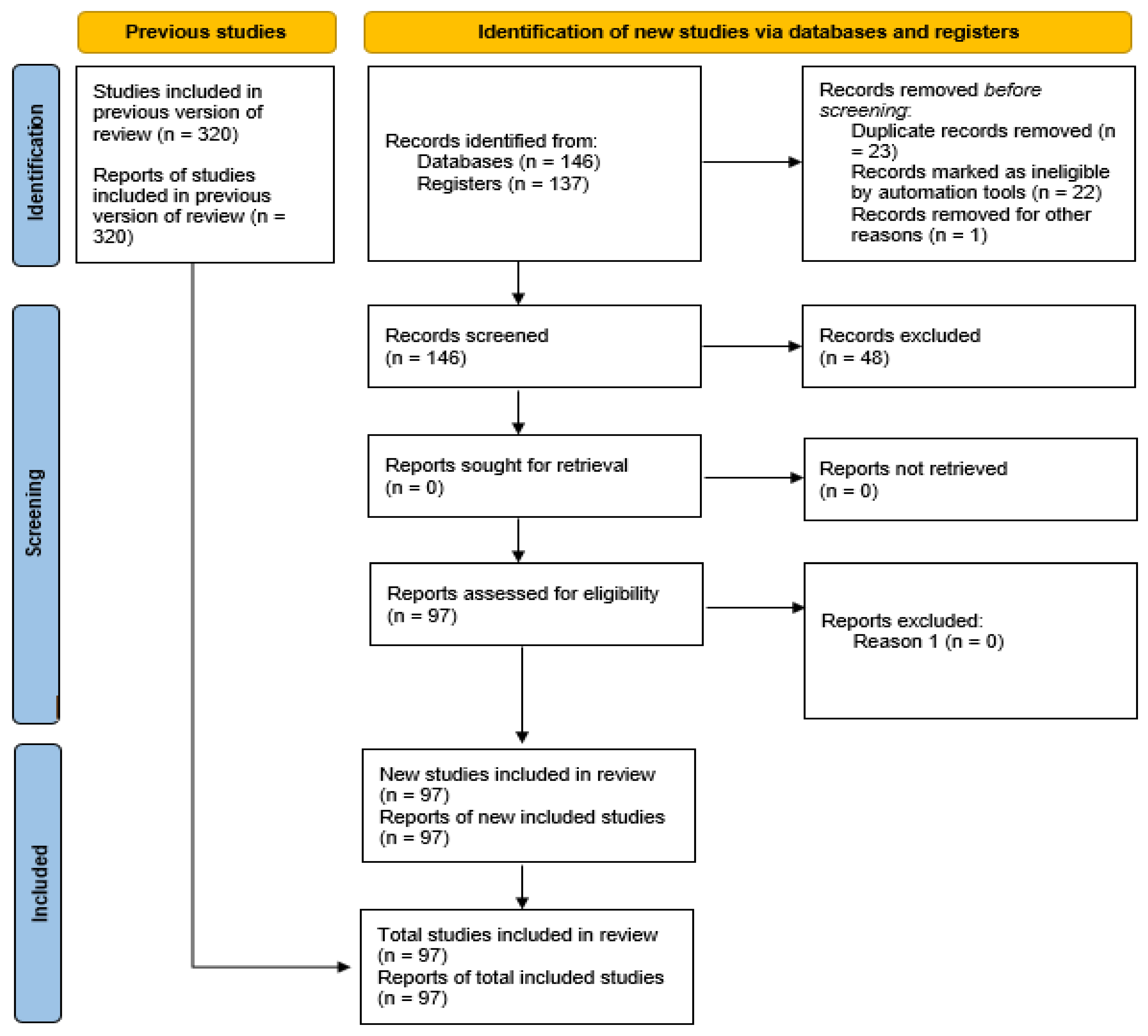
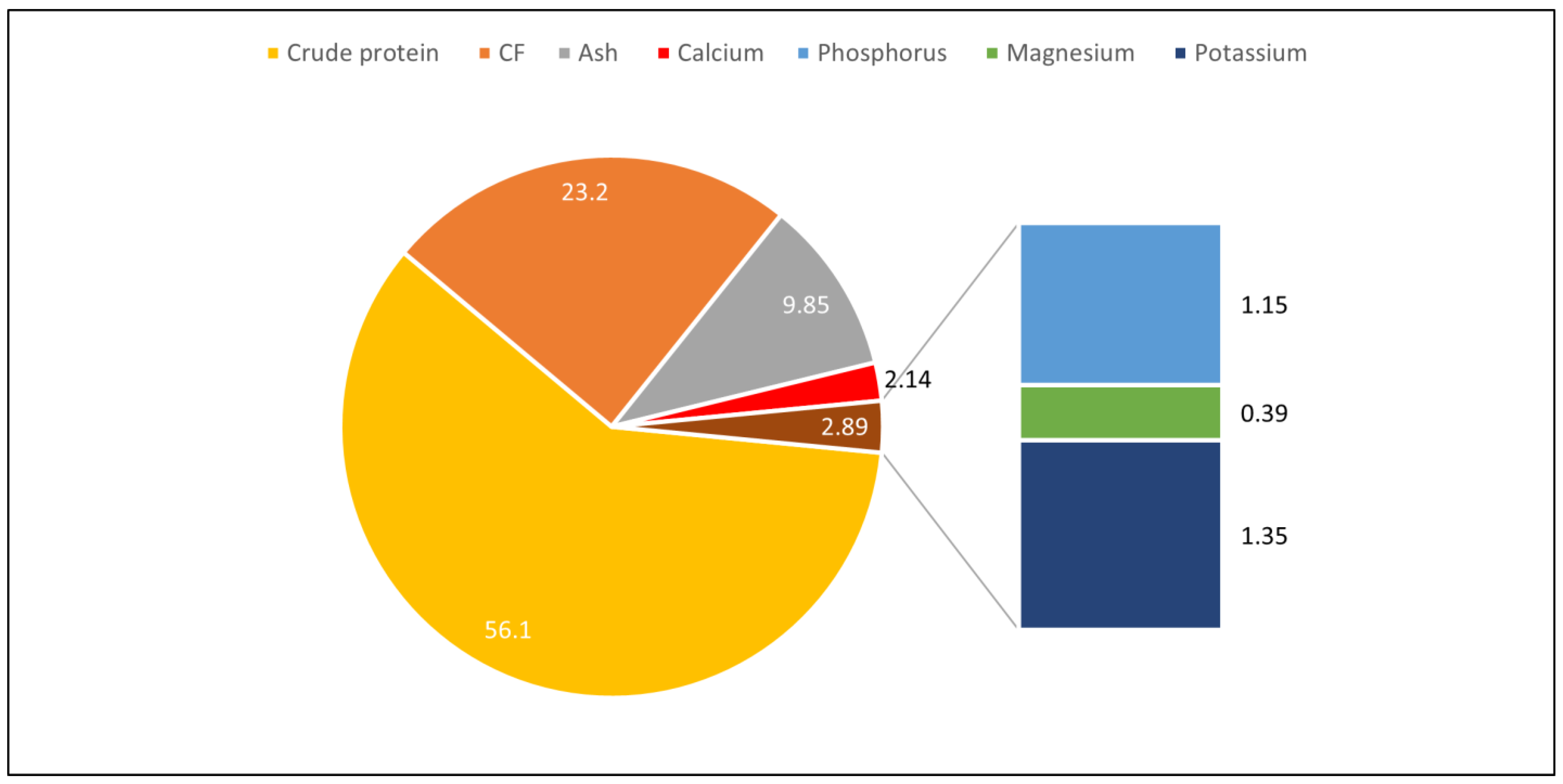

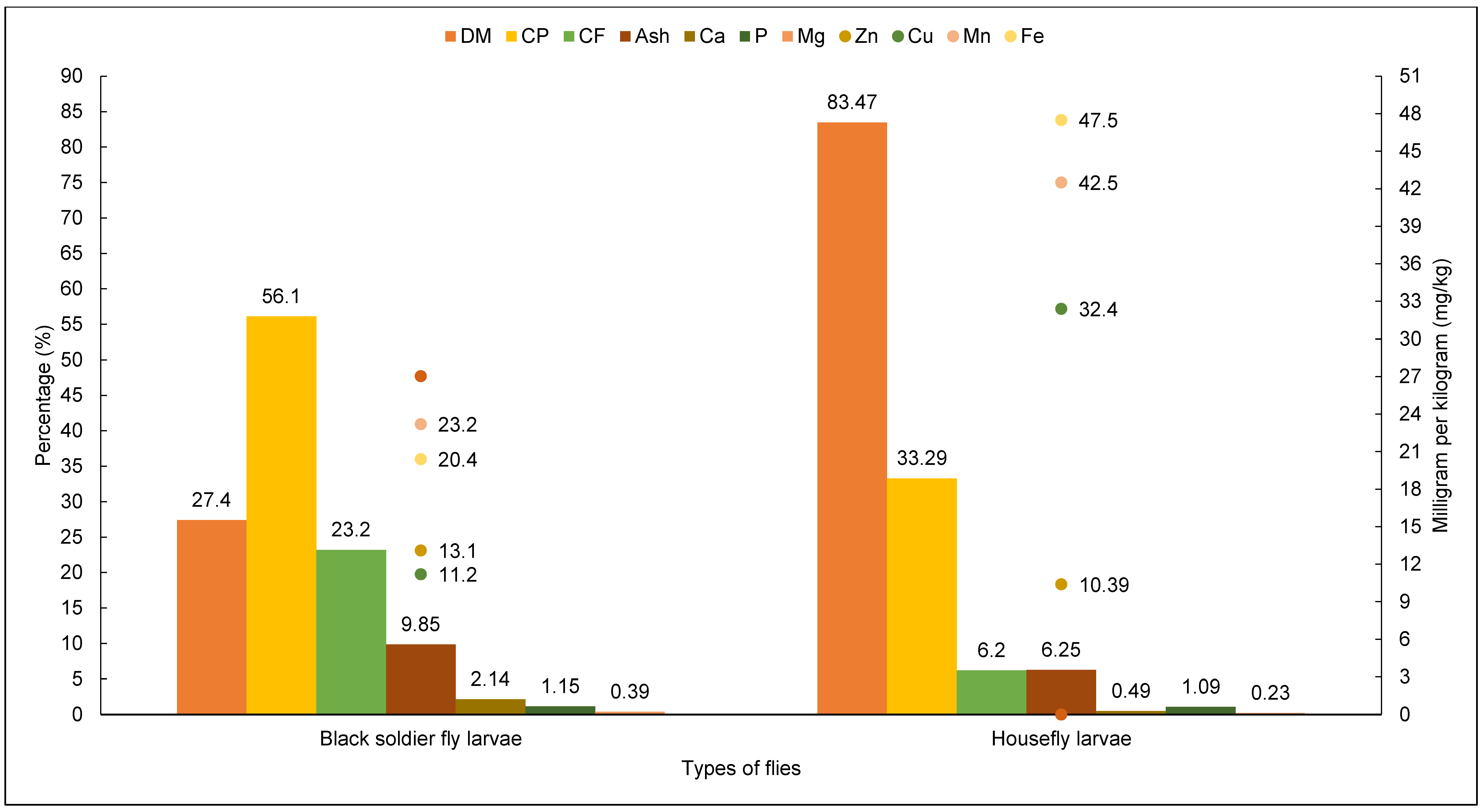
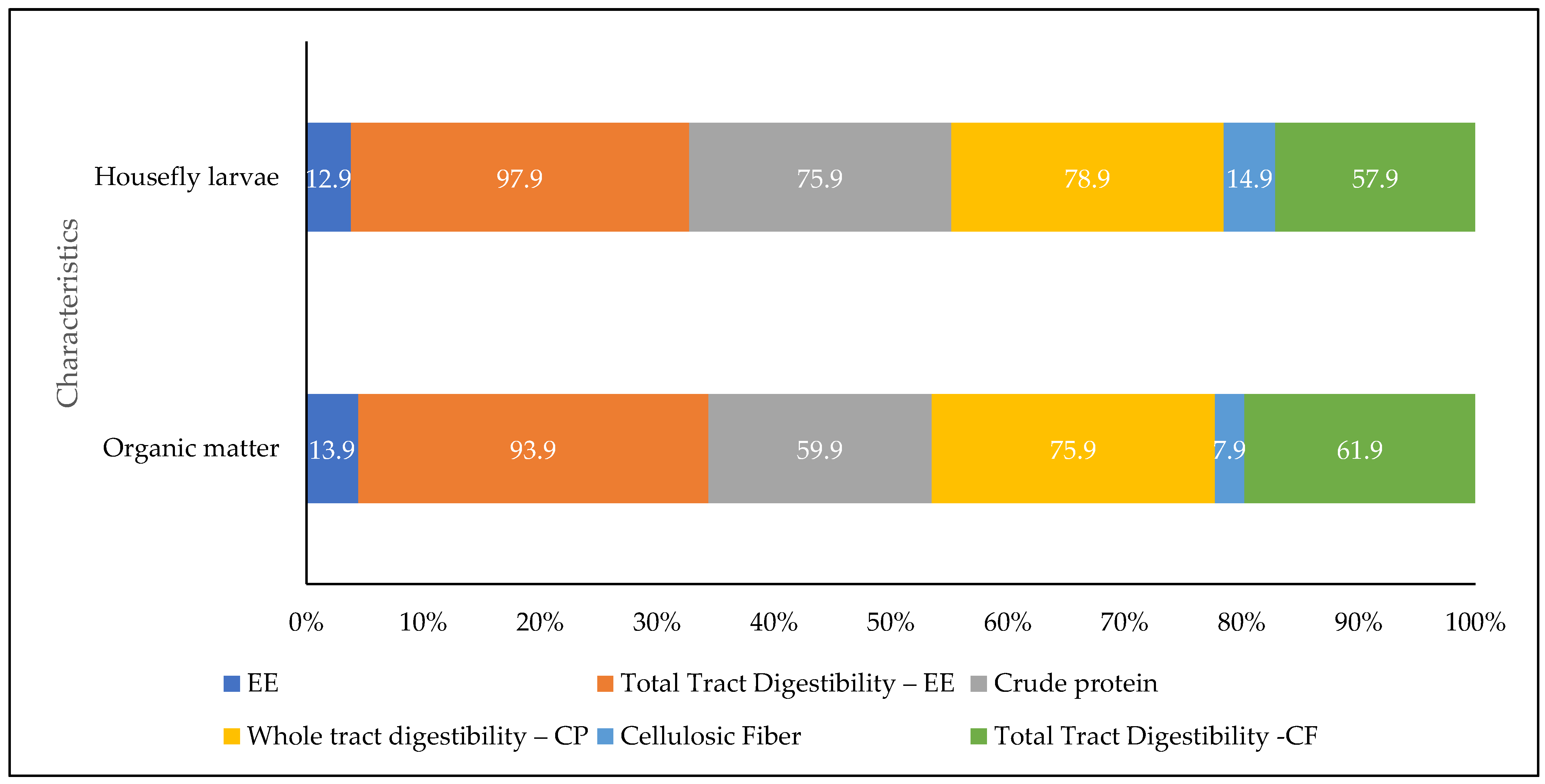
| ¥ Year | Authors | Title | Species | Breed | NA | Experimental Design and Diet |
|---|---|---|---|---|---|---|
| 2007 | St-Hilaire et al. [40] | Fly Prepupae as a Feedstuff for Rainbow Trout, Oncorhynchus mykiss. | Fish—Rainbow trout | Oncorhynchus mykiss | ¥¥ | Three test diets were formulated, which consisted of partially replacing the fishmeal of the control diet with larvae of different flies: Hermetia illucens prepupae (25% and 50% replacement) and Musca domestica pupae (25% replacement). |
| 2009 | Hwangbo et al. [41] | Utilization of house fly maggots, a feed supplement in the production of broiler chickens. | Male Cutting Chick | Ross Lineage | 150 | A total of 600 birds were divided into five groups, one fed a control diet and the other with a basal diet supplemented with 5.0, 10.0, 15.0, and 20.0% housefly larvae; however, on day 35, 30 birds from each treatment group were chosen for the analyses. |
| 2013 | Pieterse and Pretorius [42] | Nutritional evaluation of dried larvae and pupae meal of the housefly (Musca domestica) using chemical and broiler-based biological assays. | Chick | Cobb 500 lineage | 120 | The animals were distributed in metabolic cages, with five chicks per cage, totaling 24 cages used in the experiment, in which, at 25 days of age, they were fed one of three diets, which were based on corn (the reference diet), larvae, or pupae. |
| 2014 | Schiavone et al. [43] | Nutrient digestibility of Hermetia illucens and Tenebrio molitor meal in broiler chickens. | Chickens | Ross Lineage 706 | 30 | The diets were randomly allocated to cages on the 26th day, consisting of a control diet and two experimental diets with larvae, in which 25% (w/w) of the basal diet was replaced by Tenebrio molitor larvae meal or Hermetia illucens larvae meal. |
