Combined Toxic Effects of Lead and Glyphosate on Apis cerana cerana
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Breeding
2.2. Chemicals and Reagents
2.3. Acute Toxicity Test
2.4. Chronic Toxicity Test
2.5. Proboscis Extension Reflex (PER) Experiments on Worker Bees
2.6. Biochemical and Molecular Tests
2.6.1. Sample Preparation
2.6.2. Biochemical Tests
2.6.3. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Toxic Effects of Lead and GY in Isolation and Combination on A. c. cerana
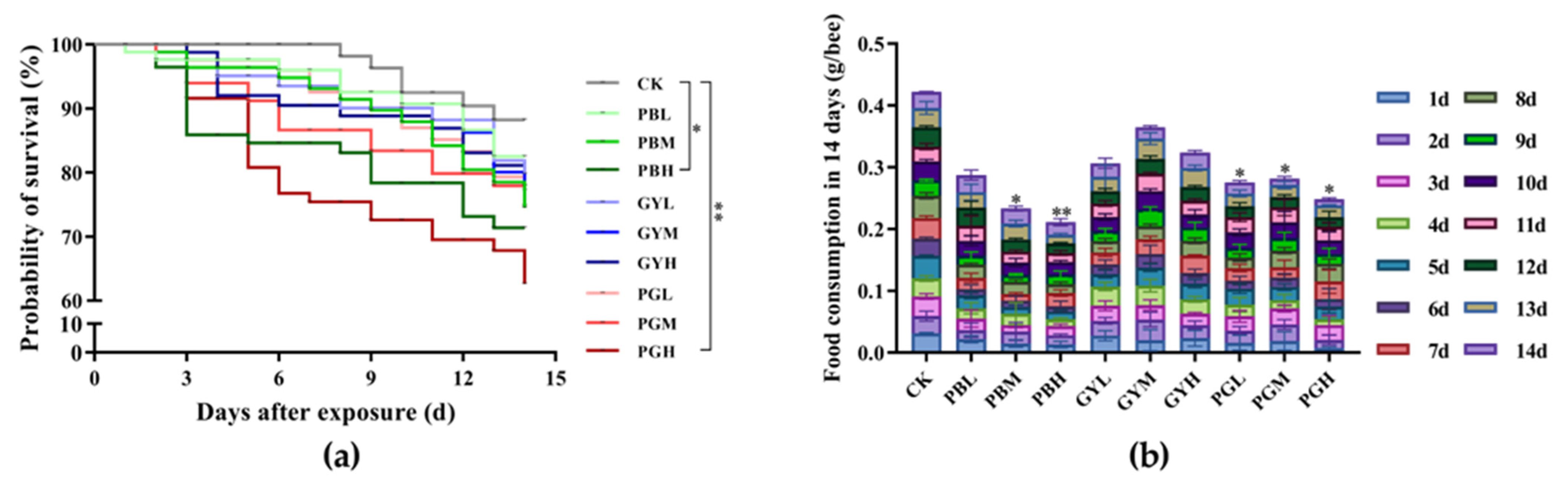
3.2. Combined Toxic Effects of Lead and Glyphosate on Survival and Feed Intake of A. c. cerana
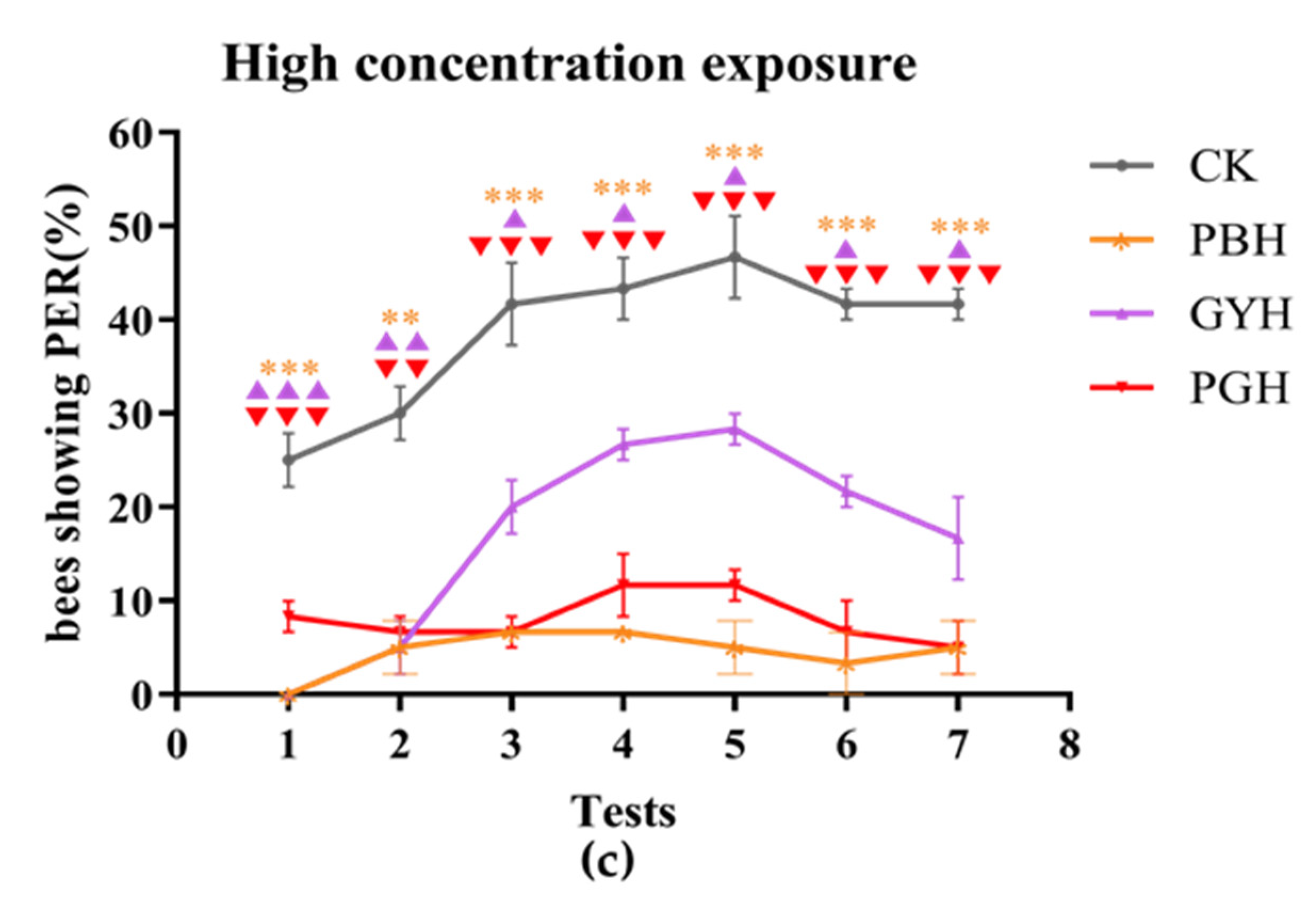
3.3. Combined Toxic Effects of Lead and Glyphosate on Proboscis Extension Response of A. c. cerana
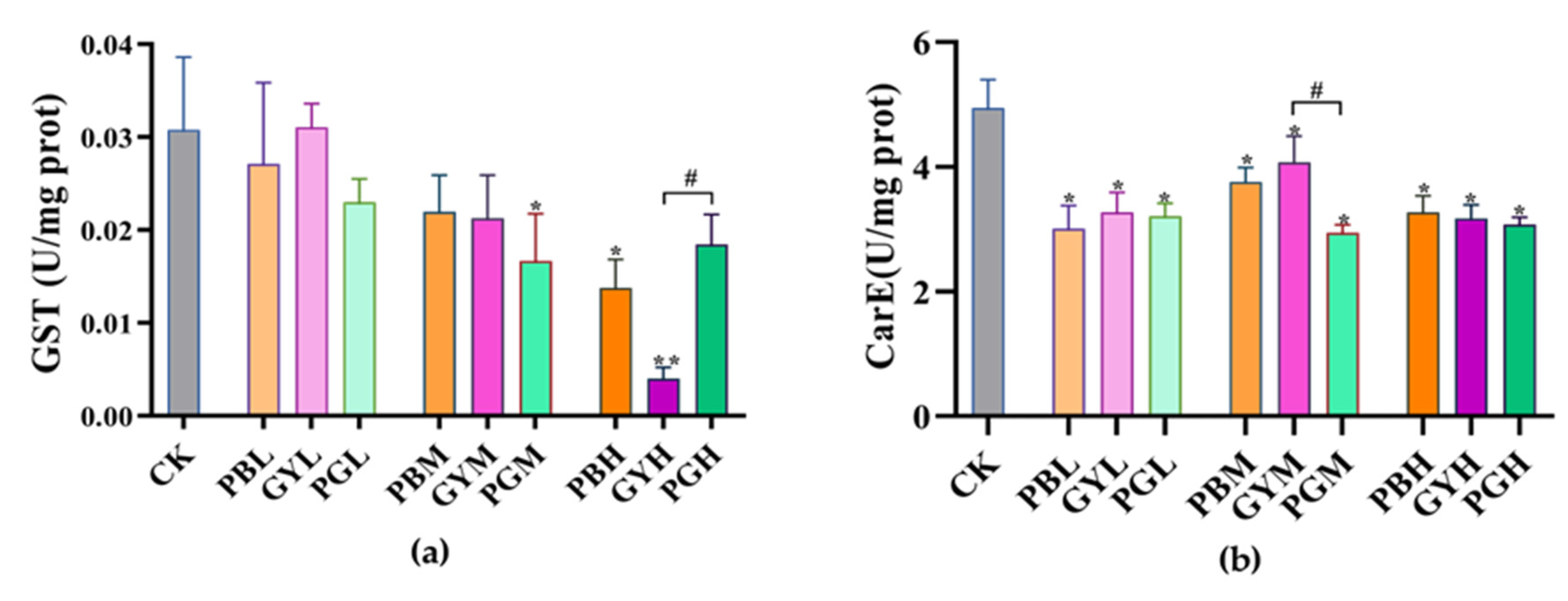
3.4. Combined Toxic Effects of Lead and Glyphosate on Enzyme Activity in A. c. cerana
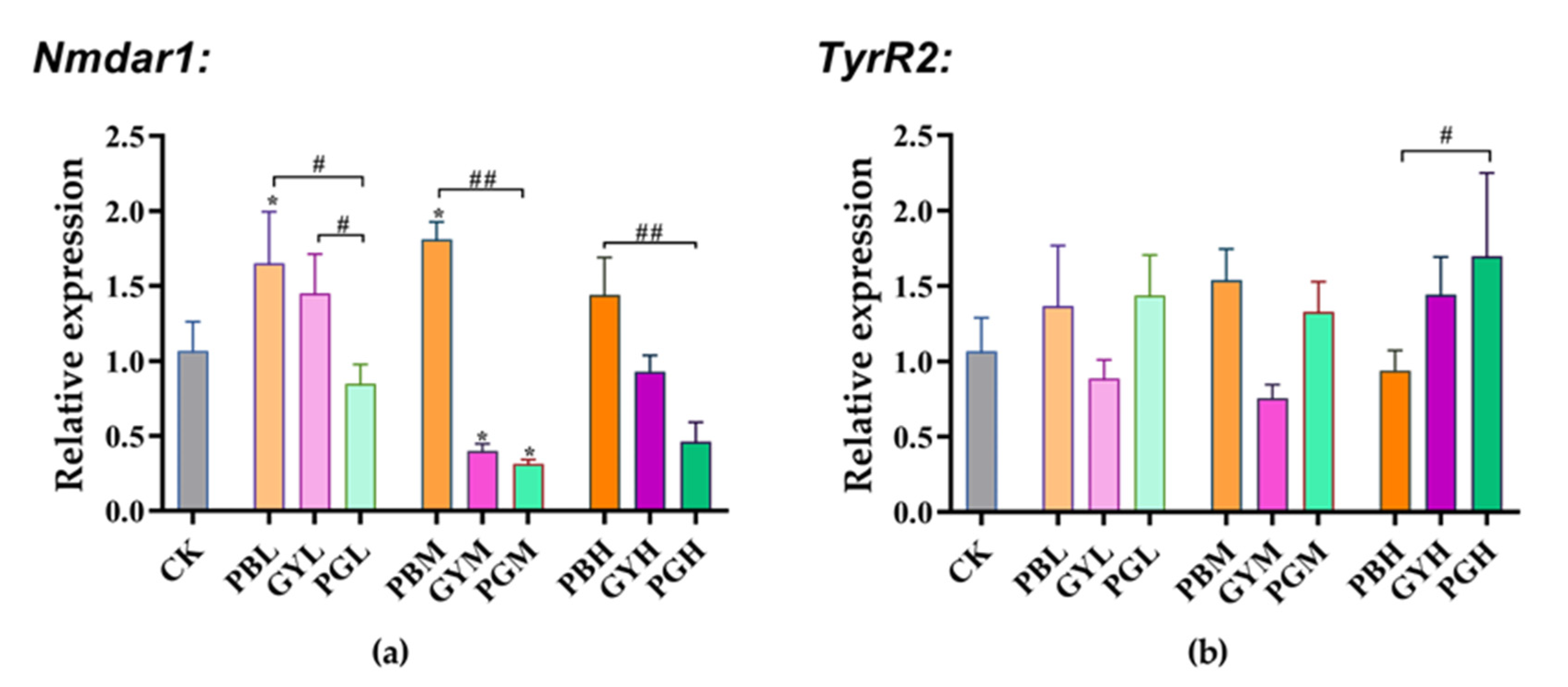
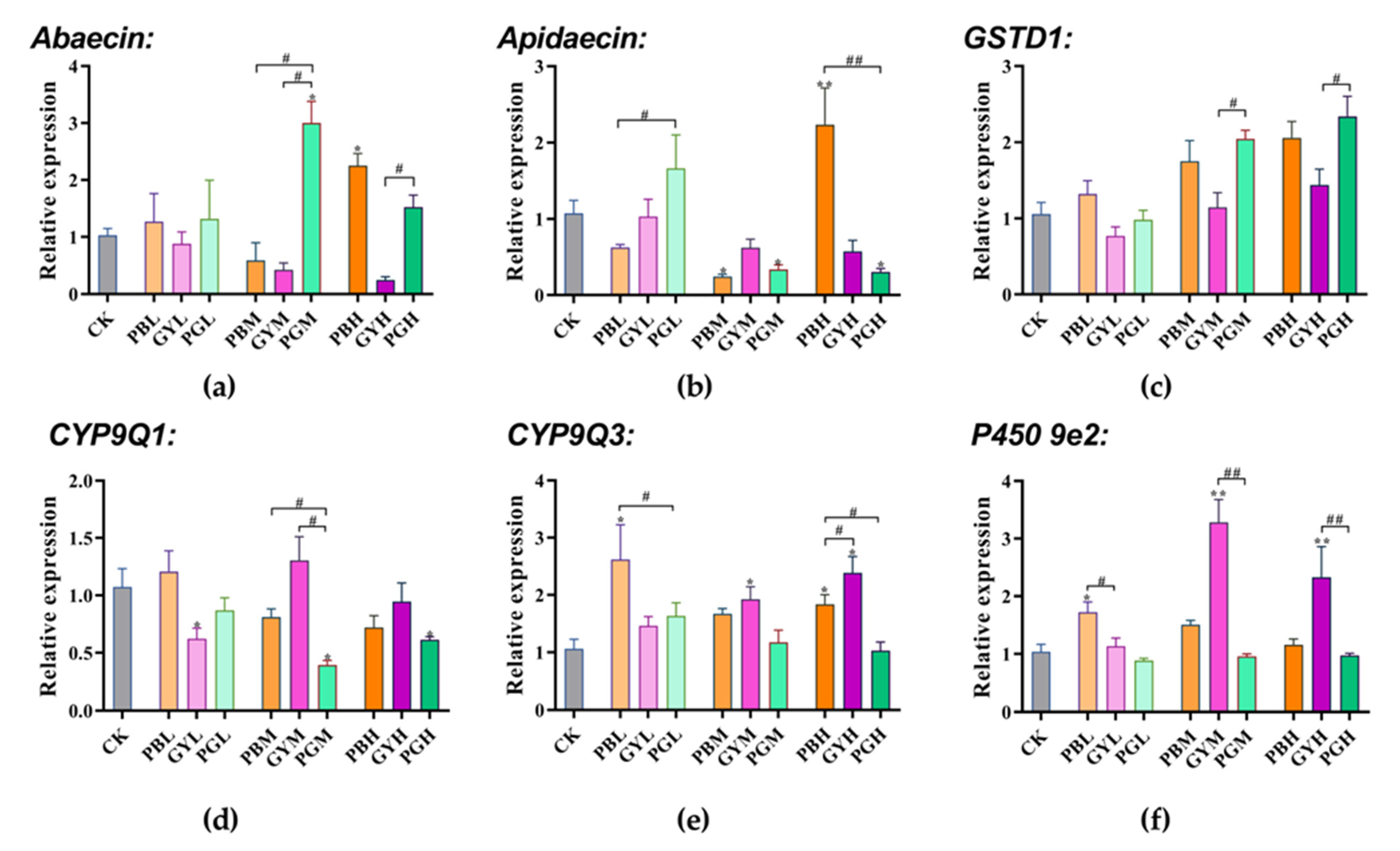
3.5. Combined Toxic Effects of Lead and Glyphosate on Gene Expression in A. c. cerana
3.5.1. Expression of Memory Genes
3.5.2. Expression of Abdominal Immunity and Detoxification Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallberg, A.; Han, F.; Wellhagen, G.; Dahle, B.; Kawata, M.; Haddad, N.; Simões, Z.L.P.; Allsopp, M.H.; Kandemir, I.; De la Rúa, P. A worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis mellifera. Nat. Genet. 2014, 46, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Lin, Z.; Page, P.; Li, L.; Qin, Y.; Zhang, Y.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Go east for better honey bee health: Apis cerana is faster at hygienic behavior than A. mellifera. PLoS ONE 2016, 11, e0162647. [Google Scholar] [CrossRef]
