Leg Attachment Devices of Tiger Beetles (Coleoptera, Cicindelidae) and Their Relationship to Their Habitat Preferences
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Terminology
2.3. Photographic System
2.4. Scanning Electron Microscopy (SEM)
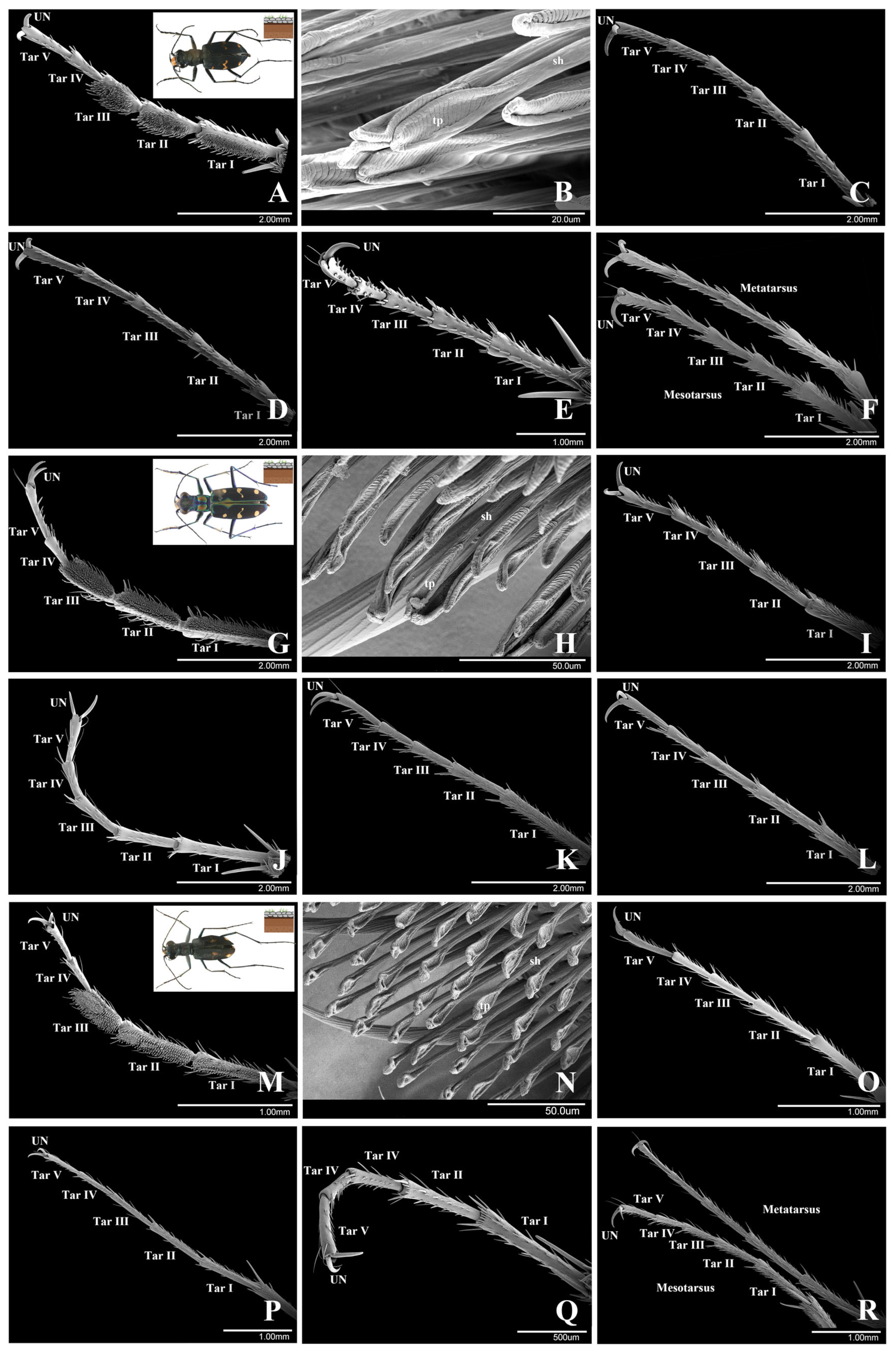
Morphometry of the Attachment System
3. Results
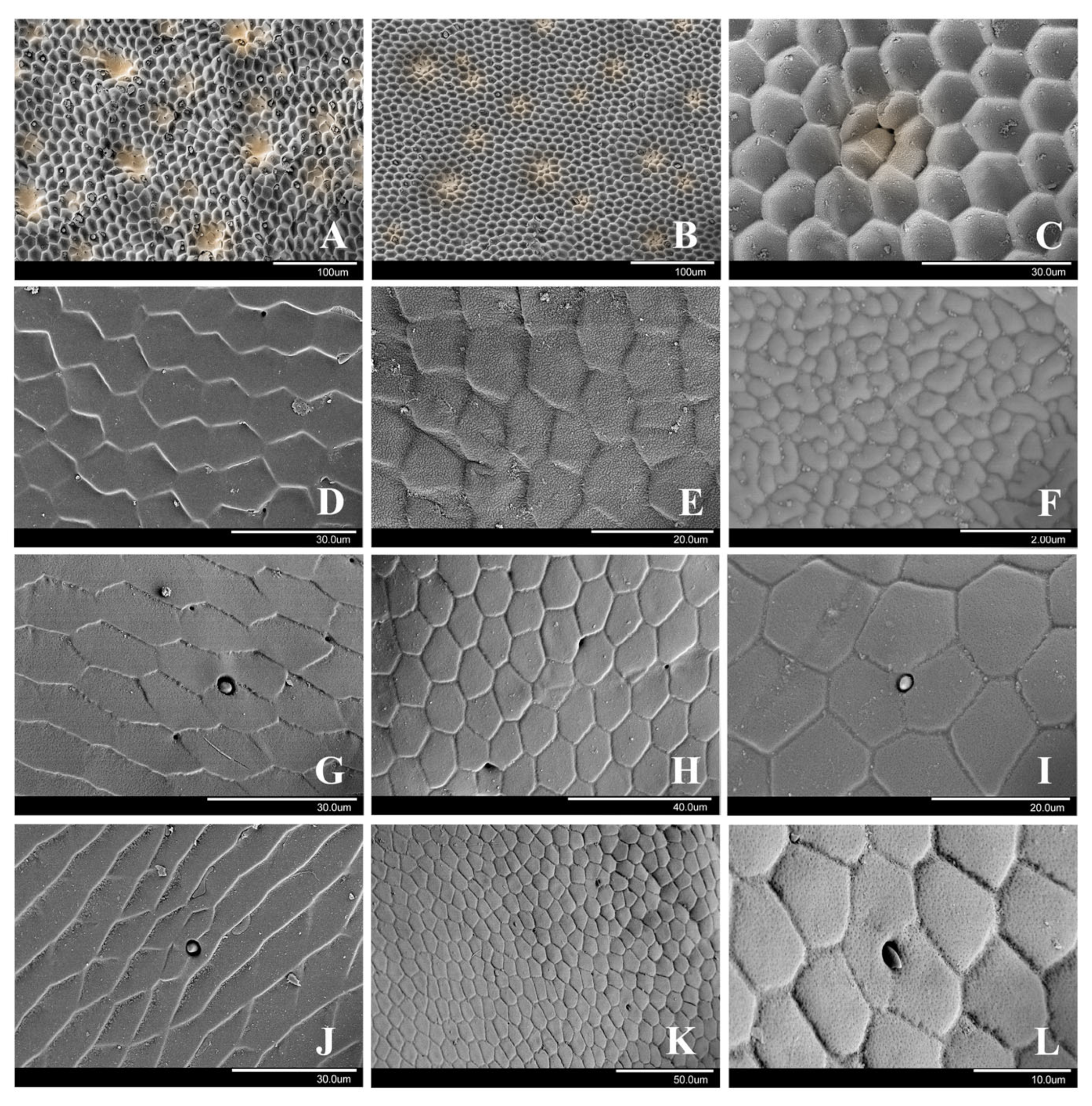
3.1. Cicindelini
3.1.1. Cicindela sachalinensis Morawitz, 1862
3.1.2. Cosmodela separata (Fleutiaux, 1894)
3.1.3. Cylindera kaleea (Bates, 1866)
3.2. Collyridini
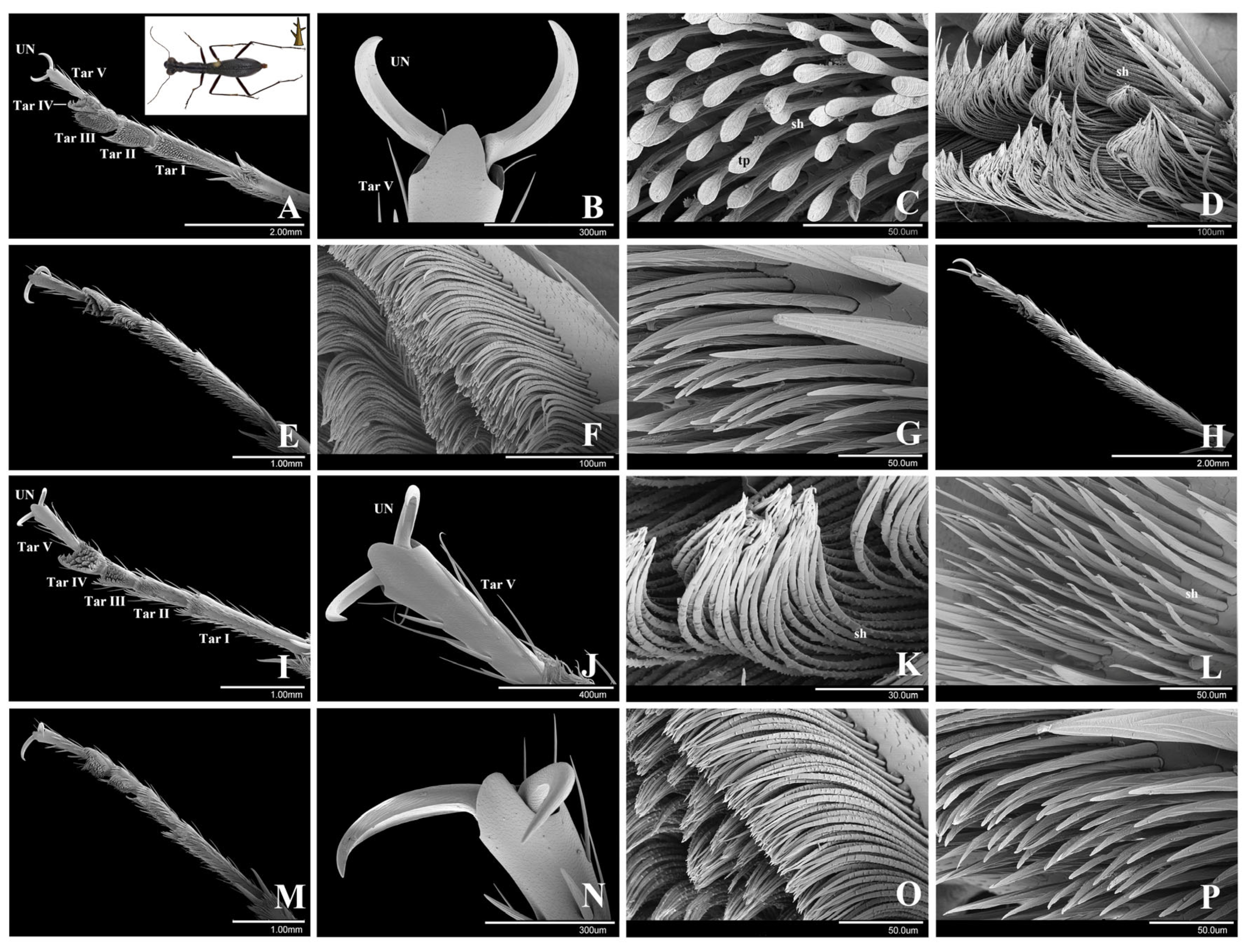
3.2.1. Subtribe Tricondylina: Tricondyla pulchripes White, 1844
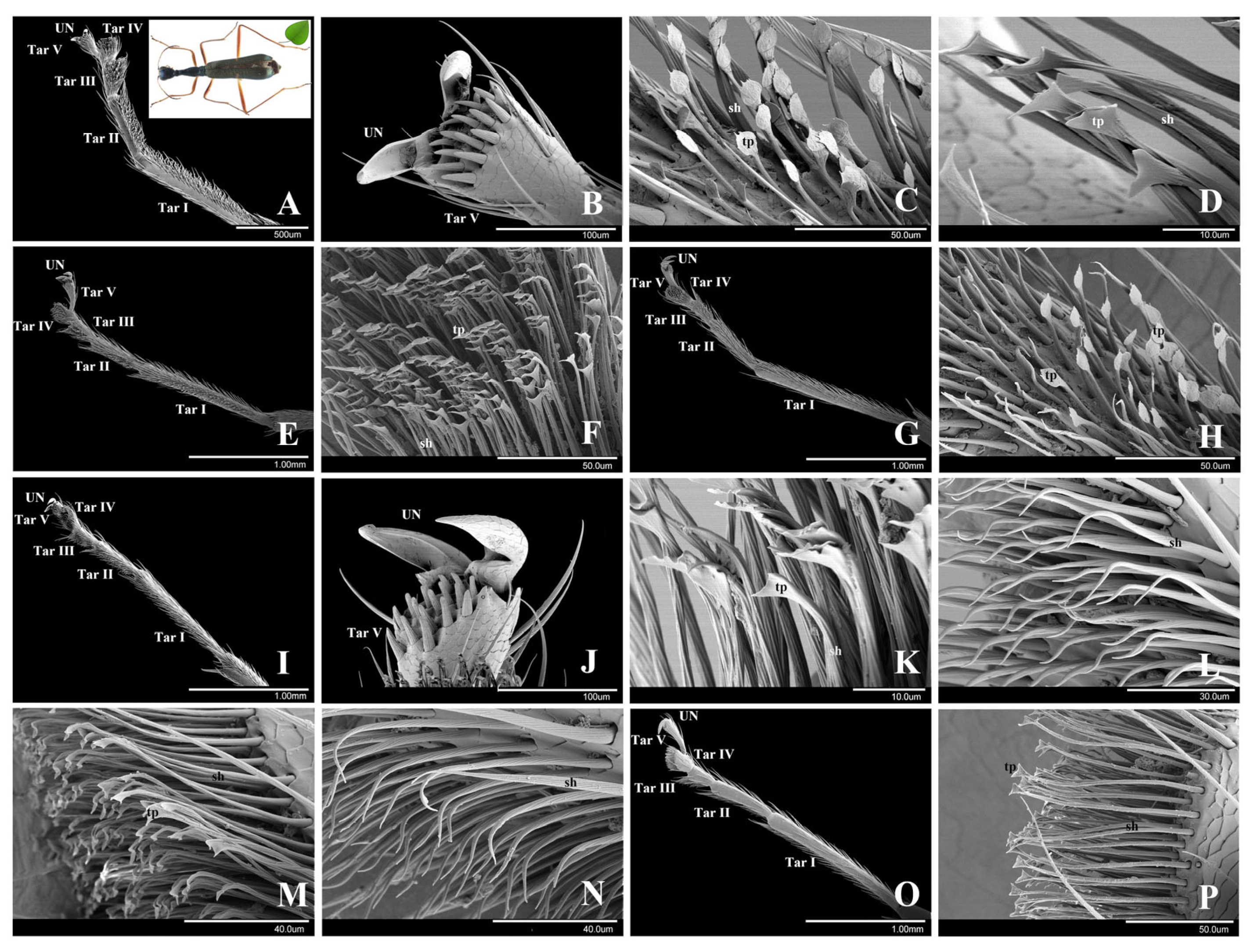
3.2.2. Subtribe Collyridina: Neocollyris linearis (Schmidt-Göbel, 1846)
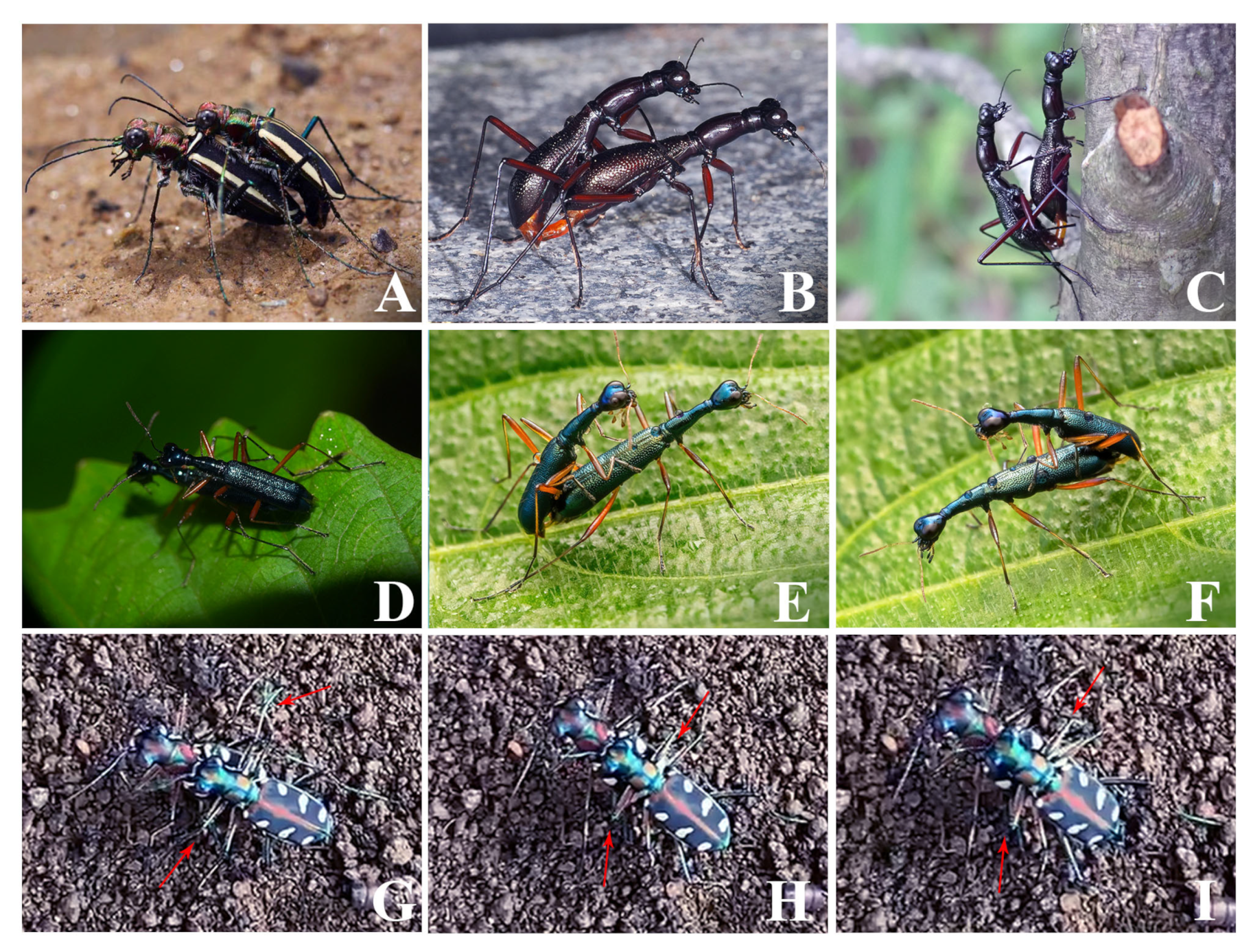
3.3. Microstructure of the Female Cuticle Surface and the Male Behavior during Mating
3.3.1. Cicindelini
Cicindela sachalinensis and Cosmodela separata
3.3.2. Collyridini (Subtribe Tricondylina)
Tricondyla pulchripes
3.3.3. Collyridini (Subtribe Collyridina)
Neocollyris linearis
4. Discussion
4.1. Correlation between Morphology of Adhesive Setae, Beetle Habitat and Gender
4.2. Comparison of Adhesive Devices within Tiger Beetles
4.3. Comparison of Adhesive Devices between Tiger Beetles and Other Beetles
4.4. Key Drivers of the Specific Structure of Adhesive Setae
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, T. The foot of the fly: Its structure and action elucidated by comparison with the feet of other insect. Trans. Linn. Soc. Lond. 1862, 23, 393–421. [Google Scholar] [CrossRef]
