The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. BSFL Incubation
2.2. Larvae Feeding
2.3. Component Analysis
2.3.1. Total Lipid Content Detection
2.3.2. Total Protein and Moisture Content Detection
2.3.3. Total Sugar Content Detection
2.3.4. Analysis of the Fatty Acid Composition of BSFL
2.3.5. Analysis of Amino Acid Composition of BSFL
2.4. Statistical Processing
3. Results
3.1. BSFL Growth Performance
3.2. Growth Kinetics of BSFL
3.3. Composition of BSFL and Feeding Substrates
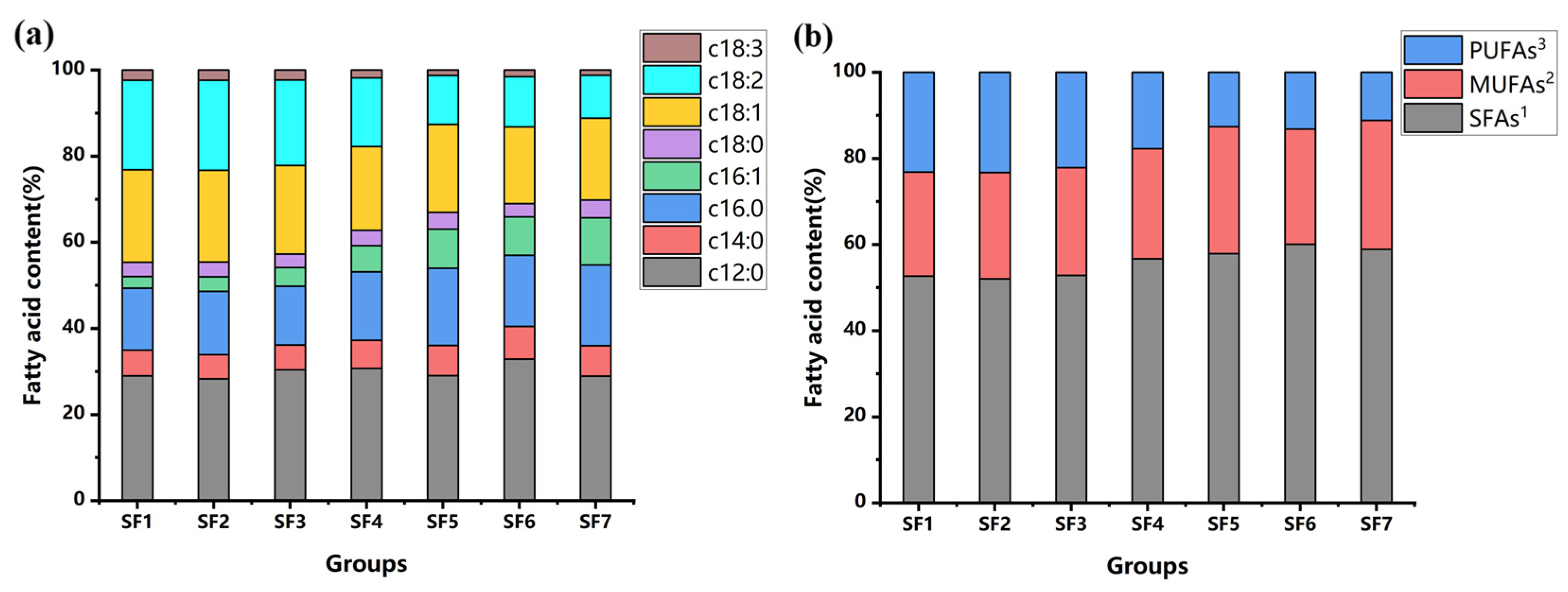
3.4. Fatty Acid Composition of BSFL
3.5. Amino Acid Content of BSFL
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivanchenko, O.; Khabibullin, R.; Le Huong, T.; Balanov, P.; Smotraeva, I. Toxicity Assessment of Meat-Processing Wastewater. In Proceedings of the E3S Web of Conferences, Prague, Czech Republic, 27–28 February 2020; Volume 161. [Google Scholar]
- Varelas, V. Food Wastes as a Potential New Source for Edible Insect Mass Production for Food and Feed: A Review. Fermentation 2019, 5, 81. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving Production of Volatile Fatty Acids from Food Waste Fermentation by Hydrothermal Pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Hua, P.U.; Yubing, B.A.I. Current Situation and Advices to Harmless Disposal of Animal Carcasses in China. ableSci 2014, 30, 2–5. [Google Scholar]
- Ockerman, H.W.; Hansenputri, C.L. Animal By-Product Processing & Utilization; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 2000; pp. 1–7. [Google Scholar]
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and Poultry Wastes: Management Practices, Feedstocks for Renewable Energy Production, and Recovery of Value Added Products. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef] [PubMed]
- Ovuru, K.F.; Izah, S.C.; Ogidi, O.I.; Imarhiagbe, O.; Ogwu, M.C. Slaughterhouse Facilities in Developing Nations: Sanitation and Hygiene Practices, Microbial Contaminants and Sustainable Management System. Food Sci. Biotechnol. 2024, 33, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Debsarkar, A.; Mukherjee, S. Treatment of Slaughter House Wastewater in a Sequencing Batch Reactor: Performance Evaluation and Biodegradation Kinetics. BioMed Res. Int. 2013, 2013, 134872. [Google Scholar] [CrossRef]
- Serajuddin, M. Emerging Technologies, Environment and Research for Sustainable Aquaculture; Books on Demand GmbH: Norderstedt, Germany, 2019; ISBN 9781838811990. [Google Scholar]