| 2015 | Marco et al. [44] | Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility, and apparent metabolizable energy. | Poultry (Chick) | Ross 708 Lineage | 90 | On day 19 of life, the birds were divided into three groups: the Control Group; Group TM (Tenebrio molitor); and Group HI (Hermetia illucens) to evaluate digestibility coefficients. |
| 2015 | Marono et al. [45] | In vitro CP digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. | In vitro | ¥¥ | ¥¥ | Twelve samples of insect meal (Tenebrio molitor and Hermetia illucens) obtained from small producers and submitted to centesimal and CP analysis by the two-step enzymatic method were analyzed in vitro. |
| 2016 | Bovera et al. [46] | Use of Tenebrio molitor larvae meal as a protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. | Chicken | Shaver | 32 | They were divided into 8 replicates of 5 birds, totaling 40 birds in each group (2), of which, after 62 days of age, 16 were slaughtered per group. The first group received a diet based on corn and soybean meal, and the other group received meal from Tenebrio molitor larvae. |
| 2016 | Cullere et al. [47] | BSF as a dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass, and meat traits. | Quails | Coturnix coturnix japonica | 15 | 450 birds were divided into groups that received a control diet (C) and two diets (H1 and H2) corresponding to inclusion levels of 10% and 15% of meal from defatted larvae of the BSF, of which 15 were randomly chosen for the analysis of the groups. |
| 2016 | Jin et al. [48] | Supplementation of dried mealworm (Tenebrio molitor larva) affects growth performance, nutrient digestibility, and blood profiles in weaning pigs. | Piglets | Mestizo | 120 | They were divided into five feeding groups, in which there was a control group based on corn-barley-soybean meal and four groups with a diet of 1.5%, 3.0%, 4.5%, or 6.0% dry meal powder for 35 days after weaning. |
| 2017 | Borrelli et al. [28] | Insect-based diets, a promising nutritional source, modulate gut microbiota composition and SCFA production in laying hens | Pruning chickens | Lohmann Brown Classic | 24 | They were divided into two groups with different diets, one based on corn and soybeans and another with a defatted flour of H. larvae of illucens. |
