Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insect
2.2. Identification of LdKnk-Family Genes
2.3. Bioinformatics and Phylogenetic Analysis
2.4. Expression Analysis of LdKnk-Family Genes
2.5. Preparation of dsRNA Using Bacterial Expression
2.6. Dietary dsRNA Bioassays
2.7. Transmission Electron Microscopy (TEM) Analysis
2.8. Data Analysis
3. Results
3.1. Identification and Characterization of LdKnk-Family Genes in L.decemlineata
3.2. Spatio-Temporal Expression Patterns of LdKnk-Family Genes
3.3. Effects of Silencing LdKnk Genes on Larval Development and Epidermis Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Guo, X.; Tian, S.; Yang, Q.; Kim, M.; Mun, S.; Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. AA15 lytic polysaccharide monooxygenase is required for efficient chitinous cuticle turnover during insect molting. Commun. Biol. 2022, 5, 518. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. A Life’s Journey Through Insect Metamorphosis. Annu. Rev. Entomol. 2020, 65, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, X.; Li, H.; Lan, Q.; Wu, H.; Wang, Y.; Zhang, J.; Zhao, X. Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria. Insects 2024, 15, 237. [Google Scholar] [CrossRef]
- Yang, W.J.; Xu, K.K.; Yan, Y.; Li, C.; Jin, D.C. Role of Chitin Deacetylase 1 in the Molting and Metamorphosis of the Cigarette Beetle Lasioderma serricorne. Int. J. Mol. Sci. 2020, 21, 2449. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Noh, M.Y.; Moussian, B.; Specht, C.A.; Kramer, K.J.; Beeman, R.W.; Arakane, Y.; Muthukrishnan, S. Knickkopf and retroactive proteins are required for formation of laminar serosal procuticle during embryonic development of Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 60, 1–6. [Google Scholar] [CrossRef]
- Tonning, A.; Helms, S.; Schwarz, H.; Uv, A.E.; Moussian, B. Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development 2006, 133, 331–341. [Google Scholar] [CrossRef]