- Stork, N.E.; Evans, M.E.G. Tarsal setae in Coleoptera. Int. J. Insect Morphol. Embryol. 1976, 5, 219–221. [Google Scholar] [CrossRef]
- Gorb, S.N.; Beutel, R.G. Evolution of locomotory attachment pads of hexapods. Naturwissenschaften 2001, 88, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Gorb, S.N. Convergent evolution of adhesive properties in leaf insect eggs and plant seeds: Cross-kingdom bioinspiration. Biomimetics 2022, 7, 173. [Google Scholar] [CrossRef]
- Beutel, R.G.; Gorb, S.N. Ultrastructure of attachment specializations of hexapods (Arthropoda): Evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001, 39, 177–207. [Google Scholar] [CrossRef]
- Federle, W.; Brainerd, E.L.; McMahon, T.A.; Hölldobler, B. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl. Acad. Sci. USA 2001, 98, 6215–6220. [Google Scholar] [CrossRef]
- Federle, W. Why are so many adhesive pads hairy? J. Exp. Biol. 2006, 209, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, Z.D.; Wang, Z.Y.; Ji, A.H.; Gorb, S.N. The synergy between the insect-inspired claws and adhesive pads increases the attachment ability on various rough surfaces. Sci. Rep. 2016, 6, 26219. [Google Scholar] [CrossRef]
- Gorb, S.N. The design of the fly adhesive pad: Distal tenent setae are adapted to the delivery of an adhesive secretion. Proc. R. Soc. Lond. B 1998, 265, 747–752. [Google Scholar] [CrossRef]
- Niederegger, S.; Gorb, S.N.; Jiao, Y. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J. Comp. Physiol. A 2002, 187, 961–970. [Google Scholar] [CrossRef]