| 2017 | Valle et al. [49] | Dietary chitosan improves nitrogen use and feed conversion in diets for mid-lactation dairy cows. | Cows | Holstein Breed | 24 | Cows were segregated into six groups based on the presence of a rumen cannula, days in lactation, and milk production. These animals were randomly allocated to a treatment sequence: control; chitosan; soybean oil, and chitosan + soybean oil. |
| 2017 | Dias et al. [50] | Increasing doses of chitosan to grazing beef steers: Nutrient intake and digestibility, ruminal fermentation, and nitrogen utilization. | Steers | Rumen cannulated crossbred steers | 5 | Five different treatments were designated, in which each bovine was randomly raised. For 21 days, the animals received doses of chitosan added to the concentrate fed to the animals, with doses of 0, 400, 800, 1200 or 1600 mg/kg of dry matter (DM) of the concentrate |
| 2017 | Jayanegara et al. [11] | Use of BSF larvae (Hermetia illucens) to substitute soybean meal in ruminant diets: An in vitro rumen fermentation study. | In vitro | Rumen microorganisms from a non-lactating fistulated Friesian-Holstein cow | ¥¥ | Evaluation of rumen fermentation in vitro, in which soybean meal was replaced by BSF larvae in a Napier grass diet, with analysis of six treatments with different percentages of dietary treatments. |
| 2017 | Jayanegara et al. [12] | Evaluation of some insects as potential feed ingredients for ruminants: chemical composition, in vitro rumen fermentation, and methane emissions. | In vitro | Rumen fluid collected from a Friesian Holstein cow with rumen fistula | ¥¥ | In vitro ruminal fermentation tests were carried out, in which Jamaican cricket, mealworm, and larvae of the BSF at 1 and 2 weeks of age were used to evaluate methane emission, chemical composition, in vitro ruminal fermentation, and digestibility. |
