Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management
Abstract
Simple Summary
Abstract
1. Introduction
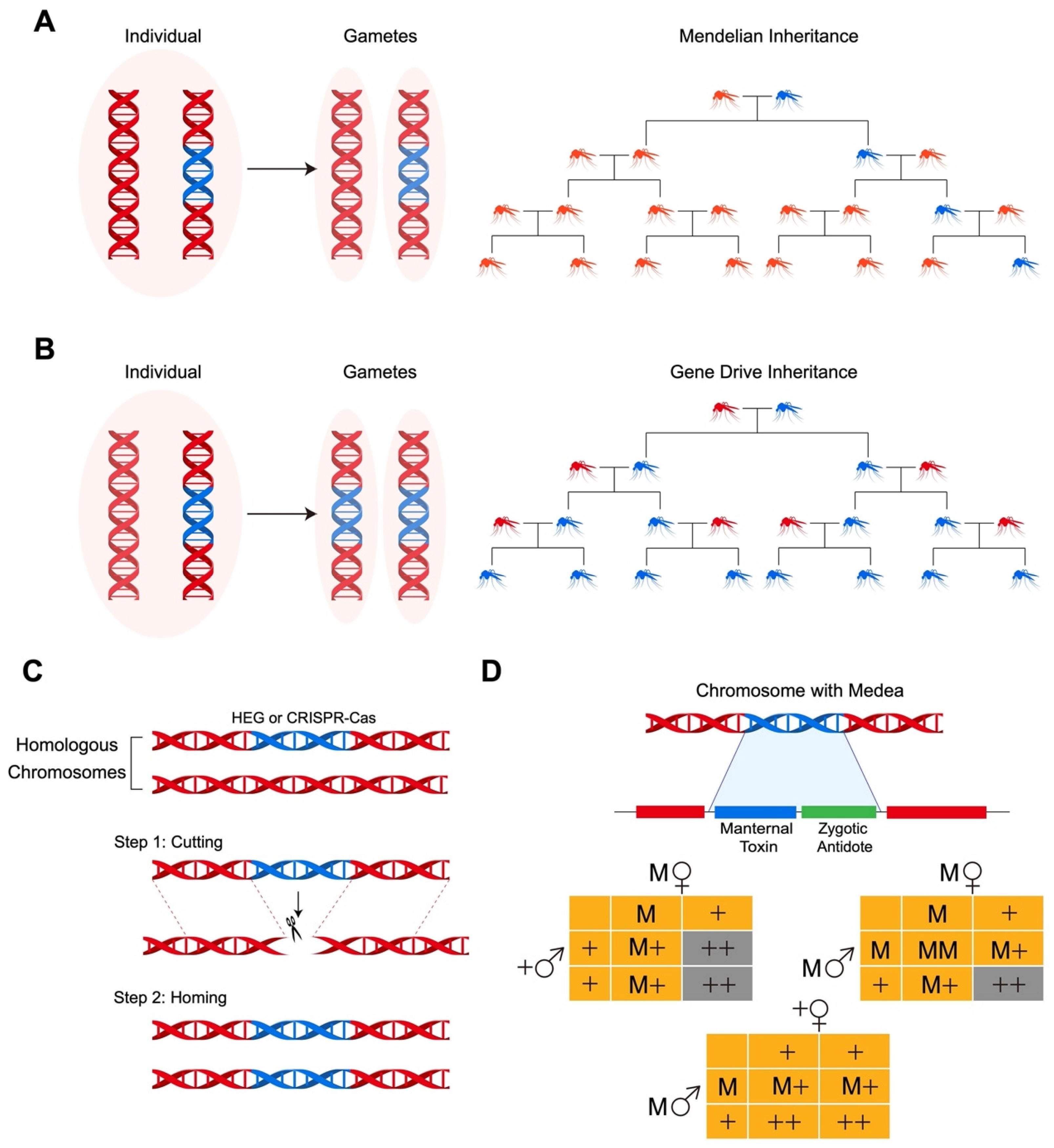
2. Population Replacement Using Gene Drive Systems
2.1. Molecular Genetic Manipulation of Insects
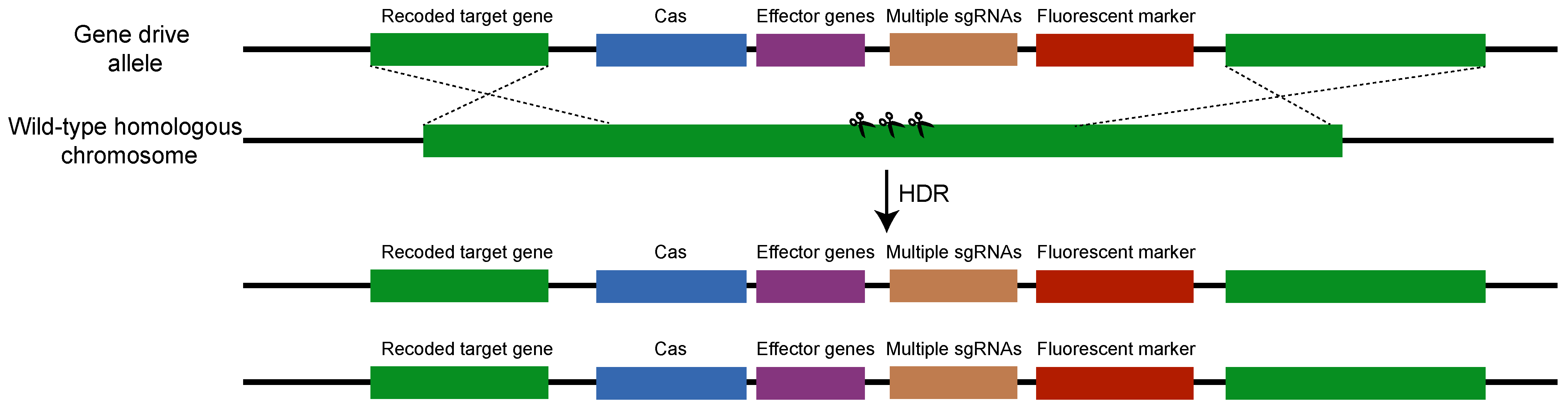
2.2. Performance of CRISPR-Cas-Mediated Gene Drive Populations
3. Ideal Traits for Gene Drives in Pest Control
3.1. Blocking Pathogen Transmission
3.2. Manipulation of Sex Ratios
3.3. Manipulation of Feeding Behaviors
3.4. Manipulation of Migration
4. How Is a CRISPR-Cas-Mediated Gene Drive Made and Introduced?
5. Benefits and Risks of Gene Drives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chapman, A.D. Numbers of Living Species in Australia and the World, 2nd ed.; Australian Government, Department of the Environment and Energy: Canberra, Australia, 2009.
- Van Der Goes Van Naters, W.; Carlson, J.R. Insects as chemosensors of humans and crops. Nature 2006, 444, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Ferry, N.; Gatehouse, A. Transgenic crop plants for resistance to biotic stress. In Transgenic Crop Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–65. [Google Scholar]
- Knipling, E. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998, 43, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef]
- Xi, Z.; Dean, J.L.; Khoo, C.; Dobson, S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005, 35, 903–910. [Google Scholar] [CrossRef]
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Sanders, P.R.; Flick, J.S.; Adams, S.P.; Bittner, M.L.; Brand, L.A.; Fink, C.L.; Fry, J.S. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4803–4807. [Google Scholar] [CrossRef]
- Charlesworth, B.; Langley, C.H. The population genetics of Drosophila transposable elements. Annu. Rev. Genet. 1989, 23, 251–287. [Google Scholar] [CrossRef] [PubMed]
- Ranner, H. Investigations on the biology and control of the cherry fruit fly, Rhagoletis cerasi L. (Diptera, Trypetidae)—V. Experiments on the control of the cherry fruit fly by means of the incompatible insect technique (IIT). Pflanzenschutzberichte 1990, 51, 1–16. [Google Scholar]
- Beeman, R.; Friesen, K.; Denell, R. Maternal-effect selfish genes in flour beetles. Science 1992, 256, 89–92. [Google Scholar] [CrossRef]
- Burt, A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 921–928. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; U.S. Department of Agriculture: Washington, DC, USA, 1979; pp. 1–659.
- Marec, F.; Vreysen, M.J. Advances and challenges of using the sterile insect technique for the management of pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Harris, A.F.; Nimmo, D.; McKemey, A.R.; Kelly, N.; Scaife, S.; Donnelly, C.A.; Beech, C.; Petrie, W.D.; Alphey, L. Field performance of engineered male mosquitoes. Nat. Biotechnol. 2011, 29, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 2008, 627, 104–113. [Google Scholar] [PubMed]
- Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Suckling, D.M.; Wearing, C.H. Past, present, and future of integrated control of apple pests: The New Zealand experience. Annu. Rev. Entomol. 2017, 62, 231–248. [Google Scholar] [CrossRef]
- Guedes, R.; Smagghe, G.; Stark, J.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Cayol, J.P. Changes in sexual behavior and life history traits of tephritid species caused by mass-rearing processes. In Fruit Flies (Tephritidae); Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 861–878. ISBN 978-0-429-12467-9. [Google Scholar]
- Curtis, C. Possible use of translocations to fix desirable genes in insect pest populations. Nature 1968, 218, 368–369. [Google Scholar] [CrossRef]
- Sinkins, S.P.; Gould, F. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 2006, 7, 427–435. [Google Scholar] [CrossRef]
- Windbichler, N.; Menichelli, M.; Papathanos, P.A.; Thyme, S.B.; Li, H.; Ulge, U.Y.; Hovde, B.T.; Baker, D.; Monnat, R.J.; Burt, A. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 2011, 473, 212–215. [Google Scholar] [CrossRef]
- Beeman, R.W.; Friesen, K.S. Properties and natural occurrence of maternal-effect selfish genes (‘Medea’ factors) in the Red Flour Beetle, Tribolium castaneum. Heredity 1999, 82, 529–534. [Google Scholar] [CrossRef]
- Buchman, A.; Marshall, J.M.; Ostrovski, D.; Yang, T.; Akbari, O.S. Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc. Natl. Acad. Sci. USA 2018, 115, 4725–4730. [Google Scholar] [CrossRef]