- Theisen-Jones, H.; Bienefeld, K. The Asian honey bee (Apis cerana) is significantly in decline. Bee World 2016, 93, 90–97. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the united states and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Leoci, R.; Ruberti, M. Glyphosate in agriculture: Environmental persistence and effects on animals. A review. J. Agric. Environ. Int. Dev. 2020, 114, 99–122. [Google Scholar]
- Blake, R.; Pallett, K. The environmental fate and ecotoxicity of glyphosate. Outlooks Pest Manag. 2018, 29, 266–269. [Google Scholar] [CrossRef]
- Rolando, C.A.; Baillie, B.R.; Thompson, D.G.; Little, K.M. The risks associated with glyphosate-based herbicide use in planted forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef]
- Farina, W.M.; Balbuena, M.S.; Herbert, L.T.; Mengoni Goñalons, C.; Vázquez, D.E. Effects of the herbicide glyphosate on honey bee sensory and cognitive abilities: Individual impairments with implications for the hive. Insects 2019, 10, 354. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.-L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Balbuena, M.S.; Chaves, F.; Gora, J.; Menzel, R.; Farina, W.M. Sleep in honey bees is affected by the herbicide glyphosate. Sci. Rep. 2020, 10, 10516. [Google Scholar] [CrossRef] [PubMed]
- Odemer, R.; Alkassab, A.T.; Bischoff, G.; Frommberger, M.; Wernecke, A.; Wirtz, I.P.; Pistorius, J.; Odemer, F. Chronic high glyphosate exposure delays individual worker bee (Apis mellifera L.) development under field conditions. Insects 2020, 11, 664. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.N. Phytohormonal roles in plant responses to heavy metal stress: Implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (thms) (arsenic (as), cadmium (cd), chromium (cr) (vi), mercury (hg), and lead (pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey bees (Apis mellifera, L.) as active samplers of airborne particulate matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]