| 2017 | Marono et al. [51] | Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as a total replacement of soybean meal from 24 to 45 weeks of age | Laying hens | Lohmann Brown Classic | 108 | The chickens were equally segregated into two groups, with distinction in feeding, the first based on soybean meal and the other on defatted meal from Hermetia illucens larvae. |
| 2018 | Rashmi et al. [13] | Effect of dietary incorporation of silkworm pupae meal on in vitro rumen fermentation and digestibility | In vitro—rumen content of three steers | Mestizos | ¥¥ | From the analysis of eleven mixtures, and before feeding and morning water supply of the three steers, the inclusion of defatted silkworm pupae meal (DSWP) in rumen fermentation and in vitro digestibility were analyzed. |
| 2019 | Benzertiha et al. [29] | Tenebrio molitor and Zophobas morio full-fat meals in broiler chicken diets: Effects on nutrient digestibility, digestive enzyme activities, and cecal microbiome. | Broiler chicks (Female) | Ross Lineage | 600 | Six experimental groups were used in the present study with two different levels of yellow flour and whole wheat superflour (0.2% and 0.3%), a positive control with the addition of salinomycin, and a negative control. |
| 2019 | Biasato et al. [30] | Partially defatted BSF larval meal inclusion in piglet diets: effects on growth performance, nutrient digestibility, blood profile, gut morphology, and histological features | Piglets | Topigs | 48 | Three different feeding treatments, with four boxes as replicates for each treatment and four animals per box. FSB larval meal was added at increasing levels (0%, 5%, and 10%) in diets formulated for two feeding phases: phase I (from day 1 to day 23) and phase II (from day 24 to day 61). |
| 2019 | Cullere et al. [31] | Hermetia illucens larvae reared on different substrates in broiler quail diets: effect on the physicochemical and sensory quality of the quail meat. | Quails | ¥¥ | 300 | They were divided into three experimental groups: control; soldier fly larvae reared on conventional substrate; and soldier fly larvae reared on substrate composed of 50% chicken layer must and 50% fish offal. |