- Barrangou, R.; van der Oost, J. RNA-mediated Adaptive Immunity in Bacteria and Archaea. In CRISPR-Cas Systems; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef]
- Wang, H.; Russa, M.L.; Qi, L.S. CRISPR/Cas9 in genome editing and beyond. Annu. Rev. Biochem. 2016, 85, 227–264. [Google Scholar] [CrossRef]
- Raban, R.; Marshall, J.M.; Hay, B.A.; Akbari, O.S. Manipulating the destiny of wild populations using CRISPR. Annu. Rev. Genet. 2023, 57, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Gantz, V.M.; Bier, E. The mutagenic chain reaction: A method for converting heterozygous to homozygous mutations. Science 2015, 348, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, G.; Ivy, T.; Hay, B.A. Cleave and Rescue, a novel selfish genetic element and general strategy for gene drive. Proc. Natl. Acad. Sci. USA 2019, 116, 6250–6259. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, T.; Kandul, N.P.; Bui, M.; Gamez, S.; Raban, R.; Bennett, J.; Sánchez C, H.M.; Lanzaro, G.C.; Schmidt, H.; et al. Development of a confinable gene drive system in the human disease vector Aedes aegypti. eLife 2020, 9, e51701. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Butler, C.; Yamamoto, A.; Patil, A.A.; Lloyd, A.L.; Scott, M.J. CRISPR/Cas9-based split homing gene drive targeting doublesex for population suppression of the global fruit pest Drosophila suzukii. Proc. Natl. Acad. Sci. USA 2023, 120, e2301525120. [Google Scholar] [CrossRef]
- Meccariello, A.; Hou, S.; Davydova, S.; Fawcett, J.D.; Siddall, A.; Leftwich, P.T.; Krsticevic, F.; Papathanos, P.A.; Windbichler, N. Gene drive and genetic sex conversion in the global agricultural pest Ceratitis capitata. Nat. Commun. 2024, 15, 372. [Google Scholar] [CrossRef]
- Xu, X.; Harvey-Samuel, T.; Siddiqui, H.A.; Ang, J.X.D.; Anderson, M.E.; Reitmayer, C.M.; Lovett, E.; Leftwich, P.T.; You, M.; Alphey, L. Toward a CRISPR-Cas9-based gene drive in the diamondback moth Plutella xylostella. CRISPR J. 2022, 5, 224–236. [Google Scholar] [CrossRef]
- Oberhofer, G.; Ivy, T.; Hay, B.A. Gene drive and resilience through renewal with next generation Cleave and Rescue selfish genetic elements. Proc. Natl. Acad. Sci. USA 2020, 117, 9013–9021. [Google Scholar] [CrossRef]
- Champer, J.; Lee, E.; Yang, E.; Liu, C.; Clark, A.G.; Messer, P.W. A toxin-antidote CRISPR gene drive system for regional population modification. Nat. Commun. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Champer, J.; Yang, E.; Lee, E.; Liu, J.; Clark, A.G.; Messer, P.W. A CRISPR homing gene drive targeting a haplolethal gene removes resistance alleles and successfully spreads through a cage population. Proc. Natl. Acad. Sci. USA 2020, 117, 24377–24383. [Google Scholar] [CrossRef]
- Terradas, G.; Buchman, A.B.; Bennett, J.B.; Shriner, I.; Marshall, J.M.; Akbari, O.S.; Bier, E. Inherently confinable split-drive systems in Drosophila. Nat. Commun. 2021, 12, 1480. [Google Scholar] [CrossRef]
- Nash, A.; Capriotti, P.; Hoermann, A.; Papathanos, P.A.; Windbichler, N. Intronic gRNAs for the construction of minimal gene drive systems. Front. Bioeng. Biotechnol. 2022, 10, 857460. [Google Scholar] [CrossRef]
- Sanz Juste, S.; Okamoto, E.M.; Nguyen, C.; Feng, X.; López Del Amo, V. Next-generation CRISPR gene-drive systems using Cas12a nuclease. Nat. Commun. 2023, 14, 6388. [Google Scholar] [CrossRef]
- Carballar-Lejarazú, R.; Ogaugwu, C.; Tushar, T.; Kelsey, A.; Pham, T.B.; Murphy, J.; Schmidt, H.; Lee, Y.; Lanzaro, G.C.; James, A.A. Next-generation gene drive for population modification of the malaria vector mosquito, Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2020, 117, 22805–22814. [Google Scholar] [CrossRef] [PubMed]
- Adolfi, A.; Gantz, V.M.; Jasinskiene, N.; Lee, H.-F.; Hwang, K.; Terradas, G.; Bulger, E.A.; Ramaiah, A.; Bennett, J.B.; Emerson, J. Efficient population modification gene-drive rescue system in the malaria mosquito Anopheles stephensi. Nat. Commun. 2020, 11, 5553. [Google Scholar] [CrossRef]
- Hammond, A.; Karlsson, X.; Morianou, I.; Kyrou, K.; Beaghton, A.; Gribble, M.; Kranjc, N.; Galizi, R.; Burt, A.; Crisanti, A. Regulating the expression of gene drives is key to increasing their invasive potential and the mitigation of resistance. PLoS Genet. 2021, 17, e1009321. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Liu, D.; Li, J.; Chen, J.; Yang, G. Development of CRISPR/Cas9-mediated gene-drive construct targeting the phenotypic gene in Plutella xylostella. Front. Physiol. 2022, 13, 938621. [Google Scholar] [CrossRef]
