Population Dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in Four Counties of Yunnan, China, by Electronic Monitoring System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
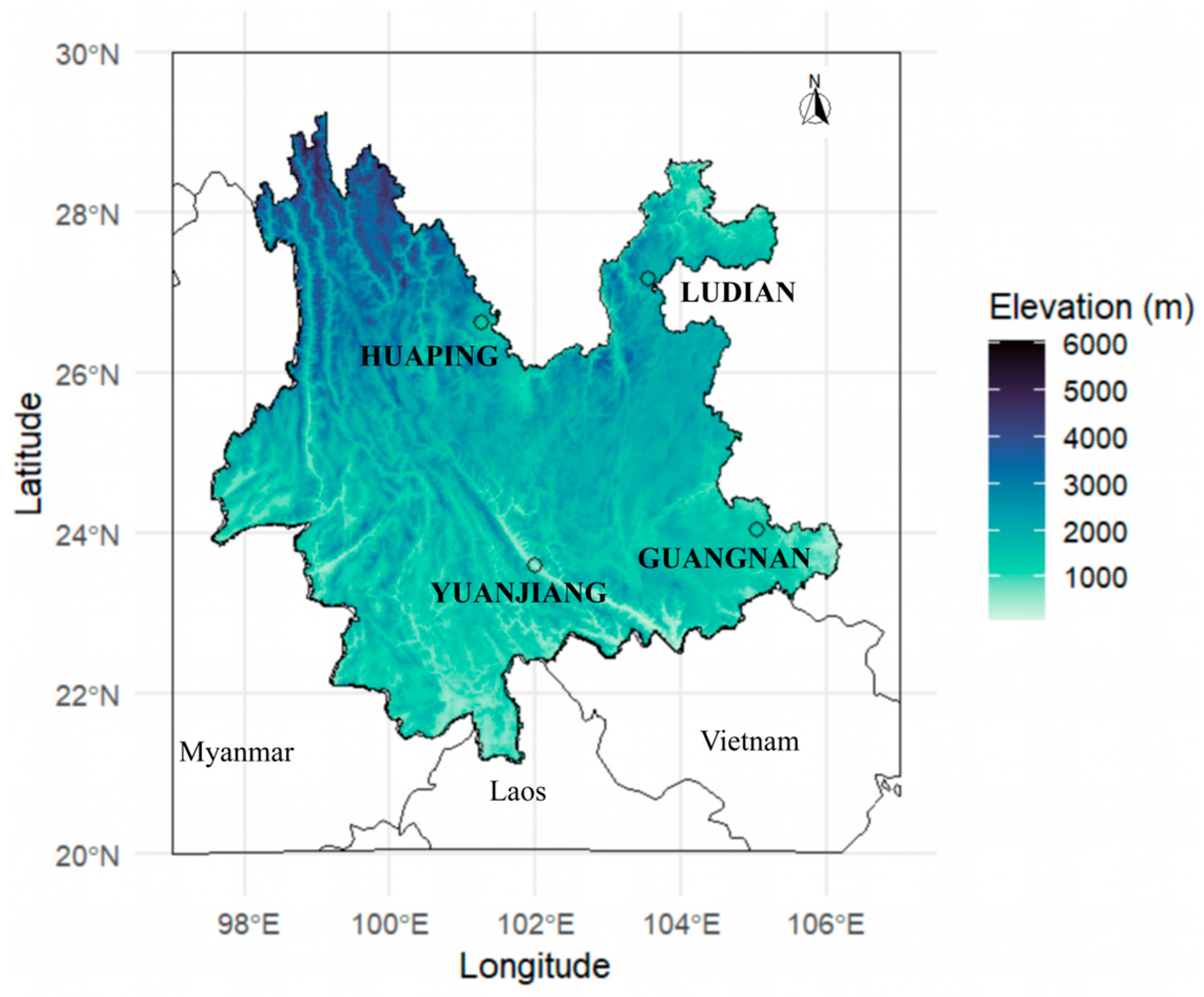
2.1. Study Area
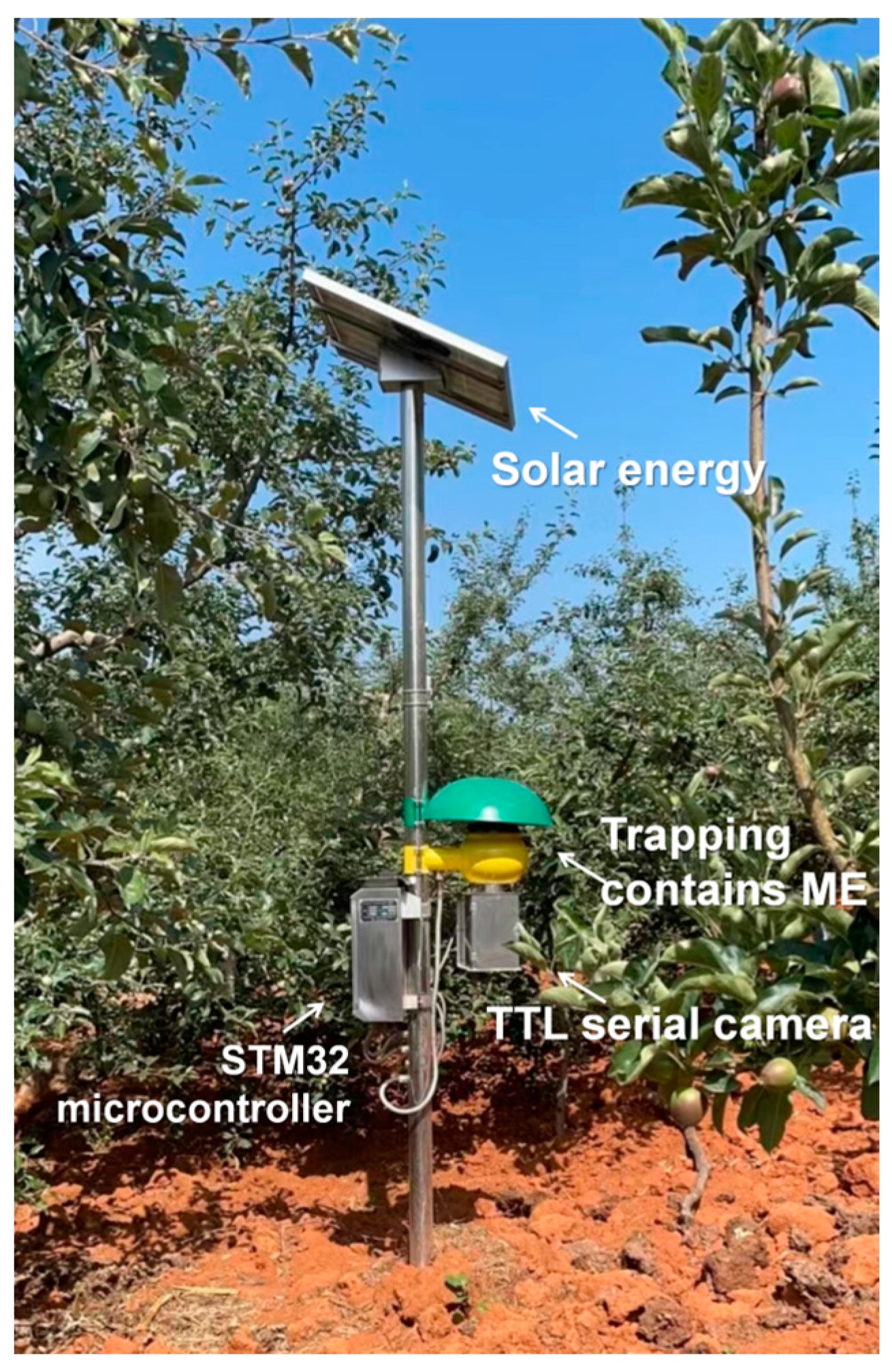
2.2. Monitoring the Population Dynamics of B. dorsalis
2.3. Data Analysis
3. Results
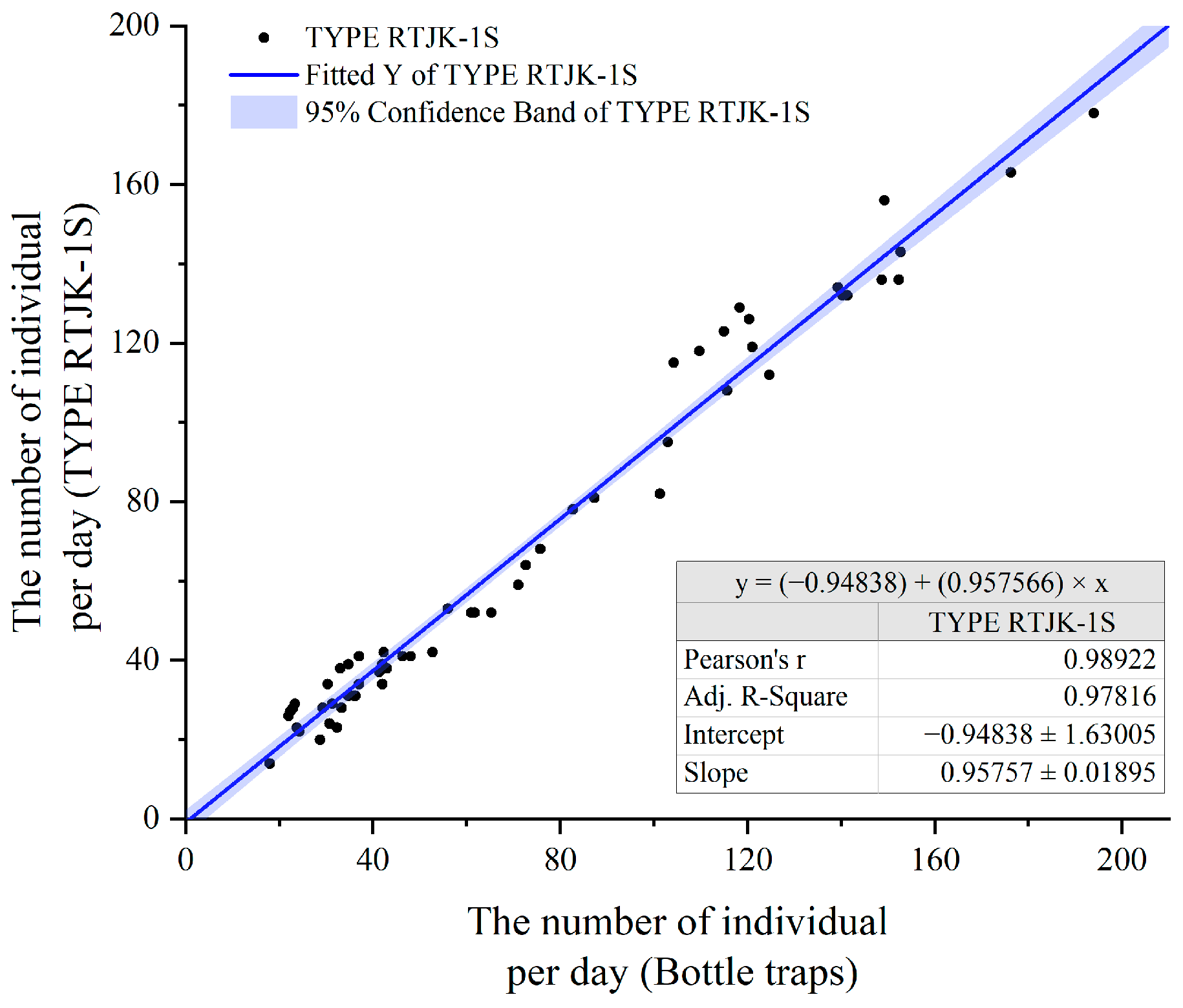
3.1. Accuracy Assessment of Automated Monitoring System
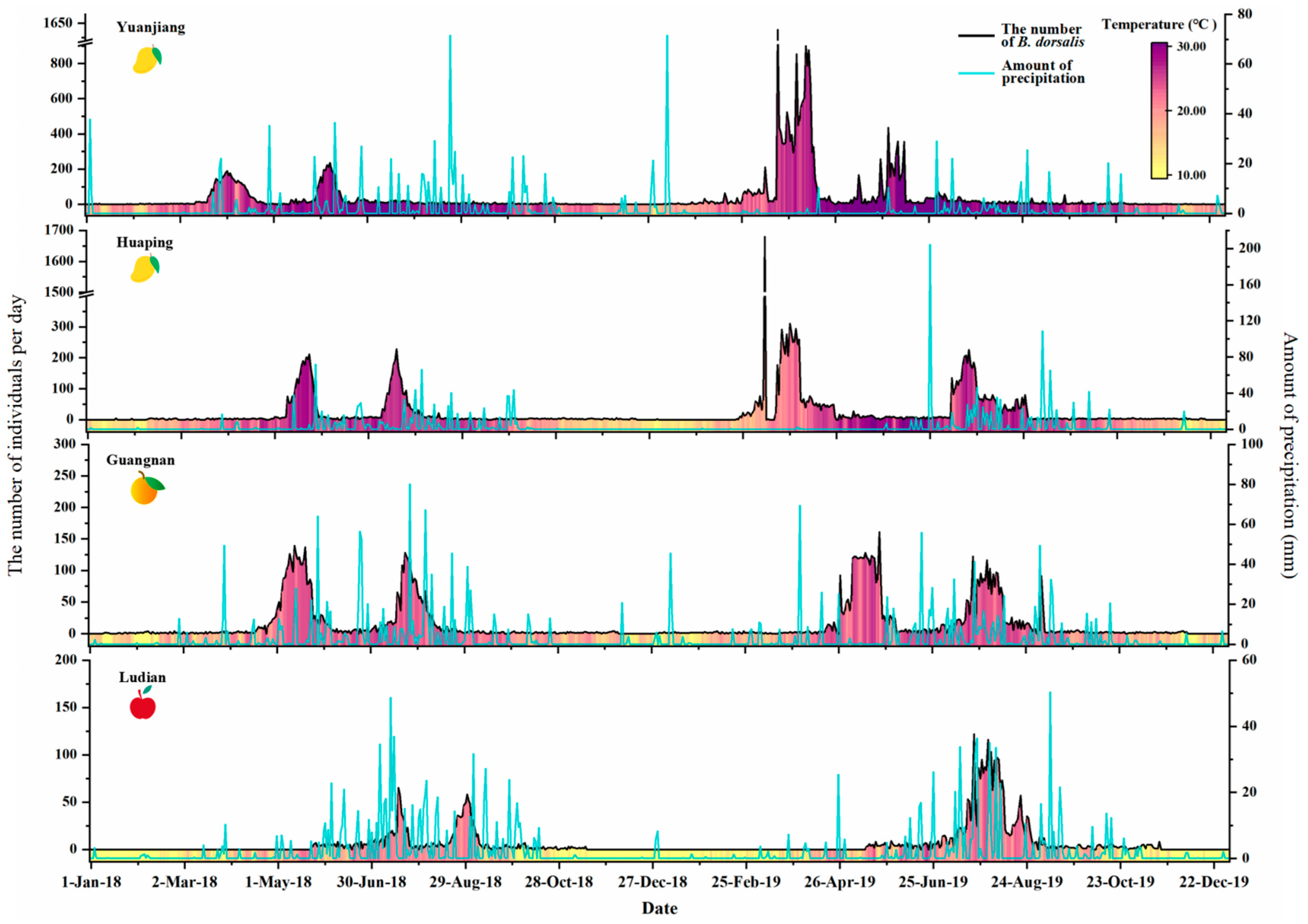
3.2. Bactrocera dorsalis Occurrence Dynamics in Four Counties
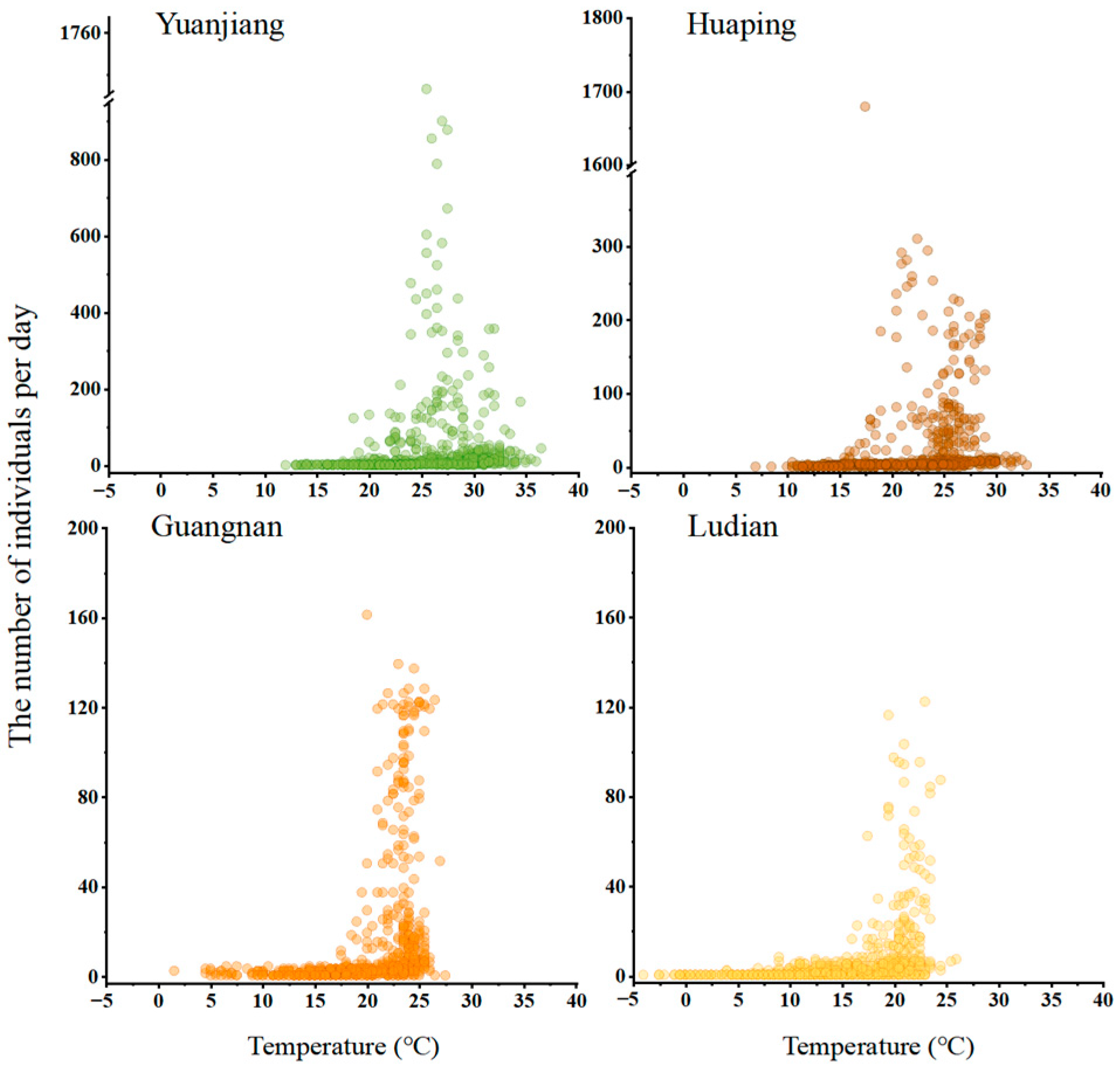