- Burden, C.M.; Morgan, M.O.; Hladun, K.R.; Amdam, G.V.; Trumble, J.J.; Smith, B.H. Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Sci. Rep. 2019, 9, 4253. [Google Scholar] [CrossRef]
- Kiljanek, T.; Niewiadowska, A.; Gaweł, M.; Semeniuk, S.; Borzęcka, M.; Posyniak, A.; Pohorecka, K. Multiple pesticide residues in live and poisoned honeybees–preliminary exposure assessment. Chemosphere 2017, 175, 36–44. [Google Scholar] [CrossRef]
- Jumarie, C.; Aras, P.; Boily, M. Mixtures of herbicides and metals affect the redox system of honey bees. Chemosphere 2017, 168, 163–170. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Pioz, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Mixtures of an insecticide, a fungicide and a herbicide induce high toxicities and systemic physiological disturbances in winter Apis mellifera honey bees. Ecotoxicol. Environ. Saf. 2020, 203, 111013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Adamczyk, J.; Luttrell, R. Synergistic toxicity and physiological impact of imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera). PLoS ONE 2017, 12, e0176837. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, B.; Faggella, G. Comparison of laboratory and field observations of fish exposed to the herbicides molinate and thiobencarb. Trans. Am. Fish. Soc. 1986, 115, 882–890. [Google Scholar] [CrossRef]
- Xun, E.; Zhang, Y.; Zhao, J.; Guo, J. Heavy metals in nectar modify behaviors of pollinators and nectar robbers: Consequences for plant fitness. Environ. Pollut. 2018, 242, 1166–1175. [Google Scholar] [CrossRef]