- Champer, J.; Reeves, R.; Oh, S.Y.; Liu, C.; Liu, J.; Clark, A.G.; Messer, P.W. Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLoS Genet. 2017, 13, e1006796. [Google Scholar] [CrossRef] [PubMed]
- Champer, J.; Liu, J.; Oh, S.Y.; Reeves, R.; Luthra, A.; Oakes, N.; Clark, A.G.; Messer, P.W. Reducing resistance allele formation in CRISPR gene drive. Proc. Natl. Acad. Sci. USA 2018, 115, 5522–5527. [Google Scholar] [CrossRef]
- Carrami, E.M.; Eckermann, K.N.; Ahmed, H.M.; Sánchez, C.H.M.; Dippel, S.; Marshall, J.M.; Wimmer, E.A. Consequences of resistance evolution in a Cas9-based sex conversion-suppression gene drive for insect pest management. Proc. Natl. Acad. Sci. USA 2018, 115, 6189–6194. [Google Scholar] [CrossRef]
- López Del Amo, V.; Bishop, A.L.; Sánchez C, H.M.; Bennett, J.B.; Feng, X.; Marshall, J.M.; Bier, E.; Gantz, V.M. A transcomplementing gene drive provides a flexible platform for laboratory investigation and potential field deployment. Nat. Commun. 2020, 11, 352. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Bennett, J.B.; Marshall, J.M.; Akbari, O.S. A confinable home-and-rescue gene drive for population modification. eLife 2021, 10, e65939. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Metzloff, M.; Langmüller, A.M.; Xu, X.; Clark, A.G.; Messer, P.W.; Champer, J. A homing suppression gene drive with multiplexed gRNAs maintains high drive conversion efficiency and avoids functional resistance alleles. G3 2022, 12, jkac081. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Champer, J. Assessment of distant-site rescue elements for CRISPR toxin-antidote gene drives. Front. Bioeng. Biotechnol. 2023, 11, 1138702. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.; Liu, Y.; Champer, J. Population suppression by release of insects carrying a dominant sterile homing gene drive targeting doublesex in Drosophila. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ma, S.; Ni, X.; Chen, S.; Qiao, X.; Xu, X.; Champer, J.; Huang, J. A small-molecule approach to restore female sterility phenotype targeted by a homing suppression gene drive in the fruit pest Drosophila suzukii. PLoS Genet. 2024, 20, e1011226. [Google Scholar] [CrossRef] [PubMed]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Gribble, M.; Morselli, G.; Burt, A. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat. Biotechnol. 2020, 38, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Aumann, R.A.; Häcker, I.; Schetelig, M.F. CRISPR-based genetic control strategies for insect pests. J. Integr. Agric. 2023, 22, 651–668. [Google Scholar]
- Burt, A. Heritable strategies for controlling insect vectors of disease. Phil. Trans. R. Soc. B 2014, 369, 20130432. [Google Scholar] [CrossRef]
- Akbari, O.S.; Matzen, K.D.; Marshall, J.M.; Huang, H.; Ward, C.M.; Hay, B.A. A synthetic gene drive system for local, reversible modification and suppression of insect populations. Curr. Biol. 2013, 23, 671–677. [Google Scholar] [CrossRef]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 2014, 5, 3977. [Google Scholar] [CrossRef]
- Fasulo, B.; Meccariello, A.; Morgan, M.; Borufka, C.; Papathanos, P.A.; Windbichler, N. A fly model establishes distinct mechanisms for synthetic CRISPR/Cas9 sex distorters. PLoS Genet. 2020, 16, e1008647. [Google Scholar] [CrossRef] [PubMed]
- Deredec, A.; Burt, A.; Godfray, H.C. The population genetics of using homing endonuclease genes in vector and pest management. Genetics 2008, 179, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Osta, M.A.; Christophides, G.K.; Kafatos, F.C. Effects of mosquito genes on Plasmodium development. Science 2004, 303, 2030–2032. [Google Scholar] [CrossRef]
- Franz, A.W.; Sanchez-Vargas, I.; Adelman, Z.N.; Blair, C.D.; Beaty, B.J.; James, A.A.; Olson, K.E. Engineering RNA interference–based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. USA 2006, 103, 4198–4203. [Google Scholar] [CrossRef]
- Isaacs, A.T.; Jasinskiene, N.; Tretiakov, M.; Thiery, I.; Zettor, A.; Bourgouin, C.; James, A.A. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc. Natl. Acad. Sci. USA 2012, 109, E1922–E1930. [Google Scholar] [CrossRef]