3.3. Correlation between Meteorological Factors and Population Dynamics of B. dorsalis
4. Discussion
4.1. Overview of B. dorsalis Population Dynamics in Yunnan
4.2. Impact of Environmental Factors on B. dorsalis Population Dynamics
4.3. Regional Recommendations for B. dorsalis Management
4.4. Automated Electronic Monitoring Devices in Pest Monitoring: Current Applications and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Follett, P.A.; Pinero, J.; Souder, S.; Jamieson, L.; Waddell, B.; Wall, M. Host status of ‘Scifresh’ apples to the invasive fruit fly species Bactrocera dorsalis, Zeugodacus cucurbitae, and Ceratitis capitata (Diptera: Tephritidae). J. Asia-Pac. Entomol. 2019, 22, 458–470. [Google Scholar] [CrossRef]
- Abro, Z.A.; Baloch, N.; Memon, R.M.; Khuhro, N.H.; Shaikh, I. Population fluctuations of Bactrocera species (Diptera: Tephritidae) in guava and mango orchards at different climatic conditions of Sindh. PJZ 2022, 55, 37–42. [Google Scholar] [CrossRef]
- Hoskins, J.L.; Rempoulakis, P.; Stevens, M.M.; Dominiak, B.C. Biosecurity and management strategies for economically important exotic Tephritid fruit fly species in Australia. Insects 2023, 14, 801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, G.; Xu, P.; Gao, B.; Liu, X.; Qi, X.; Zhang, G.; Cao, S.; Li, Z.; Ren, X.; et