- Yu, Z.; Sun, Z.; Ou, B.; Zhou, M.; Huang, Y.; Tan, X. Effects of Partial Replacement of Fish Meal with Black Soldier Fly (Hermetia illucens) Larvae Meal on Growth Performance, Lipid Metabolism and Hepatointestinal Health of Juvenile Golden Pompano (Trachinotus ovatus). Aquac. Rep. 2023, 33, 101824. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H.; Chapman, S.J. Pretreatment Is an Important Method for Increasing the Conversion Efficiency of Rice Straw by Black Soldier Fly Larvae Based on the Function of Gut Microorganisms. Sci. Total Environ. 2021, 762, 144118. [Google Scholar] [CrossRef]
- Jiang, C.L.; Jin, W.Z.; Tao, X.H.; Zhang, Q.; Zhu, J.; Feng, S.Y.; Xu, X.H.; Li, H.Y.; Wang, Z.H.; Zhang, Z.J. Black Soldier Fly Larvae (Hermetia illucens) Strengthen the Metabolic Function of Food Waste Biodegradation by Gut Microbiome. Microb. Biotechnol. 2019, 12, 528–543. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and Characterization of a Novel Antibacterial Peptide from Black Soldier Fly (Hermetia illucens) Larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional Value of a Partially Defatted and a Highly Defatted Black Soldier Fly Larvae (Hermetia illucens L.) Meal for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Metabolizable Energy and Apparent Ileal Amino Acid Digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H. Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals 2019, 9, 349. [Google Scholar] [CrossRef]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the Potential of Lipids from Black Soldier Fly: New Paradigm for Biodiesel Production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Fliedner, A.; Schäfers, C. Determination of Lipid Content in Fish Samples from Bioaccumulation Studies: Contributions to the Revision of Guideline OECD 305. Environ. Sci. Eur. 2012, 24, 13. [Google Scholar] [CrossRef]
- Olson, B.J.S.C. Assays for Determination of Protein Concentration. Curr. Protoc. Pharmacol. 2016, 73, A.3A.1–A.3A.32. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of Monosaccharide Composition on Quantitative Analysis of Total Sugar Content by Phenol-Sulfuric Acid Method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef] [PubMed]
- Mihai, A.L.; Negoiță, M.; Belc, N. Evaluation of Fatty Acid Profile of Oils/Fats by GC-MS through Two Quantification Approaches. Rom. Biotechnol. Lett. 2019, 24, 973–985. [Google Scholar] [CrossRef]
- Kambhampati, S.; Li, J.; Evans, B.S.; Allen, D.K. Accurate and Efficient Amino Acid Analysis for Protein Quantification Using Hydrophilic Interaction Chromatography Coupled Tandem Mass Spectrometry. Plant Methods 2019, 15, 46. [Google Scholar] [CrossRef]