- Langer, M.G.; Ruppersberg, J.P.; Gorb, S.N. Adhesion forces measured at the level of a terminal plate of the fly’s seta. Proc. R. Soc. Lond. B 2004, 271, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, K.; Schneeberg, K.; Beutel, R.G. Fly on the wall—Attachment structures in lower Diptera. Syst. Entomol. 2014, 39, 460–473. [Google Scholar] [CrossRef]
- Walker, G.; Yulf, A.B.; Ratcliffe, J. The adhesive organ of the blowfly, Calliphora vomitoria: A functional approach (Diptera: Calliphoridae). J. Zool. 1985, 205, 297–307. [Google Scholar] [CrossRef]
- Stork, N.E. A scanning electron microscope study of tarsal adhesive setae in the Coleoptera. Zool. J. Linn. Soc. 1980, 68, 173–306. [Google Scholar] [CrossRef]
- Eisner, T.; Aneshansley, D.J. Defense by foot adhesion in a beetle (Hemisphaerota cyanea). Proc. Natl. Acad. Sci. USA 2000, 97, 6568–6573. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.M.R.; Federle, W. Beetle adhesive hairs differ in stiffness and stickiness: In vivo adhesion measurements on individual setae. Naturwissenschaften 2011, 98, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, A.P. Ultramorphology of the tarsal adhesive structures of eight leaf beetle species (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 2016, 89, 215–230. [Google Scholar] [CrossRef]
- Voigt, D.; Tsipenyuk, A.; Varenberg, M. How tight are beetle hugs? Attachment in mating leaf beetles. Roy. Soc. Open Sci. 2017, 4, 171108. [Google Scholar] [CrossRef]