al. Behavioral and genomic divergence between a generalist and a specialist fly. Cell Rep. 2022, 41, 111654. [Google Scholar] [CrossRef]
- Ansari, M.S.; Hasan, F.; Ahmad, N. Threats to fruit and vegetable crops: Fruit flies (Tephritidae)—Ecology, behaviour, and management. J. Crop Sci. Biotechnol. 2012, 15, 169–188. [Google Scholar] [CrossRef]
- Schutze, M.K.; Virgilio, M.; Norrbom, A.; Clarke, A.R. Tephritid integrative taxonomy: Where we are now, with a focus on the resolution of three tropical fruit fly species complexes. Annu. Rev. Entomol. 2017, 62, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef]
- Dongmo, M.A.K.; Fiaboe, K.K.M.; Kekeunou, S.; Nanga, S.N.; Kuate, A.F.; Tonnang, H.E.Z.; Gnanvossou, D.; Hanna, R. Temperature-based phenology model to predict the development, survival, and reproduction of the oriental fruit fly Bactrocera dorsalis. J. Therm. Biol. 2021, 97, 102877. [Google Scholar] [CrossRef]
- Zeng, Y.; Reddy, G.V.P.; Li, Z.; Qin, Y.; Wang, Y.; Pan, X.; Jiang, F.; Gao, F.; Zhao, Z.H. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2019, 143, 165–176. [Google Scholar] [CrossRef]
- Manrakhan, A. Bactrocera dorsalis (Oriental Fruit Fly). CABI Compendium 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.17685 (accessed on 13 August 2024).