- Tan, K.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 2015, 5, 10989. [Google Scholar] [CrossRef]
- Xu, S.; Hao, Z.; Li, Y.; Zhou, Y.; Shao, R.; Chen, R.; Zheng, M.; Xu, Y.; Wang, H. Biochemical toxicity and transcriptome aberration induced by dinotefuran in bombyx mori. Environ. Pollut. 2022, 307, 119562. [Google Scholar] [CrossRef]
- Scharlaken, B.; de Graaf, D.C.; Goossens, K.; Brunain, M.; Peelman, L.J.; Jacobs, F.J. Reference gene selection for insect expression studies using quantitative real-time PCR: The head of the honeybee, Apis mellifera, after a bacterial challenge. J. Insect Sci. 2008, 8, 33. [Google Scholar] [CrossRef]
- Shi, T.; Burton, S.; Zhu, Y.; Wang, Y.; Xu, S.; Yu, L. Effects of field-realistic concentrations of carbendazim on survival and physiology in forager honey bees (Hymenoptera: Apidae). J. Insect Sci. 2018, 18, 6. [Google Scholar] [CrossRef]
- Zannat, M.T.; Locatelli, F.; Rybak, J.; Menzel, R.; Leboulle, G. Identification and localisation of the nr1 sub-unit homologue of the nmda glutamate receptor in the honeybee brain. Neurosci. Lett. 2006, 398, 274–279. [Google Scholar] [CrossRef]
- Danihlík, J.; Aronstein, K.; Petřivalský, M. Antimicrobial peptides: A key component of honey bee innate immunity: Physiology, biochemistry, and chemical ecology. J. Apic. Res. 2015, 54, 123–136. [Google Scholar] [CrossRef]
- Tan, S.; Li, G.; Liu, Z.; Wang, H.; Guo, X.; Xu, B. Effects of glyphosate exposure on honeybees. Environ. Toxicol. Pharmacol. 2022, 90, 103792. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-X.; Li, W.-L.; Li, X.-J.; Li, J.-L.; Zhang, J.; Zhao, C.-H.; Huang, Q.; Cao, Z.; Chen, Y.; Zhao, H.-M. Glyphosate exposure affected longevity-related pathways and reduced survival in asian honey bees (Apis cerana). Chemosphere 2024, 351, 141199. [Google Scholar]