- Voigt, D.; Varenberg, M.; Schuppert, J.; Gorb, S.N. Comparison of tarsal attachment in two closely related leaf beetle species. J. Insect Physiol. 2020, 127, 104158. [Google Scholar] [CrossRef]
- Gorb, S.N.; Scherge, M. Biological microtribology: Anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. R. Soc. Lond. B 2000, 267, 1239–1244. [Google Scholar] [CrossRef]
- Schulmeister, S. Morphology and evolution of the tarsal plantulae in Hymenoptera (Insecta), focusing on the basal lineages. Zool. Scr. 2003, 32, 153–172. [Google Scholar] [CrossRef]
- Weirauch, C. Hairy attachment structures in Reduviidae (Cimicomorpha, Heteroptera), with observations on the Fossula spongiosa in some other Cimicomorpha. Zool. Anz. 2007, 246, 155–175. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Gorb, E.V.; Gorb, S.N. Attachment devices and the tarsal gland of the bug Coreus marginatus (Hemiptera: Coreidae). Zoomorphology 2021, 140, 85–102. [Google Scholar] [CrossRef]
- Hayer, S.; Sturm, B.P.; Büsse, S.; Büscher, T.H.; Gorb, S.N. Louse flies holding on mammals’ hair: Comparative functional morphology of specialized attachment devices of ectoparasites (Diptera: Hippoboscoidea). J. Morphol. 2022, 283, 1561–1576. [Google Scholar] [CrossRef]
- Orivel, J.; Malherbe, M.C.; Dejean, A. Relationships between pretarsus morphology and arboreal life in ponerine ants of the genus Pachycondyla (Formicidae: Ponerinae). Ann. Entomol. Soc. Am. 2001, 94, 449–456. [Google Scholar] [CrossRef]