- Dong, Y.; Simões, M.L.; Dimopoulos, G. Versatile transgenic multistage effector-gene combinations for Plasmodium falciparum suppression in Anopheles. Sci. Adv. 2020, 6, eaay5898. [Google Scholar] [CrossRef]
- Hamilton, W.D. Extraordinary sex ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Courtier-Orgogozo, V.; Morizot, B.; Boëte, C. Agricultural pest control with CRISPR-based gene drive: Time for public debate: Should we use gene drive for pest control? EMBO Rep. 2017, 18, 878–880. [Google Scholar] [CrossRef]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Jury, S.; Chabot, C.C.; Goldstein, J.; Harzsch, S. Sensory biology and behaviour. In Ecophysiology of the European Green Crab (Carcinus Maenas) and Related Species; Elsevier: Amsterdam, The Netherlands, 2024; pp. 123–157. [Google Scholar]
- Jiang, C.; Xu, H.; Yang, L.; Liu, J.; Li, Y.; Takei, K.; Xu, W. Neuromorphic antennal sensory system. Nat. Commun. 2024, 15, 2109. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Pírez, N.; Klappenbach, M.; Locatelli, F.F. Experience-dependent tuning of the olfactory system. Curr. Opin. Insect Sci. 2023, 60, 101117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zung, J.L.; Hinze, A.; Kriete, A.L.; Iqbal, A.; Younger, M.A.; Matthews, B.J.; Merhof, D.; Thiberge, S.; Ignell, R. Mosquito brains encode unique features of human odour to drive host seeking. Nature 2022, 605, 706–712. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, A.Q.; Ryu, J.; Del Mármol, J. Structural basis of odor sensing by insect heteromeric odorant receptors. Science 2024, 384, eadn6384. [Google Scholar] [CrossRef]
- Gomes, J.V.; Singh-Bhagania, S.; Cenci, M.; Chacon Cordon, C.; Singh, M.; Butterwick, J.A. The molecular basis of sugar detection by an insect taste receptor. Nature 2024, 629, 228–234. [Google Scholar] [CrossRef]
- Ma, D.; Hu, M.; Yang, X.; Liu, Q.; Ye, F.; Cai, W.; Wang, Y.; Xu, X.; Chang, S.; Wang, R. Structural basis for sugar perception by Drosophila gustatory receptors. Science 2024, 383, eadj2609. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wang, P.C.; Ning, C.; Yang, K.; Li, G.C.; Cao, L.L.; Huang, L.Q.; Wang, C.Z. The larva and adult of Helicoverpa armigera use differential gustatory receptors to sense sucrose. eLife 2024, 12, RP91711. [Google Scholar] [CrossRef]
- Arntsen, C.; Guillemin, J.; Audette, K.; Stanley, M. Tastant-receptor interactions: Insights from the fruit fly. Front. Nutr. 2024, 11, 1394697. [Google Scholar] [CrossRef]
- Pentzold, S.; Burse, A.; Boland, W. Contact chemosensation of phytochemicals by insect herbivores. Nat. Prod. Rep. 2017, 34, 478–483. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, J.; Li, Y.; Li, D.; Chen, G.; Chen, L.; Yang, Z.; He, N. The silkworm gustatory receptor BmGr63 is dedicated to the detection of isoquercetin in mulberry. Proc. Biol. Sci. 2022, 289, 20221427. [Google Scholar] [CrossRef]
- Frank, H.M.; Walujkar, S.; Walsh, R.M.; Laursen, W.J.; Theobald, D.L.; Garrity, P.A.; Gaudet, R. Structural basis of ligand specificity and channel activation in an insect gustatory receptor. Cell Rep. 2024, 43, 4. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Houshyani, B.; Overeem, A.J.; Bouwmeester, H.J.; Weldegergis, B.T.; van Loon, J.J.; Dicke, M.; Vet, L.E. Genetic engineering of plant volatile terpenoids: Effects on a herbivore, a predator and a parasitoid. Pest Manag. Sci. 2013, 69, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, C.; Jian, G.; Zeng, L.; Yang, Y. Release patterns and potential utility of herbivore-induced plant volatiles in crops: A review. Environ. Exp. Bot. 2024, 219, 105659. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef]
- Volpe, H.X.; Carmo-Sousa, M.; Luvizotto, R.A.; de Freitas, R.; Esperança, V.; Darolt, J.C.; Pegoraro, A.A.; Magalhães, D.M.; Favaris, A.P.; Wulff, N.A. The greening-causing agent alters the behavioral and electrophysiological responses of the Asian citrus psyllid to a putative sex pheromone. Sci. Rep. 2024, 14, 455. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; ISBN 13 978-0-521-82149-0. [Google Scholar]
- Xu, H.J.; Zhang, C.X. Insulin receptors and wing dimorphism in rice planthoppers. Philos. Trans. R. Soc. Lond. B. Biol Sci. 2017, 372, 20150489. [Google Scholar] [CrossRef]
- Wagner, D.L.; Liebherr, J.K. Flightlessness in insects. Trends Ecol. Evol. 1992, 7, 216–220. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef]