- Ornelas, A.; Delgado-Vences, F.; Morales-Bojórquez, E.; Cruz-Escalona, V.H.; Marín-Enríquez, E.; Hernández-Camacho, C.J. Modeling the Biological Growth with a Random Logistic Differential Equation. Environ. Ecol. Stat. 2023, 30, 233–260. [Google Scholar] [CrossRef]
- Fu, S.F.; Wang, D.H.; Xie, Z.; Zou, H.; Zheng, Y. Producing Insect Protein from Food Waste Digestate via Black Soldier Fly Larvae Cultivation: A Promising Choice for Digestate Disposal. Sci. Total Environ. 2022, 830, 154654. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Black Soldier Fly Larvae for Treatment and Segregation of Commingled Municipal Solid Waste at Different Environmental Conditions. J. Environ. Manag. 2022, 302, 114060. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Klammsteiner, T.; Dregulo, A.M.; Kumar, V.; Zhou, Y.; Zhang, Z.; Awasthi, M.K. Black Soldier Fly Larvae for Organic Manure Recycling and Its Potential for a Circular Bioeconomy: A Review. Sci. Total Environ. 2022, 833, 155122. [Google Scholar] [CrossRef] [PubMed]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Wangu, M.M.; Dubois, T.; Ekesi, S.; et al. Low-Cost Technology for Recycling Agro-Industrial Waste into Nutrient-Rich Organic Fertilizer Using Black Soldier Fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.P.; Calado, R.; Pinho, M.; Rosário Domingues, M.; Antonio Vázquez, J.; Ameixa, O.M.C.C. Bioconversion and Performance of Black Soldier Fly (Hermetia illucens) in the Recovery of Nutrients from Expired Fish Feeds. Waste Manag. 2022, 141, 183–193. [Google Scholar] [CrossRef]
- Peters, L.; Chikweto, A.; Mckibben, J.; Gibson, K. Potential for Scombroid Poisoning from Ingestion of Selar Crumenophthalmus Due to Increased Histamine Levels in Grenada, West Indies. J. Food Prot. 2021, 84, 368–371. [Google Scholar] [CrossRef]
- Paulsen, P.; Hagen, U.; Bauer, F. Changes in Biogenic Amine Contents, Non-Protein Nitrogen and Crude Protein during Curing and Thermal Processing of M. Longissimus, Pars Lumborum of Pork. Eur. Food Res. Technol. 2006, 223, 603–608. [Google Scholar] [CrossRef]
- Huang, J. Molecular Pharmacology and Physiology of Insect Biogenic Amine Receptors. ACS Symp. Ser. 2017, 1265, 127–138. [Google Scholar] [CrossRef]