- Moussian, B.; Tang, E.; Tonning, A.; Helms, S.; Schwarz, H.; Nusslein-Volhard, C.; Uv, A.E. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 2006, 133, 163–171. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle formation and pigmentation in beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef]
- Mun, S.; Noh, M.Y.; Geisbrecht, E.R.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. Chitin deacetylases are necessary for insect femur muscle attachment and mobility. Proc. Natl. Acad. Sci. USA 2022, 119, e2120853119. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Shi, J.F.; Fu, J.; Mu, L.L.; Guo, W.C.; Li, G.Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylases are specialized for chitin synthesis in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochem. Mol. Biol. 2016, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet. 2018, 14, e1007307. [Google Scholar] [CrossRef]
- Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Kanost, M.R.; Beeman, R.W.; Arakane, Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem. Mol. Biol. 2014, 53, 22–29. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet. 2015, 11, e1004963. [Google Scholar] [CrossRef]
- Mun, S.; Noh, M.Y.; Dittmer, N.T.; Muthukrishnan, S.; Kramer, K.J.; Kanost, M.R.; Arakane, Y. Cuticular protein with a low complexity sequence becomes cross-linked during insect cuticle sclerotization and is required for the adult molt. Sci. Rep. 2015, 5, 10484. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yi, L.; Lu, Z. Silencing of Chitin-Binding Protein with PYPV-Rich Domain Impairs Cuticle and Wing Development in the Asian Citrus Psyllid, Diaphorina citri. Insects 2022, 13, 353. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Yang, Q. Chitin Organizing and Modifying Enzymes and Proteins Involved In Remodeling of the Insect Cuticle. Adv. Exp. Med. Biol. 2019, 1142, 83–114. [Google Scholar] [CrossRef]