- Zhao, Z.; Zera, A.J. Differential lipid biosynthesis underlies a tradeoff between reproduction and flight capability in a wing-polymorphic cricket. Proc. Natl. Acad. Sci. USA 2002, 99, 16829–16834. [Google Scholar] [CrossRef]
- Simpson, S.J.; Sword, G.A.; Lo, N. Polyphenism in insects. Curr. Biol. 2011, 21, R738–R749. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. Control mechanisms of polyphenic development in insects: In polyphenic development, environmental factors alter some aspects of development in an orderly and predictable way. Bioscience 1999, 49, 181–192. [Google Scholar] [CrossRef]
- Roff, D.A. The genetic basis of wing dimorphism in the sand cricket, Gryllus firmus and its relevance to the evolution of wing dimorphisms in insects. Heredity 1986, 57, 221. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, W.; Chen, X.; Lu, H.; Xiao, Y.; Li, Q.; Luo, L.; Kang, L.; Cui, F. A plant virus manipulates the long-winged morph of insect vectors. Proc. Natl. Acad. Sci. USA 2024, 121, e2315341121. [Google Scholar] [CrossRef]
- Roff, D.A.; Fairbairn, D.J. The evolution and genetics of migration in insects. Bioscience 2007, 57, 155–164. [Google Scholar] [CrossRef]
- Korkmaz, R.; Rajabi, H.; Eshghi, S.; Gorb, S.N.; Büscher, T.H. The frequency of wing damage in a migrating butterfly. Insect Sci. 2023, 30, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Gene drives gaining speed. Nat. Rev. Genet. 2022, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, T.S.; Baudrier, L.; Billon, P.; Ciccia, A. CRISPR-based genome editing through the lens of DNA repair. Mol. Cell 2022, 82, 348–388. [Google Scholar] [CrossRef]
- Li, M.; Bui, M.; Yang, T.; Bowman, C.S.; White, B.J.; Akbari, O.S. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc. Natl. Acad. Sci. USA 2017, 114, E10540–E10549. [Google Scholar] [CrossRef]
- Port, F.; Boutros, M. Tissue-specific CRISPR-Cas9 screening in Drosophila. Methods Mol. Biol. 2022, 2540, 157–176. [Google Scholar] [PubMed]
- Yajima, M.; Wessel, G.M. Essential elements for translation: The germline factor Vasa functions broadly in somatic cells. Development 2015, 142, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Port, F.; Chen, H.M.; Lee, T.; Bullock, S.L. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Zhao, F. Synonymous but not silent: The codon usage code for gene expression and protein folding. Annu. Rev. Biochem. 2021, 90, 375–401. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Cha, J.; Chae, M.; Chen, S.; Barral, J.M.; Sachs, M.S.; Liu, Y. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature 2013, 495, 111. [Google Scholar] [CrossRef]
- Gao, J.; Wang, G.; Ma, S.; Xie, X.; Wu, X.; Zhang, X.; Wu, Y.; Zhao, P.; Xia, Q. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 2015, 87, 99–110. [Google Scholar] [CrossRef]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, B.; Weeks, D.P.; Spalding, M.H.; Yang, B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014, 42, 10903–10914. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Yu, W.; Moon, A.S.; Pandey, A.; Hanley, K.A.; Xu, J. Programmable CRISPR interference for gene silencing using Cas13a in mosquitoes. J. Genom. 2020, 8, 30. [Google Scholar] [CrossRef]
- Anderson, M.A.; Gonzalez, E.; Edgington, M.P.; Ang, J.X.; Purusothaman, D.K.; Shackleford, L.; Nevard, K.; Verkuijl, S.A.; Harvey-Samuel, T.; Leftwich, P.T. A multiplexed, confinable CRISPR/Cas9 gene drive can propagate in caged Aedes aegypti populations. Nat. Commun. 2024, 15, 729. [Google Scholar] [CrossRef]
- Rylee, J.C.; Nin-Velez, A.; Mahato, S.; Helms, K.J.; Wade, M.J.; Zentner, G.E.; Zelhof, A.C. Generating and testing the efficacy of transgenic Cas9 in Tribolium castaneum. Insect. Mol. Biol. 2022, 31, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Tracey Jr, W.D.; Ning, X.; Klingler, M.; Kramer, S.G.; Gergen, J.P. Quantitative analysis of gene function in the Drosophila embryo. Genetics 2000, 154, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Duncker, B.; Davies, P.; Walker, V. Introns boost transgene expression in Drosophila melanogaster. Mol. Gen. Genet. 1997, 254, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Haber, D.A.; Arien, Y.; Lamdan, L.B.; Alcalay, Y.; Zecharia, C.; Krsticevic, F.; Yonah, E.S.; Avraham, R.D.; Krzywinska, E.; Krzywinski, J. Targeting mosquito X-chromosomes reveals complex transmission dynamics of sex ratio distorting gene drives. Nat. Commun. 2024, 15, 4983. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Zhan, S.; Wang, Y.; Huang, Y.; Xu, J.; Liu, Q.; Li, Z.; Huang, Y.; Tan, A. Expansion of CRISPR targeting sites in Bombyx mori. Insect Biochem. Mol. Biol. 2016, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Zeng, B.; Liu, Z.; Xu, X.; Meng, Q.; Huang, Y.; Yang, G.; Vasseur, L.; Gurr, G.M. Functional characterization of Pol III U6 promoters for gene knockdown and knockout in Plutella xylostella. Insect Biochem. Mol. Biol. 2017, 89, 71–78. [Google Scholar] [CrossRef]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-programmed genome editing in human cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef]