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The Evolution of Tarsal Adhesive Microstructures in Stick and Leaf Insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 69. [Google Scholar] [CrossRef]
- Matsumura, Y.; Gorb, E.V.; Gorb, S.N. The tight attachment achieved by the male discoidal setae is possibly acounter-adaptation to the grease layer on female integument surfaces in green dock beetles. J. R. Soc. Interface 2023, 20, 20230324. [Google Scholar] [CrossRef]
- Voigt, D.; Schuppert, J.M.; Dattinger, S.; Gorb, S.N. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 2008, 54, 765–776. [Google Scholar] [CrossRef]
- Büscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 20180281. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Gorb, E.V.; Gorb, S.N. Mechanical ecology of fruit-insect interaction in the adult Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Zoology 2020, 139, 125748. [Google Scholar] [CrossRef]
- Labandeira, C. Early history of arthropod and vascular plant associations. Annu. Rev. Earth Planet. Sci. 1998, 26, 329–377. [Google Scholar] [CrossRef]
- Wolff, J.O.; Gorb, S.N. Adhesive foot pads: An adaptation to climbing? An ecological survey in hunting spiders. Zoology 2015, 118, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beutel, R.G.; Leschen, R.A.B.; Coleoptera, B. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). In Handbook of Zoology, Arthropoda: Insecta, 2nd ed.; Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter GmbH & Co.: Berlin, Germany; New York, NY, USA, 2016; Volume 1, pp. 173–174. [Google Scholar]
