Enhancing Biological Control Efficacy: Insights into the Feeding Behavior and Fitness of the Omnivorous Pest Lygus lineolaris
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Rearing
2.3. Biological Trials
2.4. Statistical Analyses
3. Results
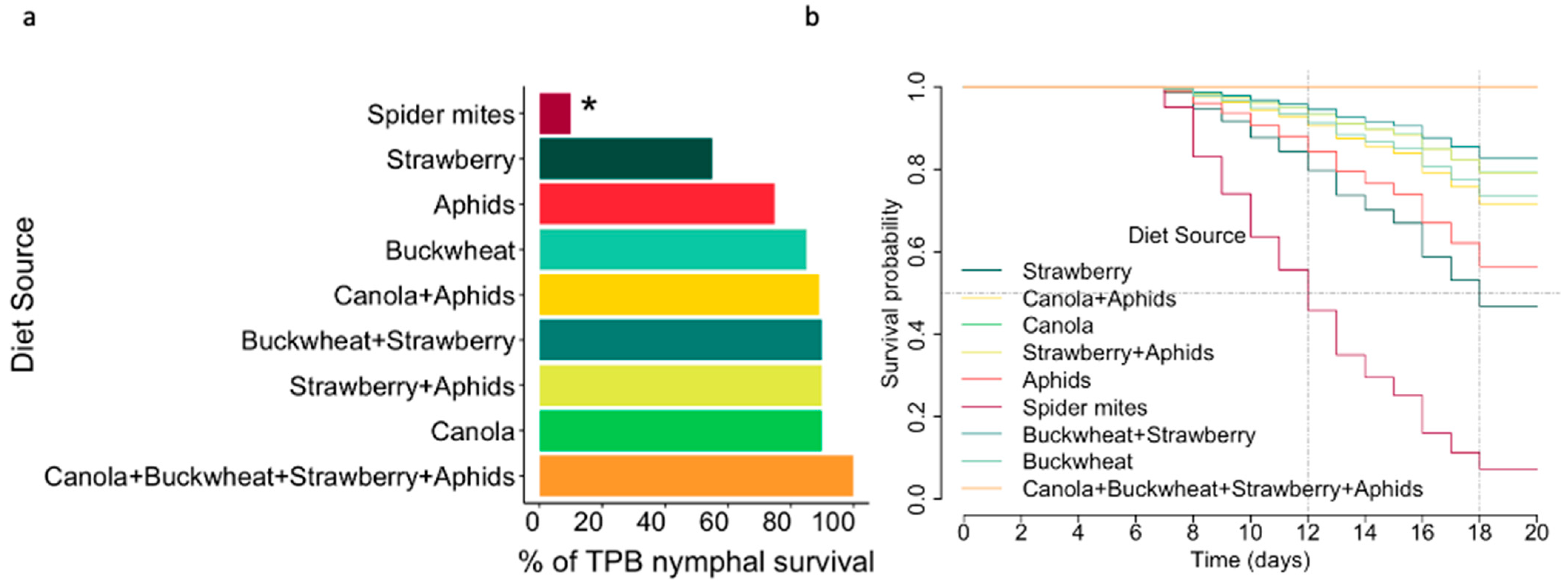
3.1. Diet Source