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 2014, 32, 279. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Dang, Y.; Choi, J.G.; Zhang, J.; Wu, H. Pol III promoters to express small RNAs: Delineation of transcription initiation. Mol. Ther. Nucleic Acids 2014, 3, E161. [Google Scholar] [CrossRef]
- Yoshioka, S.; Fujii, W.; Ogawa, T.; Sugiura, K.; Naito, K. Development of a mono-promoter-driven CRISPR/Cas9 system in mammalian cells. Sci. Rep. 2015, 5, 18341. [Google Scholar] [CrossRef]
- Nissim, L.; Perli, S.D.; Fridkin, A.; Perez-Pinera, P.; Lu, T.K. Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol. Cell 2014, 54, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; Dicarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, L.; Gao, Y.; Xu, J.; Han, R. Empower multiplex cell and tissue-specific CRISPR-mediated gene manipulation with self-cleaving ribozymes and tRNA. Nucleic Acids Res. 2017, 45, e28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Mon, H.; Xu, J.; Lee, J.M.; Kusakabe, T. CRISPR/Cas9-mediated knockout of factors in non-homologous end joining pathway enhances gene targeting in silkworm cells. Sci. Rep. 2015, 5, 18103. [Google Scholar] [CrossRef] [PubMed]
- Reed, F.A. CRISPR/Cas9 gene drive: Growing pains for a new technology. Genetics 2017, 205, 1037–1039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Unckless, R.L.; Clark, A.G.; Messer, P.W. Evolution of resistance against CRISPR/Cas9 gene drive. Genetics 2017, 205, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.B.; Phong, C.H.; Bennett, J.B.; Hwang, K.; Jasinskiene, N.; Parker, K.; Stillinger, D.; Marshall, J.M.; Carballar-Lejarazú, R.; James, A.A. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 2019, 15, e1008440. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Dai, X.Y.; Wang, W.T.; Yang, Z.X.; Zhao, J.J.; Zhang, J.P.; Wen, W.; Zhang, F.; Oberg, K.C.; Zhang, L. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 2021, 49, 969–985. [Google Scholar] [CrossRef]
- Roy, S.; Juste, S.S.; Sneider, M.; Auradkar, A.; Klanseck, C.; Li, Z.; Julio, A.H.F.; Del Amo, V.L.; Bier, E.; Guichard, A. Cas9/Nickase-induced allelic conversion by homologous chromosome-templated repair in Drosophila somatic cells. Sci. Adv. 2022, 8, eabo0721. [Google Scholar] [CrossRef]
- Eckhoff, P.A.; Wenger, E.A.; Godfray, H.C.J.; Burt, A. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, E255–E264. [Google Scholar] [CrossRef]
- Kim, J.; Harris, K.D.; Kim, I.K.; Shemesh, S.; Messer, P.W.; Greenbaum, G. Incorporating ecology into gene drive modelling. Ecol. Lett. 2023, 26, S62–S80. [Google Scholar] [CrossRef] [PubMed]
- Guichard, A.; Haque, T.; Bobik, M.; Xu, X.-R.S.; Klanseck, C.; Kushwah, R.B.S.; Berni, M.; Kaduskar, B.; Gantz, V.M.; Bier, E. Efficient allelic-drive in Drosophila. Nat. Commun. 2019, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.; Olejarz, J.; Esvelt, K.M.; Church, G.M.; Nowak, M.A. Evolutionary dynamics of CRISPR gene drives. Sci. Adv. 2017, 3, e1601964. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Fujiwara, H. Electroporation-mediated somatic transgenesis for rapid functional analysis in insects. Development 2013, 140, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Dion, S.L.; Kutny, P.M.; Zhang, Y.; Cheng, A.W.; Jillette, N.L.; Malhotra, A.; Geurts, A.M.; Chen, Y.G.; Wang, H. Efficient CRISPR/Cas9-mediated genome editing in mice by zygote electroporation of nuclease. Genetics 2015, 200, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chaverra-Rodriguez, D.; Macias, V.M.; Hughes, G.L.; Pujhari, S.; Suzuki, Y.; Peterson, D.R.; Kim, D.; McKeand, S.; Rasgon, J.L. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat. Commun. 2018, 9, 3008. [Google Scholar] [CrossRef] [PubMed]