- Wiesner, J. Checklist of the Tiger Beetles of the World, 2nd ed.; Winterwork: Borsdorf, Germany, 2020. [Google Scholar]
- Pearson, D.L.; Wiesner, J. The use of tiger beetles (Coleoptera: Cicindelidae) in adapting hotspot conservation to global, regional, and local scales. J. Insect. Conserv. 2023, 27, 19–48. [Google Scholar] [CrossRef]
- Pearson, D.L. Biology of tiger beetles. Annu. Rev. Entomol. 1988, 33, 123–147. [Google Scholar] [CrossRef]
- Duran, D.P.; Gough, H.M. Validation of tiger beetles as distinct family (Coleoptera: Cicindelidae), review and reclassification of tribal relationships. Syst. Entomol. 2020, 45, 723–729. [Google Scholar] [CrossRef]
- Gough, H.M.; Allen, J.M.; Toussaint, E.F.A.; Storer, C.G.; Kawahara, A.Y. Transcriptomics illuminate the phylogenetic backbone of tiger beetles. Biol. J. Linn. Soc. 2020, 195, 740–751. [Google Scholar] [CrossRef]
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Reid, C.A.M.; Schmitt, M.; Ślipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar]
- Betz, O. Structure of the tarsi in some Stenus species (Coleoptera, Staphylinidae): External morphology, ultrastructure, and tarsal secretion. J. Morphol. 2003, 255, 24–43. [Google Scholar] [CrossRef]
- Pei, X.J.; Wang, J.T.; Cong, Y.; Fu, J. Recent progress in polymer hydrogel bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Spinner, M.; Westhoff, G.; Gorb, S.N. Subdigital setae of chameleon feet: Friction-enhancing microstructures for a wide range of substrate roughness. Sci. Rep. 2014, 4, 5481. [Google Scholar] [CrossRef]