- Qu, H.X.; Sun, J.S. Observation of the living habit of Bactrocera dorsalis. Chin. Hortic. Abstr. 2013, 29, 51–62. (In Chinese) [Google Scholar]
- Rot, M.; Persolja, J.; Bohinc, T.; Zezlina, I.; Trdan, S. Seasonal dynamics of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), in apple orchards of western Slovenia using two trap types. Agriculture 2023, 13, 1500. [Google Scholar] [CrossRef]
- Abro, Z.U.A.; Baloch, N.; Memon, R.M.; Khuhro, N.H. Population fluctuation of Bactrocera zonata and Bactrocera dorsalis in guava orchard agro-ecosystem in Sindh region. Pak. J. Zool. 2021, 53, 1969–1972. [Google Scholar] [CrossRef]
- Awarikabey, E.N.; Afun, J.V.K.; Osekre, E.A.; Billah, M.K. Mango phenology and fruit fly population dynamics in the transition zone of Ghana. Bull. Entomol. Res. 2023, 113, 169–179. [Google Scholar] [CrossRef]
- Soares, G.K.A.; Fidelis, E.G.; Farias, E.S.; Rodrigues, G.S.; Paes, J.L.A. Range expansion and population dynamics of Bactrocera carambolae in Roraima, Brazil. Crop Prot. 2023, 165, 106167. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.-A. Automated surveillance of fruit Flies. Sensors 2017, 17, 110. [Google Scholar] [CrossRef]
- Sandrini Moraes, F.; Edson Nava, D.; Scheunemann, T.; Santos da Rosa, V. Development of an optoelectronic sensor for detecting and classifying fruit fly (Diptera: Tephritidae) for use in real-time intelligent traps. Sensors 2019, 19, 1254. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.A.; Lin, T.S.; Yang, E.C.; Tseng, C.L.; Chen, C.P.; Yen, C.W.; Zheng, X.Y.; Liu, C.Y.; Liu, R.H.; Chen, Y.F. Application of a web-based remote agro-ecological monitoring system for observing spatial distribution and dynamics of Bactrocera dorsalis in fruit orchards. Precis. Agric. 2013, 14, 323–342. [Google Scholar] [CrossRef]
- Liu, L.; Xie, C.; Wang, R.; Yang, P.; Sudirman, S.; Zhang, J.; Li, R.; Wang, F. Deep learning based automatic multiclass wild pest monitoring approach using hybrid global and local activated features. IEEE Trans. Ind. Inform. 2021, 17, 7589–7598. [Google Scholar] [CrossRef]
- Albanese, A.; Nardello, M.; Brunelli, D. Automated pest detection with DNN on the edge for precision agriculture. IEEE J. Emerg. Sel. Top. Circuits 2021, 11, 458–467. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X. Plant diseases and pests detection based on deep learning: A review. Plant Methods 2021, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Choudhury, P.; Pati, B. Modified ride-NN optimizer for the IoT based plant disease detection. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 691–703. [Google Scholar] [CrossRef]
- Wang, S.; Qi, P.; Zhang, W.; He, X. Development and application of an intelligent plant protection monitoring system. Agronomy 2022, 12, 1046. [Google Scholar] [CrossRef]
- Shaked, B.; Amore, A.; Ioannou, C.; Valdés, F.; Alorda, B.; Papanastasiou, S.; Goldshtein, E.; Shenderey, C.; Leza, M.; Pontikakos, C.; et al. Electronic traps for detection and population monitoring of adult fruit flies (Diptera: Tephritidae). J. Appl. Entomol. 2018, 142, 43–51. [Google Scholar] [CrossRef]
- Shi, W.; Kerdelhue, C.; Ye, H. Population genetics of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), in Yunnan (China) based on mitochondrial DNA sequences. Environ. Entomol. 2005, 34, 977–983. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Ma, D.Y.; Liu, W.X.; Wang, Y.S.; Fu, W.J.; Gao, Y.H.; Wan, F.H. The arrival of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), in China. J. Biosaf. 2019, 28, 200–203. (In Chinese) [Google Scholar] [CrossRef]
- Cang, X.Y.; Li, W.F.; Ying, J.; Shan, H.L.; Wang, C.M.; Wang, X.Y.; Li, J.; Zhang, Y.R.; Huang, Y.K. Prevention and control measures of the occurrence of Ceracris kiangsu Tsai in sugarcane areas of Yunnan Province. Sugarcane Canesugar. 2020, 49, 49–51. (In Chinese) [Google Scholar]
- Hui, Y.E. Distribution of the oriental fruit fly (Diptera: Tephritidae) in Yunnan Province. Insect Sci. 2010, 8, 175–182. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 26 July 2024).
- Hijmans, R.J.; Barbosa, M.; Ghosh, A.; Mandel, A. _geodata: Download Geographic Data. R Package Version 0.6-2. 2024. Available online: https://CRAN.R-project.org/package=geodata (accessed on 26 July 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- OriginLab Corporation. Origin Version 2021; OriginLab Corporation: Northampton, MA, USA, 2021. [Google Scholar]
- Wei, T.; Viliam, S. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 30 July 2024).