| 2019 | Fontes et al. [32] | Digestibility of insect meals for Nile tilapia fingerlings. | Male Nile tilapia fingerlings | Oreochromis niloticus | 900 | Six dietary treatments were used (control—no insect meal and five insect flours) in three replications (cylindro-conical fiberglass tanks with a capacity of 250 L), each containing 50 fingerlings. |
| 2019 | Gasco et al. [5] | Effect of dietary supplementation with insect fats on growth performance, digestive efficiency, and health of rabbits. | Rabbits | Mestizo | 200 | A total of 12 animals from the following groups were randomly evaluated: a control diet with 1.5% soybean oil; and four experimental diets, partially (50%) or totally (100%) replaced by fats, soldier fly larvae, and yellow cascudinha larvae. |
| 2019 | Yoo et al. [52] | Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. | Pigs | Mestizo | 12 | The pigs were surgically fitted with simple T cannulas. After the recovery period, the animals were allocated to four different treatments, which consisted of different diets, one of which was based on Tenebrio molitor. |
| 2020 | Chemello et al. [34] | Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): Effects on growth performance, diet digestibility, and metabolic responses | Fish—rainbow trout | Oncorhynchus mykiss Walbaum | 252 | The animals were randomly divided into twelve tanks and fed four experimental diets containing different levels of Tenebrio molitor larval meal inclusion (0%—control, 25%, 50%, and 100%—positive fishmeal replacement test). |
| 2020 | Dabbou et al. [35] | Yellow mealworm (Tenebrio molitor L.) larvae inclusion in diets for free-range chickens: Effects on meat quality and fatty acid profile. | Free-range chickens | Hubbard Label Hybrid Chickens | 140 | Feeding trial with two dietary treatments, a control group and a group with Tenebrio molitor larval meal, in which at 97 days of age ten, from each group were slaughtered for evaluation. |