- Bullock, J.M.R.; Federle, W. The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci. 2011, 18, 298–304. [Google Scholar] [CrossRef]
- Lee, H. Generic Revision of the Procirrina (Coleoptera: Staphylinidae: Paederinae: Pinophilini). Bull. Am. Mus. Nat. Hist. 2010, 347, 1–78. [Google Scholar]
- Moon, M.J.; Park, J.G.; Choi, W.J. Fine structural analysis of the fibrillar adhesion apparatus in ladybird beetle. Entomol. Res. 2012, 42, 196–205. [Google Scholar] [CrossRef]
- Voigt, D.; Takanashi, T.; Tsuchihara, K.; Yazaki, K.; Kuroda, K.; Tsubaki, R.; Hosoda, N. Strongest grip on the rod: Tarsal morphology and attachment of Japanese pine sawyer beetles. Zool. Lett. 2017, 3, 16. [Google Scholar] [CrossRef]
- Niederegger, S.; Gorb, S.N. Friction and adhesion in the tarsal and metatarsal scopulae of spiders. J. Comp. Physiol. A 2006, 192, 1223–1232. [Google Scholar] [CrossRef]
- Wolff, J.O.; Gorb, S.N. Comparative morphology of pretarsal scopulae in eleven spider families. Arthropod Struct. Dev. 2012, 41, 419–433. [Google Scholar] [CrossRef]
- Frost, F.; Gorb, S.N.; Wolff, J.O. Adhesion and friction in hunting spiders: The effect of contact splitting on their attachment ability. Zool. Anz. 2018, 273, 231–239. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Heepe, L.; Höft, S.; Michels, J.; Gorb, S.N. Material gradients in fibrillar insect attachment systems: The role of joint-like elements. Soft Matter 2018, 14, 7026–7033. [Google Scholar] [CrossRef]
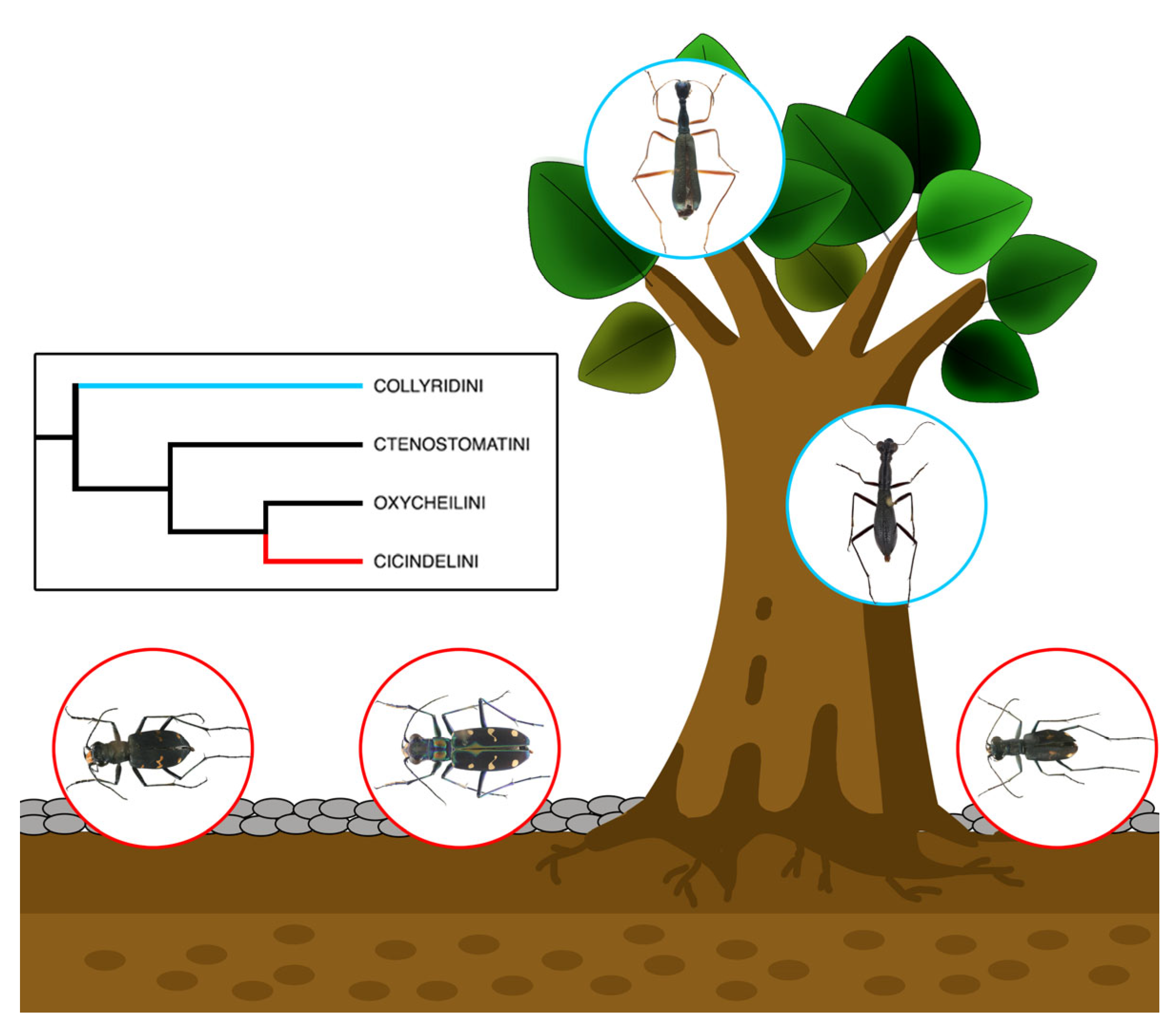