- Rehman, K.U.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of Mixtures of Dairy Manure and Soybean Curd Residue by Black Soldier Fly Larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of Feedstock on Larval Development and Process Efficiency in Waste Treatment with Black Soldier Fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Kinasih, I.; Putra, R.E.; Permana, A.D.; Gusmara, F.F.; Nurhadi, M.Y.; Anitasari, R.A. Growth Performance of Black Soldier Fly Larvae (Hermetia illucens) Fed on Some Plant Based Organic Wastes. HAYATI J. Biosci. 2018, 25, 79–84. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens Larvae to Process Biowaste from Aquaculture Production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, P.; Zhang, G.; Wang, Y.; Yang, A. Degradation Properties of Protein and Carbohydrate during Sludge Anaerobic Digestion. Bioresour. Technol. 2015, 192, 126–130. [Google Scholar] [CrossRef]
- van Baak, M.A.; Mariman, E.C.M. Mechanisms of Weight Regain after Weight Loss—the Role of Adipose Tissue. Nat. Rev. Endocrinol. 2019, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Rabelink, T.J.; Giera, M. New Insights into Energy and Protein Homeostasis by the Kidney. Nat. Rev. Nephrol. 2019, 15, 596–598. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Nadia, M.H.; Syaripudin; Manurung, R.; Putra, R.E. Factors Affecting the Bioconversion of Philippine Tung Seed by Black Soldier Fly Larvae for the Production of Protein and Oil-Rich Biomass. J. Asia. Pac. Entomol. 2018, 21, 836–842. [Google Scholar] [CrossRef]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Hoc, B.; Genva, M.; Fauconnier, M.L.; Lognay, G.; Francis, F.; Caparros Megido, R. About Lipid Metabolism in Hermetia illucens (L. 1758): On the Origin of Fatty Acids in Prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as Food: Enrichment of Larvae of Hermetia illucens with Omega 3 Fatty Acids by Means of Dietary Modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Chen, X.; Jin, J.; Hou, F.; Song, B.; Li, Z.; Zhao, Y. Effects of Black Soldier Fly Larvae Oil on Growth Performance, Immunity and Antioxidant Capacity, and Intestinal Function and Microbiota of Broilers. J. Appl. Poult. Res. 2022, 31, 100292. [Google Scholar] [CrossRef]
- Couto, A.; Serra, C.R.; Guerreiro, I.; Coutinho, F.; Castro, C.; Rangel, F.; Lavrador, A.S.; Monteiro, M.; Santos, R.; Peres, H.; et al. Black Soldier Fly Meal Effects on Meagre Health Condition: Gut Morphology, Gut Microbiota and Humoral Immune Response. J. Insects Food Feed 2022, 8, 1281–1295. [Google Scholar] [CrossRef]