| 2020 | Jayanegara et al. [53] | Fatty acid profiles of some insect oils and their effects on in vitro bovine rumen fermentation and methanogenesis. | In vitro | Rumen microorganisms—fistulated Ongole crossbred cattle | ¥¥ | Experimental trial of in vitro ruminal fermentation, with the objective of evaluating the effects of oils from different insect species on rumen fermentation and methane production |
| 2021 | Ahmed et al. [20] | Insects as novel ruminant feed and a potential mitigation strategy for methane emissions | Cows | Holstein cows | 2 | Six experimental groups with different combinations of Klein grass hay, soybean meal, and edible insects were used for the rumen fluid study (rumen fistulated cows) |
| 2021 | Matin et al. [54] | True metabolizable energy and amino acid digestibility in BSF larvae meals, cricket meal, and mealworms using a precision-fed rooster assay. | Gauls | Single Comb White Leghorn | ¥¥ | In six experiments, seven different insect meals were analyzed to determine their chemical and nutritional composition. |
| 2021 | Kar. et al. [55] | Local intestinal microbiota response and systemic effects of feeding BSF larvae to replace soybean meal in growing pigs. | Pigs | Wild boar | 16 | The animals were distributed equally and randomly into two groups, in which they were fed for three weeks with isocaloric and isoprotein experimental diets prepared with soybean meal or Hermetia illucens meal. |
| 2022 | Hervás et al. [56] | Insect oils and chitosan in sheep feeding: Effects on in vitro ruminal biohydrogenation and fermentation. | In vitro—microrganisms ruminais de ovelhas | ¥¥ | ¥¥ | Ten treatments were used, divided between one control without the addition of oil plus four oil supplements × absence or presence of chitosan. |
| 2022 | Toral et al. [14] | Insects as an alternative feed for ruminants: comparison of protein evaluation methods. | Intro—Rumen content of four sheep | Merino | ¥¥ | In vitro analysis of the potential of four insect species (Tenebrio molitor, Zophobas morio, Alphitobius diaperinus, and Acheta domesticus) as alternative protein sources for ruminants. |