- Chaverra-Rodriguez, D.; Dalla Benetta, E.; Heu, C.C.; Rasgon, J.L.; Ferree, P.M.; Akbari, O.S. Germline mutagenesis of Nasonia vitripennis through ovarian delivery of CRISPR-Cas9 ribonucleoprotein. Insect. Mol. Biol. 2020, 29, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Macias, V.M.; McKeand, S.; Chaverra-Rodriguez, D.; Hughes, G.L.; Fazekas, A.; Pujhari, S.; Jasinskiene, N.; James, A.A.; Rasgon, J.L. Cas9-mediated gene-editing in the malaria mosquito Anopheles stephensi by ReMOT Control. G3 2020, 10, 1353–1360. [Google Scholar] [CrossRef]
- Marshall, J.M.; Akbari, O.S. Can CRISPR-based gene drive be confined in the wild? A question for molecular and population biology. ACS Chem. Biol. 2018, 13, 424–430. [Google Scholar] [CrossRef]
- Unckless, R.L.; Messer, P.W.; Connallon, T.; Clark, A.G. Modeling the manipulation of natural populations by the mutagenic chain reaction. Genetics 2015, 201, 425–431. [Google Scholar] [CrossRef]
- Noble, C.; Adlam, B.; Church, G.M.; Esvelt, K.M.; Nowak, M.A. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife 2018, 7, e33423. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.A.; Golnar, A.J.; Overcash, J.M.; Lloyd, A.L.; Hayes, K.R.; O’Brochta, D.A.; Pepin, K.M. Leveraging eco-evolutionary models for gene drive risk assessment. Trends Genet. 2023, 39, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F.; Church, G.M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 2014, 3, 3277–3288. [Google Scholar] [CrossRef] [PubMed]
- Akbari, O.S.; Bellen, H.J.; Bier, E.; Bullock, S.L.; Burt, A.; Church, G.M.; Cook, K.R.; Duchek, P.; Edwards, O.R.; Esvelt, K.M. Safeguarding gene drive experiments in the laboratory. Science 2015, 349, aac7932. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.A.; Avraam, G.; Hoermann, A.; Wyer, C.A.; Ong, Y.X.; Christophides, G.K.; Windbichler, N. Testing non-autonomous antimalarial gene drive effectors using self-eliminating drivers in the African mosquito vector Anopheles gambiae. PLoS Genet. 2022, 18, e1010244. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, G.; Ivy, T.; Hay, B.A. Split versions of Cleave and Rescue selfish genetic elements for measured self limiting gene drive. PLoS Genet. 2021, 17, e1009385. [Google Scholar] [CrossRef]
- Champer, J.; Chung, J.; Lee, Y.L.; Liu, C.; Yang, E.; Wen, Z.; Clark, A.G.; Messer, P.W. Molecular safeguarding of CRISPR gene drive experiments. eLife 2019, 8, e41439. [Google Scholar] [CrossRef]
- Xu, X.-R.S.; Bulger, E.A.; Gantz, V.M.; Klanseck, C.; Heimler, S.R.; Auradkar, A.; Bennett, J.B.; Miller, L.A.; Leahy, S.; Juste, S.S. Active genetic neutralizing elements for halting or deleting gene drives. Mol. Cell 2020, 80, 246–262.e4. [Google Scholar] [CrossRef]
- Taxiarchi, C.; Beaghton, A.; Don, N.I.; Kyrou, K.; Gribble, M.; Shittu, D.; Collins, S.P.; Beisel, C.L.; Galizi, R.; Crisanti, A. A genetically encoded anti-CRISPR protein constrains gene drive spread and prevents population suppression. Nat. Commun. 2021, 12, 3977. [Google Scholar] [CrossRef]
- Chae, D.; Lee, J.; Lee, N.; Park, K.; Moon, S.J.; Kim, H.H. Chemical controllable gene drive in Drosophila. ACS Synth. Biol. 2020, 9, 2362–2377. [Google Scholar] [CrossRef]
- D’Amato, R.; Taxiarchi, C.; Galardini, M.; Trusso, A.; Minuz, R.L.; Grilli, S.; Somerville, A.G.; Shittu, D.; Khalil, A.S.; Galizi, R. Anti-CRISPR Anopheles mosquitoes inhibit gene drive spread under challenging behavioural conditions in large cages. Nat. Commun. 2024, 15, 952. [Google Scholar] [CrossRef] [PubMed]
- Trump, B.D.; Cummings, C.L.; Kuzma, J.; Linkov, I. Synthetic Biology 2020: Frontiers in Risk Analysis and Governance; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Macias, V.; James, A.A. Impact of genetic modification of vector populations on the malaria eradication agenda. In Genetic Control of Malaria and Dengue; Elsevier Academic Press: London, UK, 2015; pp. 423–444. [Google Scholar]
- Alphey, L. Genetic control of mosquitoes. Annu. Rev. Entomol. 2014, 33, 59. [Google Scholar] [CrossRef] [PubMed]

| Organism | Gene Drive Construct | Cas9 Cassette | gRNA Cassette | Marker | Target Gene | Phenotype | Homing Rate (G1) | Reference |
|---|---|---|---|---|---|---|---|---|
| D. melanogaster | MCR | vasa-Cas9 | U6:3-gRNA | – | yellow | yellow body color | 97% | [33] |
| – | nanos-Cas9 vasa-Cas9 | U6:3-gRNA | 3xP3-DsRed | yellow | yellow body color | 62% 52% | [52] | |