- Divisekara, T.; Navaratne, A.; Abeysekara, A. Impact of a commercial glyphosate formulation on adsorption of cd (ii) and pb (ii) ions on paddy soil. Chemosphere 2018, 198, 334–341. [Google Scholar] [CrossRef]
- Hernández, J.; Riveros, A.J.; Amaya-Márquez, M. Sublethal doses of glyphosate impair olfactory memory retention, but not learning in the honey bee (Apis mellifera scutellata). J. Insect Conserv. 2021, 25, 683–694. [Google Scholar] [CrossRef]
- Gong, Y.; Diao, Q. Current knowledge of detoxification mechanisms of xenobiotic in honey bees. Ecotoxicology 2017, 26, 1–12. [Google Scholar] [CrossRef]
- Claudianos, C.; Ranson, H.; Johnson, R.; Biswas, S.; Schuler, M.; Berenbaum, M.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.A.; Scharpenberg, H.; Moritz, R.F. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.). J. Comp. Physiol. A 2009, 195, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Caren, J.; Reddy, G.V.; Li, W.; Yao, J. Effect of age on insecticide susceptibility and enzymatic activities of three detoxification enzymes and one invertase in honey bee workers (Apis mellifera). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108844. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yang, Y.; Gao, J.; Zhao, D.; Ren, C.; Wang, S.; Zhao, S.; Zhong, Y. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology 2019, 28, 399–411. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Wang, C.; Chi, X.; Liu, Z.; Wang, Y.; Wang, H.; Guo, X.; Wang, N.; Xu, B. Toxic effects of the heavy metal cd on Apis cerana cerana (Hymenoptera: Apidae): Oxidative stress, immune disorders and disturbance of gut microbiota. Sci. Total Environ. 2024, 912, 169318. [Google Scholar] [CrossRef]