- Yu, R.R.; Duan, J.Q.; Zhao, X.M.; Abbas, M.; Zhang, Y.P.; Shi, X.K.; Chen, N.; Zhang, J.Z. Knickkopf (LmKnk) is required for chitin organization in the foregut of Locusta migratoria. Insect Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, X.; Zuo, Y.; Liu, W.; Zhang, J.; Moussian, B. Timed Knickkopf function is essential for wing cuticle formation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2017, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.P.; Lubarsky, B.; Shaw, K.; Luschnig, S.; Messina, L.; Krasnow, M.A. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 17014–17019. [Google Scholar] [CrossRef] [PubMed]
- Shaik, K.S.; Wang, Y.; Aravind, L.; Moussian, B. The Knickkopf DOMON domain is essential for cuticle differentiation in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2014, 86, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pesch, Y.Y.; Riedel, D.; Behr, M. Obstructor A organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J. Biol. Chem. 2015, 290, 10071–10082. [Google Scholar] [CrossRef]
- Petkau, G.; Wingen, C.; Jussen, L.C.; Radtke, T.; Behr, M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J. Biol. Chem. 2012, 287, 21396–21405. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Moussian, B.; Specht, C.A.; Arakane, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Functional specialization among members of Knickkopf family of proteins in insect cuticle organization. PLoS Genet. 2014, 10, e1004537. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Retroactive maintains cuticle integrity by promoting the trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 2013, 9, e1003268. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef]
- Yu, R.R.; Zhang, R.; Liu, W.M.; Zhao, X.M.; Zhu, K.Y.; Moussian, B.; Zhang, J.Z. The DOMON domain protein LmKnk contributes to correct chitin content, pore canal formation and lipid deposition in the cuticle of Locusta migratoria during moulting. Insect Mol. Biol. 2022, 31, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, X.; Liu, X.; Zhang, X.; Yu, R.; Ma, E.; Moussian, B.; Zhu, K.; Zhang, J. Effect of RNAi-mediated silencing of two Knickkopf family genes (LmKnk2 and LmKnk3) on cuticle formation and insecticide susceptibility in Locusta migratoria. Pest Manag. Sci. 2020, 76, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Pelissie, B.; Chen, Y.H.; Cohen, Z.P.; Crossley, M.S.; Hawthorne, D.J.; Izzo, V.; Schoville, S.D. Genome Resequencing Reveals Rapid, Repeated Evolution in the Colorado Potato Beetle. Mol. Biol. Evol. 2022, 39, msac016. [Google Scholar] [CrossRef]