| – | vasa-Cas9 | U6:3-gRNA | 3xP3-DsRed | yellow white | yellow body color white-eye | 37–53% 56% | [53] | |
| nanos-Cas9 | U6:3-gRNA | 3xP3-DsRed | white white-2gRNA cinnabar cinnabar yellow | white-eye white-eye brilliant orange eye brilliant orange eye yellow body color | 59% 76% 54% 38% 40–62% | |||
| CHE | Rcd-1r-Cas9 | U6:3-gRNA | 3xP3-DsRed | transformer | sex conversion from females to males | 56% | [54] | |
| ClvR | nanos-Cas9 | U6:3-gRNA | 3xP3-GFP | tko | lethality | 99% | [37] | |
| tGD | vasa-Cas9 | U6:3-gRNA | 3xP3-DsRed 3xP3-EGFP | yellow ebony white | yellow body color dark body color white-eye | 67–98% | [55] | |
| ClvR | nanos-Cas9 | U6:3-gRNA | 3xP3-GFP opie-td-tomato | dbe TfIIA-S | recessive lethal | >99% in ♀; >94.7 to >99% in ♂ | [42] | |
| TARE | nanos-Cas9 | U6:3-gRNA | 3xP3-DsRed 3xP3-GFP | hairy | recessive lethal | 88–95% in ♀ | [43] | |
| – | nanos-Cas9 | U6:3-gRNA | 3xP3-DsRed 3xP3-GFP | RpL35A | haplolethal | 91% | [44] | |
| sGD | nanos-Cas9 vasa-Cas9 | U6:3-gRNA | 3xP3-tdTomato 3XP3-eGFP Opie2-DsRed | rab5 rab11 spo11 prosalpha2 | recessive lethal recessive lethal sterility recessive lethal | 64.8–99.9% | [45] | |
| HomeR | nanos-Cas9 | U6:3-gRNA | 3xP3-EGFP | PolG2 | lethality | 99.6% in ♀; 75.0% in ♂ | [56] | |
| – | nanos-Cas9 | U6:3-gRNA | 3xP3-DsRed | yellow-g | recessive female sterility | 86.4% in ♀; 90.4% in ♂ | [57] | |
| – | rcd-1r-Cas9 | U6:3-gRNA | 3xP3-CFP | rcd-1r | male fertility | 77.1% in ♂; 80.5% in ♀ | [46] | |
| TARE TADE | nanos-Cas9 | U6:3-gRNA | 3xP3-DsRed | RpL35A hairy | haplolethal recessive lethal | 51–54% | [58] | |
| – | vasa-Cas12a | U6:3-gRNA | Opie2-GFP | ebony | dark body color | 52–89% | [47] | |
| – | nanos-Cas9 CG4415-Cas9 rcd-1r-Cas9 | U6:3-gRNA | 3xP3-DsRed 3xP3-GFP | doublesex | dominant female sterility | 70.0–85.5% | [59] | |
| Drosophila suzukii | – | nanos-Cas9 | U6:3-gRNA | pUb-DsRed hsp83-ZsGreen | doublesex | dominant female sterility | 94–99% | [39] |
| DsTdc2CRISPR | vasa-Cas9 | U6:3-gRNA | PUb-DsRed | Tyrosine decarboxylase 2 | recessive female fertility | 53.6–58.2% | [60] | |
| Anopheles stephensi | AsMCRkh2 | vasa-Cas9 | U6-gRNA | 3xP3-GFP | kynurenine hydroxylasewhite | white-eye | G1: 99.5% G3: 98.8% G4: 97.2% | [34] |
| Anopheles gambiae | CRISPRh | vasa2-Cas9 | U6-gRNA | 3xP3-RFP | AGAP007280 AGAP005958 AGAP011377 | sterility | G2: 99%, G3: 97.6% G2: 95.8%, G3: 92.8% G2: 82.8%, G3: 75.5%, | [35] |
| Anopheles gambiae | dsxFCRISPRh | zpg-Cas9 | U6-gRNA | 3xP3-RFP | doublesex | sterility in homozygous females | 95.9% in ♂; 99.4% in ♀ | [36] |
| Anopheles gambiae | SDGDdsx | zpg-Cas9 | U6-gRNA | 3xP3-DsRed | doublesex X chromosome | distort sex ratios (male only) | 92% in ♂; 99% in ♀ | [61] |
| Anopheles gambiae | AgNosCd-1 | nanos-Cas9 | U6-gRNA | 3xP3-CFP | Agcd | red-eye | 96.7% (G1–G4) | [48] |
| Anopheles stephensi | Reckh | vasa-Cas9 | U6A-gRNA | 3xP3-GFP | kynurenine hydroxylasewhite | white-eye | 99.8% in ♂; 57% in ♀ | [49] |
| Anopheles gambiae | nos-CRISPRh zpg-CRISPRh exu-CRISPRh | zpg-Cas9 nanos-Cas9 exu-Cas9 | U6-gRNA | 3xP3-DsRed | AGAP007280 | recessive female sterility | 93.5% in ♂, 97.8% in ♀; 99.6% in ♂, 99.1% in ♀; 65.0% in ♂; 0 in ♀ | [50] |
| Ceratitis capitata | – | vasa-Cas9 | U6-gRNA | pUb-DsRed | transformer | sex conversion from females to males | 83.1% | [40] |
| Plutella xylostella | – | vasa-Cas9 meiw68-Cas9 nanos-Cas9 | U6-gRNA | Hr5ie1-DsRed | yellow kmo | yellow-pigmentation yellow-eye | no significant deviation from 50% inheritance | [41] |
| Plutella xylostella | – | nanos-Cas9 | U6-gRNA | Hr5ie1-EGFP | yellow | yellow-pigmentation | 6.67–12.59% | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, L.; Wei, L.; Wang, Y.; Han, Z. Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management. Insects 2024, 15, 653. https://doi.org/10.3390/insects15090653
Zhao Y, Li L, Wei L, Wang Y, Han Z. Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management. Insects. 2024; 15(9):653. https://doi.org/10.3390/insects15090653
Chicago/Turabian StyleZhao, Yu, Longfeng Li, Liangzi Wei, Yifan Wang, and Zhilin Han. 2024. "Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management" Insects 15, no. 9: 653. https://doi.org/10.3390/insects15090653
APA StyleZhao, Y., Li, L., Wei, L., Wang, Y., & Han, Z. (2024). Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management. Insects, 15(9), 653. https://doi.org/10.3390/insects15090653