| 2023 | Tansil et al. [57] | Evaluation of standardized ileal digestibility of amino acids and metabolic availability of methionine, using the indicator amino acid oxidation method, in BSF larvae (Hermetia illucens) meal fed to growing pigs. | Pigs | Yorkshire | Experiment 1: 6 Experiment 2:7 | Two experiments were carried out with cannulated male pigs in order to evaluate the ileal digestibility and metabolic availability of methionine in partially defatted BSF larvae (PD-BSF) meal. |
| 2024 | AbdelHakeam, et al. [58] | Effect of insect meal as a substitute for soybean meal on performance of Ossimi lambs. | Lamb | Ossimi | 40 | Partial replacement in the diet of 0, 10, 20, and 30% of soybean meal with an equal portion of Oriental Wasp Flour. |
| Nutrients | Raw Eggs | Crushed Mango | ||
|---|---|---|---|---|
| Immediate | 120 h | Immediate | 120 h | |
| Crude protein | 45.8% | 7.6% | 39.5% | 6.1% |
| Ethereal extract | 19.3% | 24.6% | 19.1% | 5.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da-Silva, W.C.; Silva, É.B.R.d.; Silva, J.A.R.d.; Martorano, L.G.; Belo, T.S.; Sousa, C.E.L.; Camargo-Júnior, R.N.C.; Andrade, R.L.; Santos, A.G.d.S.; Carvalho, K.C.d.; et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects 2024, 15, 619. https://doi.org/10.3390/insects15080619
da-Silva WC, Silva ÉBRd, Silva JARd, Martorano LG, Belo TS, Sousa CEL, Camargo-Júnior RNC, Andrade RL, Santos AGdS, Carvalho KCd, et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects. 2024; 15(8):619. https://doi.org/10.3390/insects15080619
Chicago/Turabian Styleda-Silva, Welligton Conceição, Éder Bruno Rebelo da Silva, Jamile Andréa Rodrigues da Silva, Lucieta Guerreiro Martorano, Tatiane Silva Belo, Carlos Eduardo Lima Sousa, Raimundo Nonato Colares Camargo-Júnior, Rubens Lima Andrade, Ana Gizela de Souza Santos, Katarina Cardoso de Carvalho, and et al. 2024. "Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review" Insects 15, no. 8: 619. https://doi.org/10.3390/insects15080619