- Ma, M.Q.; He, W.W.; Xu, S.J.; Xu, L.T.; Zhang, J. RNA interference in Colorado potato beetle (Leptinotarsa decemlineata): A potential strategy for pest control. J. Integr. Agric. 2020, 19, 428–437. [Google Scholar] [CrossRef]
- Szendrei, Z.; Grafius, E.; Byrne, A.; Ziegler, A. Resistance to neonicotinoid insecticides in field populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2012, 68, 941–946. [Google Scholar] [CrossRef]
- Scott, I.M.; Tolman, J.H.; MacArthur, D.C. Insecticide resistance and cross-resistance development in Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) populations in Canada 2008–2011. Pest Manag. Sci. 2015, 71, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Kadoic Balasko, M.; Mikac, K.M.; Bazok, R.; Lemic, D. Modern Techniques in Colorado Potato Beetle (Leptinotarsa decemlineata Say) Control and Resistance Management: History Review and Future Perspectives. Insects 2020, 11, 581. [Google Scholar] [CrossRef]
- Shi, J.F.; Cheng, M.H.; Zhou, W.; Zeng, M.Z.; Chen, Y.; Yang, J.X.; Wu, H.; Ye, Q.H.; Tang, H.; Zhang, Q.; et al. Crucial roles of specialized chitinases in elytral and hindwing cuticles construction in Leptinotarsa decemlineata. Pest Manag. Sci. 2024, 80, 4437–4449. [Google Scholar] [CrossRef]
- Shi, X.Q.; Guo, W.C.; Wan, P.J.; Zhou, L.T.; Ren, X.L.; Ahmat, T.; Fu, K.Y.; Li, G.Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res. Notes 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Zhou, W.; Li, C.; Chen, Y.N.; Zhang, Q.; Fu, K.Y.; Guo, W.C.; Shi, J.F. Identification of chitinase genes and roles in the larval-pupal transition of Leptinotarsa decemlineata. Pest Manag. Sci. 2024, 80, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Schoville, S.D.; Chen, Y.H.; Andersson, M.N.; Benoit, J.B.; Bhandari, A.; Bowsher, J.H.; Brevik, K.; Cappelle, K.; Chen, M.M.; Childers, A.K.; et al. A model species for agricultural pest genomics: The genome of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Sci. Rep. 2018, 8, 1931. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, C.; Zhang, M.; Zhou, H.; Zuo, Z.; Ding, X.; Zhang, R.; Li, F.; Gao, Y. Chromosome-level genome assembly of the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Data 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Congiu, L.; Lindstrom, L.; Piiroinen, S.; Vidotto, M.; Grapputo, A. Sequencing, De Novo assembly and annotation of the Colorado Potato Beetle, Leptinotarsa decemlineata, Transcriptome. PLoS ONE 2014, 9, e86012. [Google Scholar] [CrossRef]
- De Giorgio, E.; Giannios, P.; Espinas, M.L.; Llimargas, M. A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023, 21, e3001978. [Google Scholar] [CrossRef]
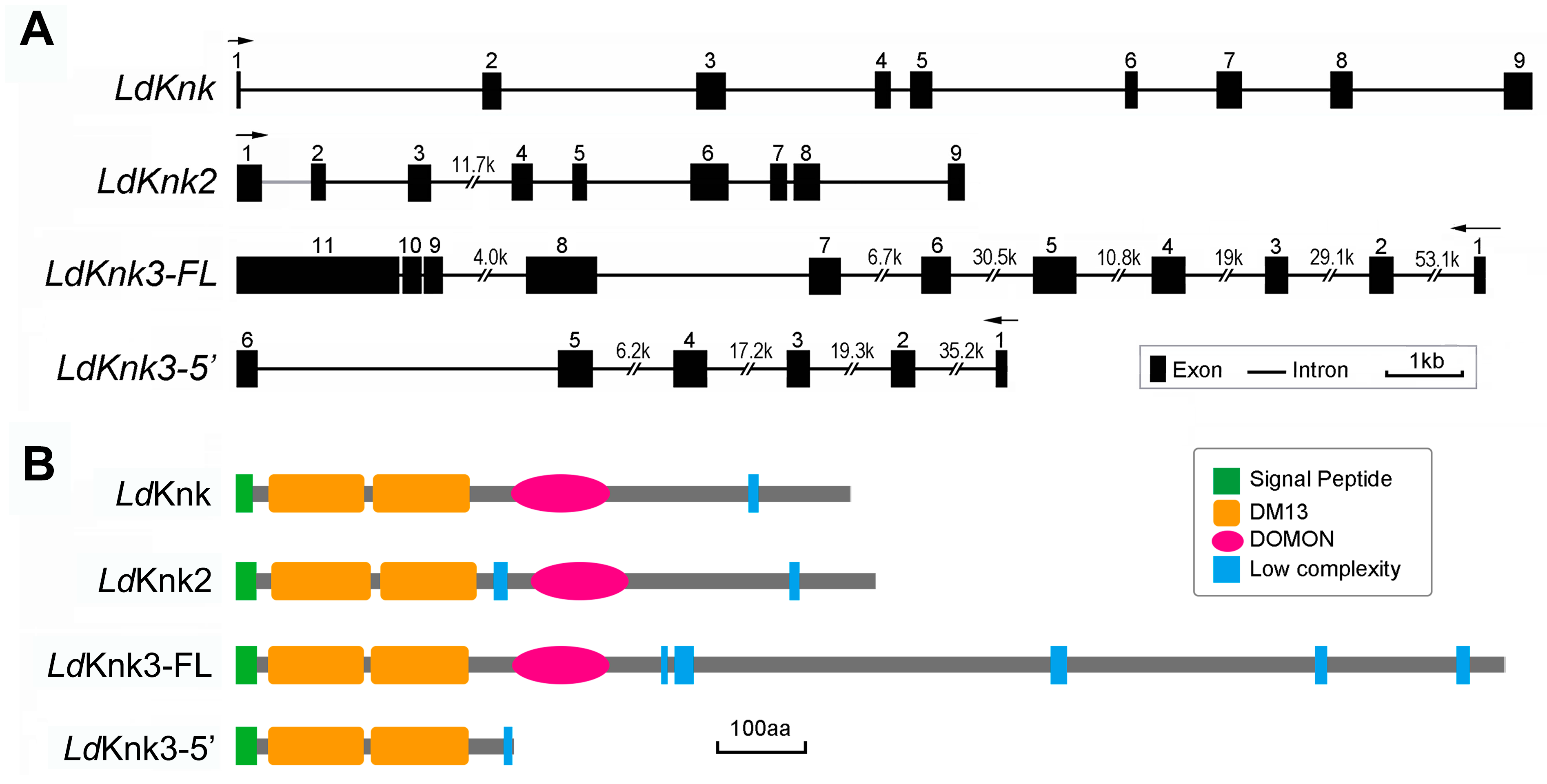


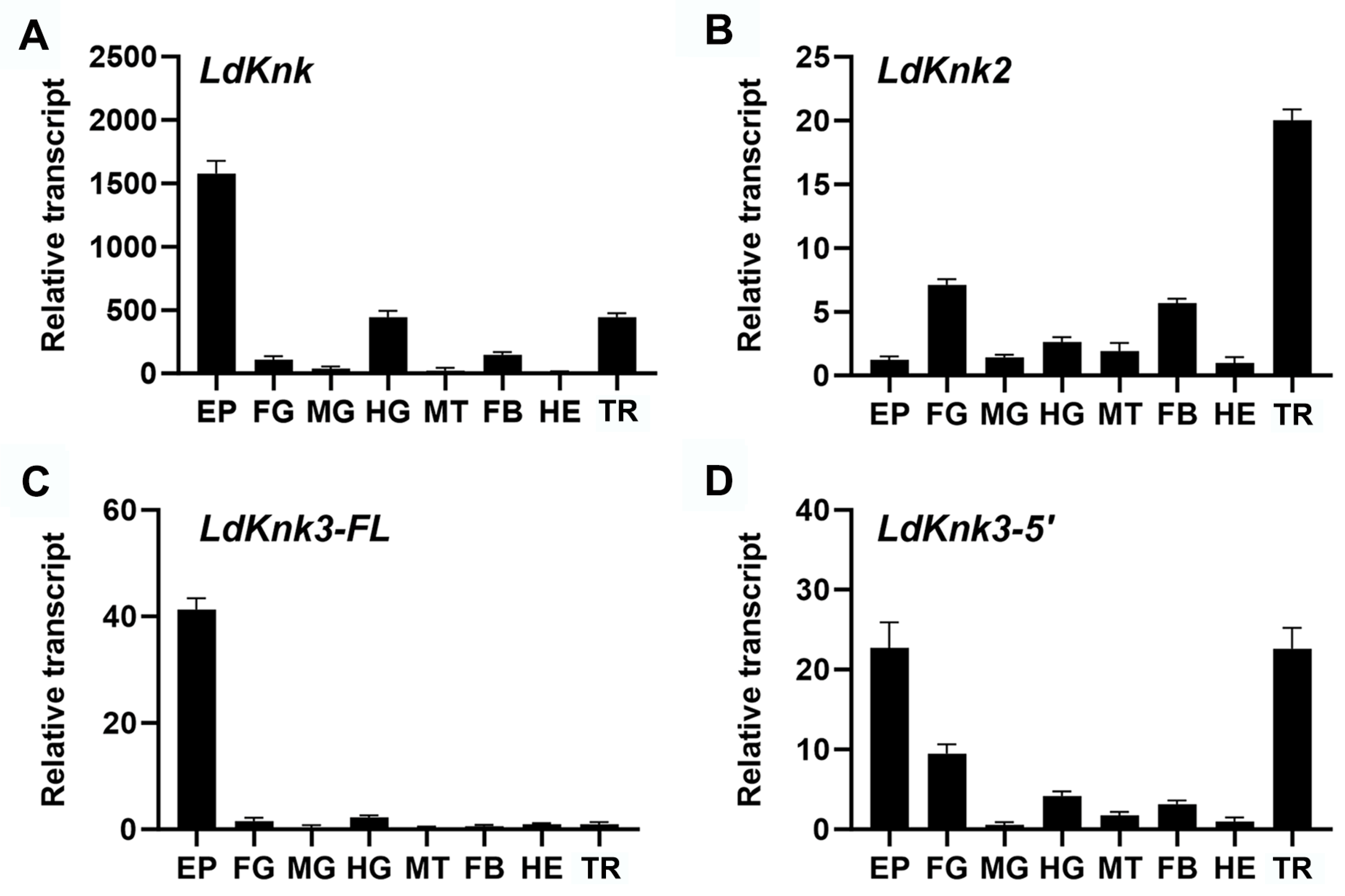
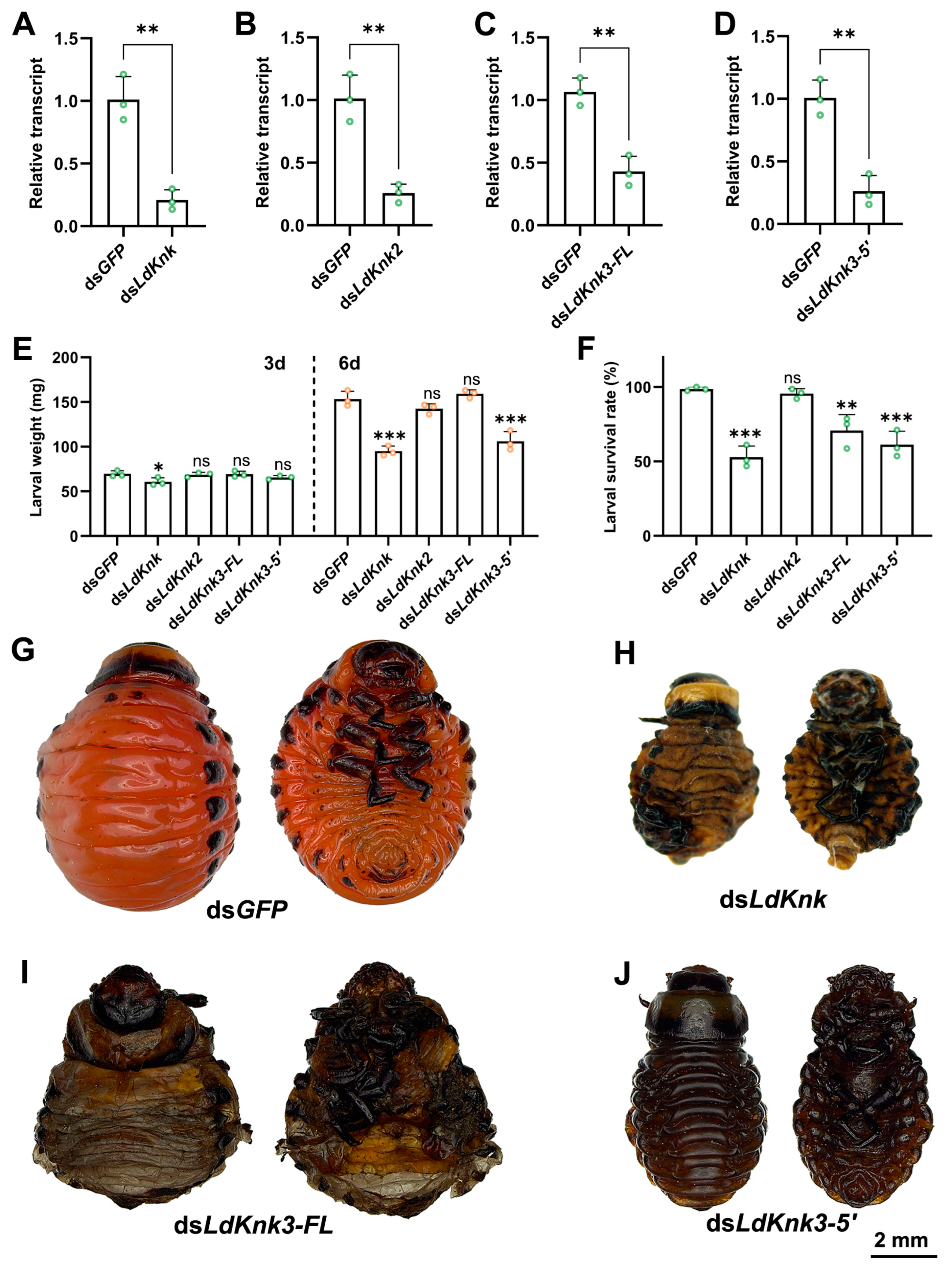

| Gene Name | Accession Number | cDNA (bp) | Coding Region | 5′-UTR (bp) | 3′-UTR (bp) | aa | pl | Mw(kDa) |
|---|---|---|---|---|---|---|---|---|
| LdKnk | PP962378 | 2118 | 61–2088 | 60 | 30 | 675 | 6.35 | 76.03 |
| LdKnk2 | PP962379 | 2142 | 1–2109 | 0 | 33 | 702 | 5.09 | 78.90 |
| LdKnk3-FL | PP962380 | 5100 | 49–4236 | 48 | 864 | 1395 | 7.41 | 156.48 |
| LdKnk3-5′ | PP962381 | 1042 | 61–975 | 60 | 67 | 304 | 7.65 | 33.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.-Z.; Zhou, W.; Wen, S.-S.; Wu, H.; Zhang, Q.; Fu, K.-Y.; Guo, W.-C.; Shi, J.-F. Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata. Insects 2024, 15, 623. https://doi.org/10.3390/insects15080623
Zeng M-Z, Zhou W, Wen S-S, Wu H, Zhang Q, Fu K-Y, Guo W-C, Shi J-F. Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata. Insects. 2024; 15(8):623. https://doi.org/10.3390/insects15080623
Chicago/Turabian StyleZeng, Mu-Zi, Wei Zhou, Shan-Shan Wen, Hao Wu, Qing Zhang, Kai-Yun Fu, Wen-Chao Guo, and Ji-Feng Shi. 2024. "Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata" Insects 15, no. 8: 623. https://doi.org/10.3390/insects15080623