- Facey, H.; Kithama, M.; Mohammadigheisar, M.; Huber, L.A.; Shoveller, A.K.; Kiarie, E.G. Complete Replacement of Soybean Meal with Black Soldier Fly Larvae Meal in Feeding Program for Broiler Chickens from Placement through to 49 Days of Age Reduced Growth Performance and Altered Organs Morphology. Poult. Sci. 2023, 102, 102293. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Composition of Commercially Raised Invertebrates Used as Food for Insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, C.; Yan, Q.; Tan, Z.; Kang, J.; Tang, S. Elucidating the Underlying Mechanism of Amino Acids to Regulate Muscle Protein Synthesis: Effect on Human Health. Nutrition 2022, 103–104, 111797. [Google Scholar] [CrossRef] [PubMed]



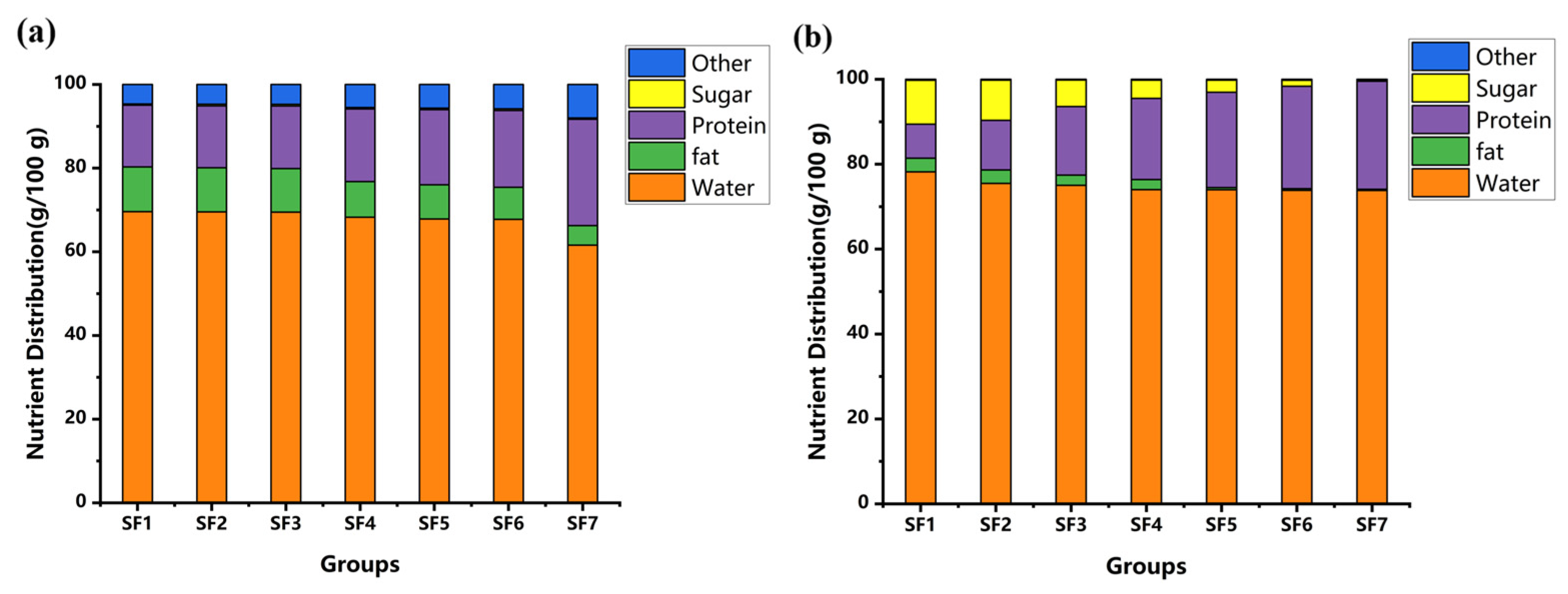

| Composition | Group Percentage Content (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | SF8 | SF9 | SF10 | SF11 | |
| Slaughter blood | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Food waste | 100 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | 10 | 0 |
| Groups | Xmax | μmax | R2 |
|---|---|---|---|
| SF1 | 102.06862 | 0.30613 | 91.33% |
| SF2 | 81.29864 | 0.32108 | 90.51% |
| SF3 | 75.74053 | 0.28956 | 89.84% |
| SF4 | 24.91285 | 0.68278 | 59.71% |
| SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | |
|---|---|---|---|---|---|---|---|
| c12:0 | 27.76 ± 2.83 a | 27.23 ± 1.19 a | 29.04 ± 2.54 a | 28.87 ± 0.9 a | 27.23 ± 0.05 a | 30.51 ± 0.37 a | 27.34 ± 0.47 a |
| c14:0 | 5.72 ± 0.17 d | 5.41 ± 0.11 e | 5.55 ± 0.26 d,e | 6.15 ± 0.04 c | 6.55 ± 0.04 b | 7.05 ± 0.09 a | 6.66 ± 0.21 b |
| c16.0 | 13.81 ± 0.58 d | 14.12 ± 0.16 d | 13.02 ± 0.63 e | 14.91 ± 0.32 c | 16.83 ± 0.1 b | 15.33 ± 0.1 c | 17.74 ± 0.07 a |
| c16:1 | 2.58 ± 0.08 g | 3.29 ± 0.03 f | 4.18 ± 0.16 e | 5.72 ± 0.17 d | 8.5 ± 0.05 b | 8.27 ± 0.16 c | 10.31 ± 0.11 a |
| c18:0 | 3.17 ± 0.16 d | 3.32 ± 0.08 c,d | 2.93 ± 0.18 e | 3.38 ± 0.06 c | 3.66 ± 0.02 b | 2.87 ± 0.05 e | 3.92 ± 0.04 a |
| c18:1 | 20.54 ± 1.04 a | 20.47 ± 0.49 a | 19.71 ± 1.07 a,b | 18.27 ± 0.22 c,d | 19.16 ± 0.17 b,c | 16.56 ± 0.26 e | 17.95 ± 0.4 d |
| c18:2 | 19.95 ± 1.01 a | 20.15 ± 0.47 a | 18.99 ± 1 b | 15.03 ± 0.17 c | 10.63 ± 0.1 d | 10.86 ± 0.16 d | 9.48 ± 0.14 e |
| c18:3 | 2.27 ± 0.12 a | 2.27 ± 0.06 a | 2.2 ± 0.12 a | 1.67 ± 0.02 b | 1.16 ± 0.02 e | 1.38 ± 0.02 d | 1.1 ± 0.02 e |
| SFAs 1 | 50.46 ± 2.28 c | 50.09 ± 1.07 c | 50.54 ± 2.25 c | 53.31 ± 0.5 b | 54.27 ± 0.15 a,b | 55.76 ± 0.38 a | 55.66 ± 0.68 a |
| MUFAs 2 | 23.12 ± 1.11 c | 23.76 ± 0.46 b,c | 23.89 ± 1.12 b,c | 23.99 ± 0.37 b,c | 27.67 ± 0.22 a | 24.84 ± 0.22 b | 28.25 ± 0.51 a |