| Tribe | Species | Collection Site | Habitat | |
|---|---|---|---|---|
| 1 | Cicindelini | Cicindela sachalinensis | Wuling Mountain, Beijing | Non-arboreal |
| 2 | Cicindelini | Cosmodela separata | Jiangpu County, Zhejiang | Non-arboreal |
| 3 | Cicindelini | Cylindera kaleea | Siming Mountain, Zhejiang | Non-arboreal |
| 4 | Collyridini | Tricondyla pulchripes | Hong Kong | Arboreal, tree stem |
| 5 | Collyridini | Neocollyris linearis | Xishuangbanna, Yunnan | Arboreal, tree leaf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gorb, S.N.; Liang, H.; Bai, M.; Lu, Y. Leg Attachment Devices of Tiger Beetles (Coleoptera, Cicindelidae) and Their Relationship to Their Habitat Preferences. Insects 2024, 15, 650. https://doi.org/10.3390/insects15090650
Liu Z, Gorb SN, Liang H, Bai M, Lu Y. Leg Attachment Devices of Tiger Beetles (Coleoptera, Cicindelidae) and Their Relationship to Their Habitat Preferences. Insects. 2024; 15(9):650. https://doi.org/10.3390/insects15090650
Chicago/Turabian StyleLiu, Zheng, Stanislav N. Gorb, Hongbin Liang, Ming Bai, and Yuanyuan Lu. 2024. "Leg Attachment Devices of Tiger Beetles (Coleoptera, Cicindelidae) and Their Relationship to Their Habitat Preferences" Insects 15, no. 9: 650. https://doi.org/10.3390/insects15090650
APA StyleLiu, Z., Gorb, S. N., Liang, H., Bai, M., & Lu, Y. (2024). Leg Attachment Devices of Tiger Beetles (Coleoptera, Cicindelidae) and Their Relationship to Their Habitat Preferences. Insects, 15(9), 650. https://doi.org/10.3390/insects15090650