- Motta, E.V.; Powell, J.E.; Moran, N.A. Glyphosate induces immune dysregulation in honey bees. Anim. Microbiome 2022, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K. Lead exposure and its impact on immune system: A review. Toxicol. Vitr. 2009, 23, 969–972. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Gao, H.; Guo, X.; Xu, B. Identification, genomic organization, and oxidative stress response of a sigma class glutathione s-transferase gene (accgsts1) in the honey bee, Apis cerana cerana. Cell Stress Chaperones 2013, 18, 415–426. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Hatjina, F.; Ioannidis, P.; Hamamtzoglou, A.; Schoonvaere, K.; Francis, F.; Meeus, I.; Smagghe, G.; de Graaf, D.C. Stress indicator gene expression profiles, colony dynamics and tissue development of honey bees exposed to sub-lethal doses of imidacloprid in laboratory and field experiments. PLoS ONE 2017, 12, e0171529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yao, Y.; Wang, H.; Liu, Z.; Ma, L.; Wang, Y.; Xu, B. The roles of four novel p450 genes in pesticides resistance in Apis cerana cerana Fabricius: Expression levels and detoxification efficiency. Front. Genet. 2019, 10, 1000. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| Nmdar1 | F:CGGGCTTCGATAGCTGCTCAAC R:CTCGACCAGGACTTCGTGGAGT |
| TyrR2 | F:CGACCACGACAACGGTTTACCA R:CACGCCCATTATCACGCCCAAT |
| Abaecin | F:CGCAGCGTTCGCATATGTACCA R:ATGGTCCCTGACCAGGAAACGT |
| Apidaecin | F:CCTTTATAGTCGCGGTATTTGG R:AGGTTCAGCTTCCGGTTCAG |
| CYP9Q1 | F:GGCTTCAGGAGGAGATCGACGA R:ACCTCATCGCCTCGCTCATCA |
| CYP9Q3 | F:ACTCCATCACCTCCGTCACCAC R:CACACCGTCGTTGCTCCTCAA |