| PUFAs 3 | 22.22 ± 1.13 a | 22.41 ± 0.53 a | 21.19 ± 1.11 b | 16.7 ± 0.19 c | 11.79 ± 0.12 d | 12.24 ± 0.18 d | 10.57 ± 0.17 e |
| SF1 | SF2 | SF3 | SF4 | SF5 | SF6 | SF7 | |
|---|---|---|---|---|---|---|---|
| Asp | 65.71 d | 76.88 b | 64.15 e | 76.77 b | 77.09 b | 75.93 c | 120.93 a |
| Thr | 34.91 f | 39.41 e | 35.18 f | 41.40 d | 42.19 c | 42.94 b | 65.07 a |
| Ser | 41.75 e | 44.18 d | 36.55 f | 48.15 c | 49.53 b | 49.80 b | 80.50 a |
| Glu | 82.72 f | 89.80 e | 65.53 g | 102.98 c | 109.02 b | 100.65 d | 179.21 a |
| Gly | 76.22 f | 84.04 e | 71.01 g | 94.01 d | 100.37 c | 108.45 b | 160.96 a |
| Ala | 111.44 f | 115.58 e | 94.49 g | 120.64 d | 128.33 c | 133.72 b | 190.68 a |
| Cys | 2.89 e | 3.03 e | 2.47 f | 3.72 d | 5.02 b | 4.06 c | 7.26 a |
| Val | 55.35 e | 62.94 d | 52.77 f | 69.34 c | 69.67 c | 71.53 b | 106.94 a |
| Met | 33.34 d | 32.15 e | 23.97 f | 35.38 c | 35.34 c | 37.62 b | 51.27 a |
| Ile | 35.44 e | 38.55 c,d | 30.95 f | 40.38 b | 38.28 d | 38.81 c | 57.54 a |
| Leu | 55.12 f | 61.99 e | 51.36 g | 66.27 d | 67.29 c | 68.26 b | 104.33 a |
| Tyr | 31.36 e | 32.73 c | 32.32 d | 34.93b | 33.01 c | 32.82 c | 48.78 a |
| Phe | 27.78 f | 31.01 e | 25.41 g | 31.58 d | 32.43 c | 33.17 b | 50.29 a |
| Lys | 51.04 f | 63.27 b | 50.04 g | 58.34 d | 56.05 e | 59.22 c | 83.46 a |
| His | 23.15 g | 32.48 d | 28.07 f | 32.05 e | 40.55 c | 42.95 b | 65.72 a |
| Arg | 29.55 f | 34.96 e | 26.16 g | 36.12 d | 37.67 b | 36.46 c | 65.74 a |
| Pro | 48.43 f | 50.48 e | 42.29 g | 57.88 d | 58.35 c | 62.92 b | 98.55 a |
| Total | 806.20 | 893.48 | 732.71 | 949.93 | 980.17 | 999.31 | 1537.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, H.; Qiao, Y.; Li, Y.; Wang, Z.; Zhao, L.; Li, Z.; Cheng, B.; Ding, G. The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood. Insects 2024, 15, 635. https://doi.org/10.3390/insects15090635
Bian H, Qiao Y, Li Y, Wang Z, Zhao L, Li Z, Cheng B, Ding G. The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood. Insects. 2024; 15(9):635. https://doi.org/10.3390/insects15090635
Chicago/Turabian StyleBian, Hao, Yuting Qiao, Yantong Li, Zifan Wang, Lei Zhao, Zhiqiang Li, Bo Cheng, and Gongtao Ding. 2024. "The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood" Insects 15, no. 9: 635. https://doi.org/10.3390/insects15090635
APA StyleBian, H., Qiao, Y., Li, Y., Wang, Z., Zhao, L., Li, Z., Cheng, B., & Ding, G. (2024). The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood. Insects, 15(9), 635. https://doi.org/10.3390/insects15090635