- Kassambara, A.; Mundt, F. _Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 30 July 2024).
- Cai, P.; Song, Y.; Meng, L.; Lin, J.; Zhao, M.; Wu, Q.; Nie, C.; Li, Y.; Ji, Q. Phenological responses of Bactrocera dorsalis (Hendel) to climate warming in china based on long-term historical data. Int. J. Trop. Insect Sci. 2023, 43, 881–894. [Google Scholar] [CrossRef]
- Jackson, C.G.; Long, J.P.; Klungness, L.M. Depth of pupation in four species of fruit flies (Diptera: Tephritidae) in sand with and without moisture. J. Econ. Entomol. 1998, 91, 138–142. [Google Scholar] [CrossRef]
- Huang, Y.B.K.; Chi, H. Fitness of Bactrocera dorsalis (Hendel) on seven host plants and an artificial diet. Turk. J. Entomol. 2014, 38, 401. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, F.; Tan, X.; Zhang, T.; Teng, Z.; Fan, Y.; Wan, F.; Zhou, H. Use of age-stage, two-sex life table to compare the fitness of Bactrocera dorsalis (Diptera: Tephritidae) on northern and southern host fruits in China. Insects 2022, 13, 258. [Google Scholar] [CrossRef]
- Cornelius, M.L.; Duan, J.J.; Messing, R.H. Visual stimuli and the response of female oriental fruit flies (Diptera: Tephritidae) to fruit-mimicking traps. J. Econ. Entomol. 1999, 92, 121–129. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.; Yao, Z.; Awan, U.A.; Zhang, Z. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef] [PubMed]
- Verghese, A.; Tandon, P.L.; Stonehouse, J.M. Economic evaluation of the integrated management of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae) in mango in India. Crop Prot. 2004, 23, 61–63. [Google Scholar] [CrossRef]
- Lima, M.; Leandro, M.E.; Pereira, L.; Valero, C.; Gonçalves Bazzo, C. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Wang, F.; Wang, R.; Xie, C.; Zhang, J.; Liu, L. Convolutional neural network based automatic pest monitoring system using hand-held mobile image analysis towards non-site-specific wild environment. Compu. Electron. Agric. 2021, 187, 106268. [Google Scholar] [CrossRef]
- Xiao, D.Q.; Yang, Q.M.; Fu, J.Q.; Deng, X.H.; Feng, J.Z.; Ye, Y.W.; Lu, Y.Y. A multi-target trapping and tracking algorithm for Bactrocera dorsalis based on cost model. Comput. Electron. Agric. 2016, 123, 224–231. [Google Scholar] [CrossRef]