| P450 9e2 | F:ATGGACGGCGAGCAATGGAAG R:TGGACAGGTGATGGGCGAATCT |
| GSTD1 | F: CATTGACGGCTGCTGCTCT R: TTCAGGCTTCAACTGTTCCCTAT |
| Arp(actin) | F:GGATTCGGGTGACGGTGTTTCG R:GCCAGCCAAGTCCAAACGAAGA |
| Exposure Time (h) | Glyphosate Alone | Lead Chloride Alone | Glyphosate Combined | Lead and Chloride Combined | Additive Index (AI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | p | LC50 (95% FL) mg a.i. L−1 | df | p | LC50 (95% FL) mg a.i. L−1 | df | p | LC50 (95% FL) mg a.i. L−1 | df | p | LC50 (95% FL) mg a.i. L−1 | ||
| 72 | 6 | 0.36 | 7121 (6089–8657) | 6 | 0.34 | 1483 (1341–1659) | 6 | 0.18 | 1448 (1263–1672) | 6 | 0.39 | 769 (701–847) | 0.43 |
| 96 | 6 | 0.23 | 4764 (4184–5505) | 6 | 0.31 | 1083 (985–1203) | 6 | 0.48 | 946 (830–1083) | 6 | 0.18 | 621 (566–682) | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhao, W.; Meng, Q.; Yang, L.; Zhi, D.; Guo, Y.; Yue, D.; Tian, Y.; Dong, K. Combined Toxic Effects of Lead and Glyphosate on Apis cerana cerana. Insects 2024, 15, 644. https://doi.org/10.3390/insects15090644
Xue Y, Zhao W, Meng Q, Yang L, Zhi D, Guo Y, Yue D, Tian Y, Dong K. Combined Toxic Effects of Lead and Glyphosate on Apis cerana cerana. Insects. 2024; 15(9):644. https://doi.org/10.3390/insects15090644
Chicago/Turabian StyleXue, Yunfei, Wenzheng Zhao, Qingxin Meng, Linfu Yang, Dandan Zhi, Yulong Guo, Dan Yue, Yakai Tian, and Kun Dong. 2024. "Combined Toxic Effects of Lead and Glyphosate on Apis cerana cerana" Insects 15, no. 9: 644. https://doi.org/10.3390/insects15090644
APA StyleXue, Y., Zhao, W., Meng, Q., Yang, L., Zhi, D., Guo, Y., Yue, D., Tian, Y., & Dong, K. (2024). Combined Toxic Effects of Lead and Glyphosate on Apis cerana cerana. Insects, 15(9), 644. https://doi.org/10.3390/insects15090644