- Diller, Y.; Shamsian, A.; Shaked, B.; Altman, Y.; Danziger, B.-C.; Manrakhan, A.; Serfontein, L.; Bali, E.; Wernicke, M.; Egartner, A.; et al. A real-time remote surveillance system for fruit flies of economic importance: Sensitivity and image analysis. J. Pest Sci. 2023, 96, 611–622. [Google Scholar] [CrossRef]
- Huang, R.; Yao, T.; Zhan, C.; Zhang, G.; Zheng, Y. A Motor-driven and computer vision-based intelligent E-trap for monitoring citrus flies. Agriculture 2021, 11, 460. [Google Scholar] [CrossRef]



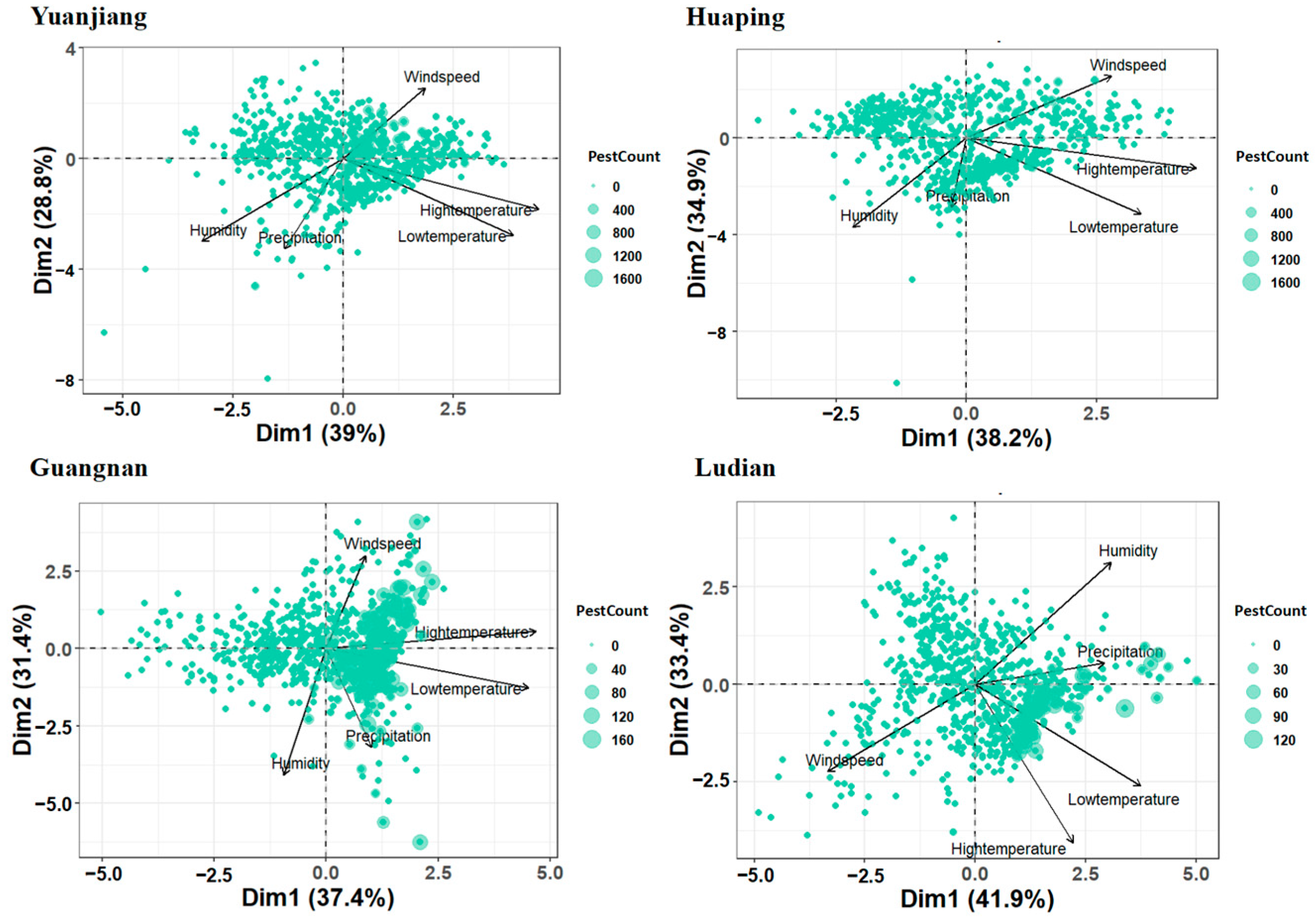

| Principal Components | Yuanjiang County | Huaping County | Guangnan County | Ludian County | ||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | |
| Eigen values | 1.949 | 1.438 | 1.910 | 1.746 | 1.872 | 1.570 | 2.096 | 1.669 |
| Factor loadings | ||||||||
| Low temperature | 0.546 | −0.454 | 0.510 | −0.498 | 0.673 | −0.208 | 0.540 | −0.420 |
| High temperature | 0.626 | −0.298 | 0.670 | −0.197 | 0.698 | 0.089 | 0.319 | −0.655 |
| Precipitation | −0.189 | −0.535 | −0.044 | −0.454 | 0.152 | −0.520 | 0.421 | 0.088 |
| Wind speed | 0.263 | 0.422 | 0.424 | 0.407 | 0.131 | 0.488 | −0.482 | −0.361 |
| Humidity | −0.453 | −0.490 | −0.332 | −0.584 | −0.141 | −0.663 | 0.444 | 0.506 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Y.; Liang, Y.; Qi, Y.; Lu, Y.; Ma, J. Population Dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in Four Counties of Yunnan, China, by Electronic Monitoring System. Insects 2024, 15, 621. https://doi.org/10.3390/insects15080621
Li Z, Li Y, Liang Y, Qi Y, Lu Y, Ma J. Population Dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in Four Counties of Yunnan, China, by Electronic Monitoring System. Insects. 2024; 15(8):621. https://doi.org/10.3390/insects15080621
Chicago/Turabian StyleLi, Ziyuan, Yan Li, Yuling Liang, Yixiang Qi, Yongyue Lu, and Jiao Ma. 2024. "Population Dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in Four Counties of Yunnan, China, by Electronic Monitoring System" Insects 15, no. 8: 621. https://doi.org/10.3390/insects15080621
APA StyleLi, Z., Li, Y., Liang, Y., Qi, Y., Lu, Y., & Ma, J. (2024). Population Dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in Four Counties of Yunnan, China, by Electronic Monitoring System. Insects, 15(8), 621. https://doi.org/10.3390/insects15080621