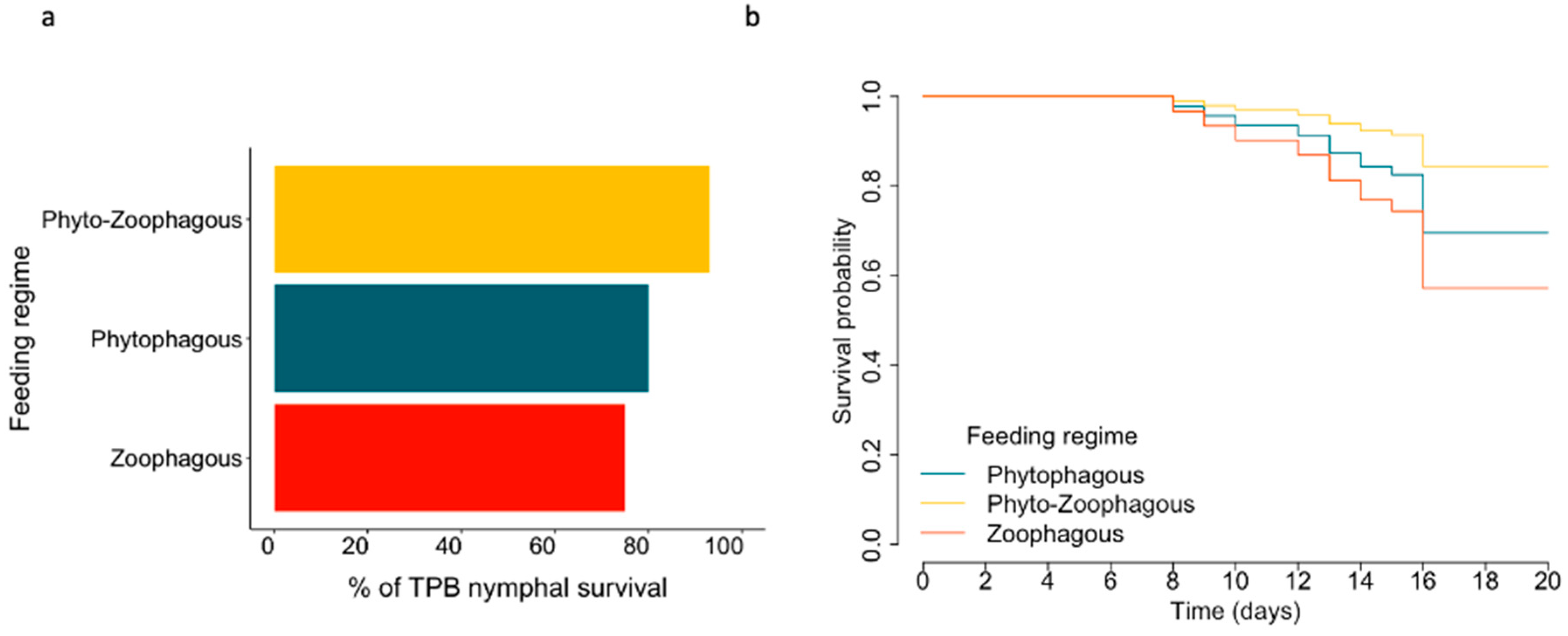
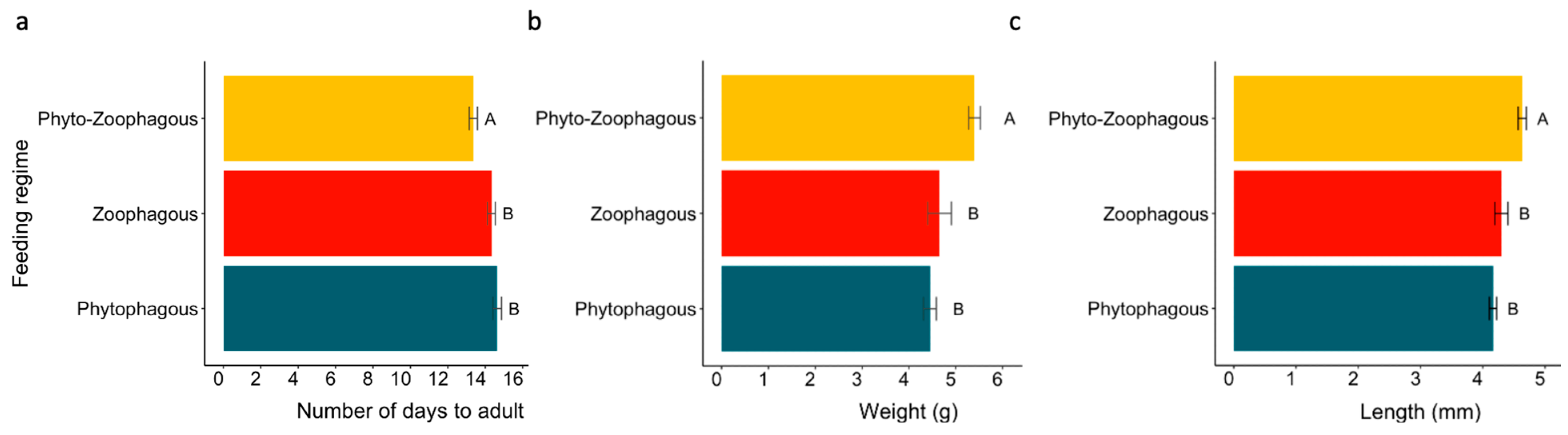
3.2. Feeding Regimes
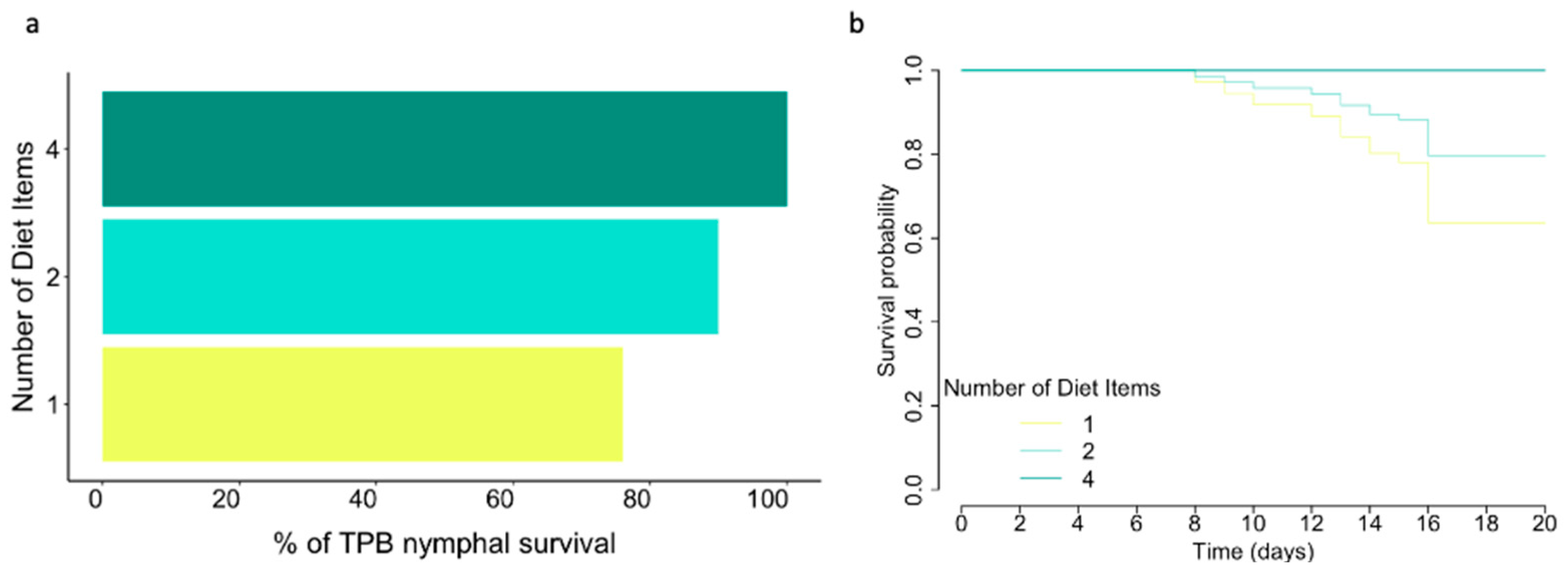
3.3. Number of Diet Items
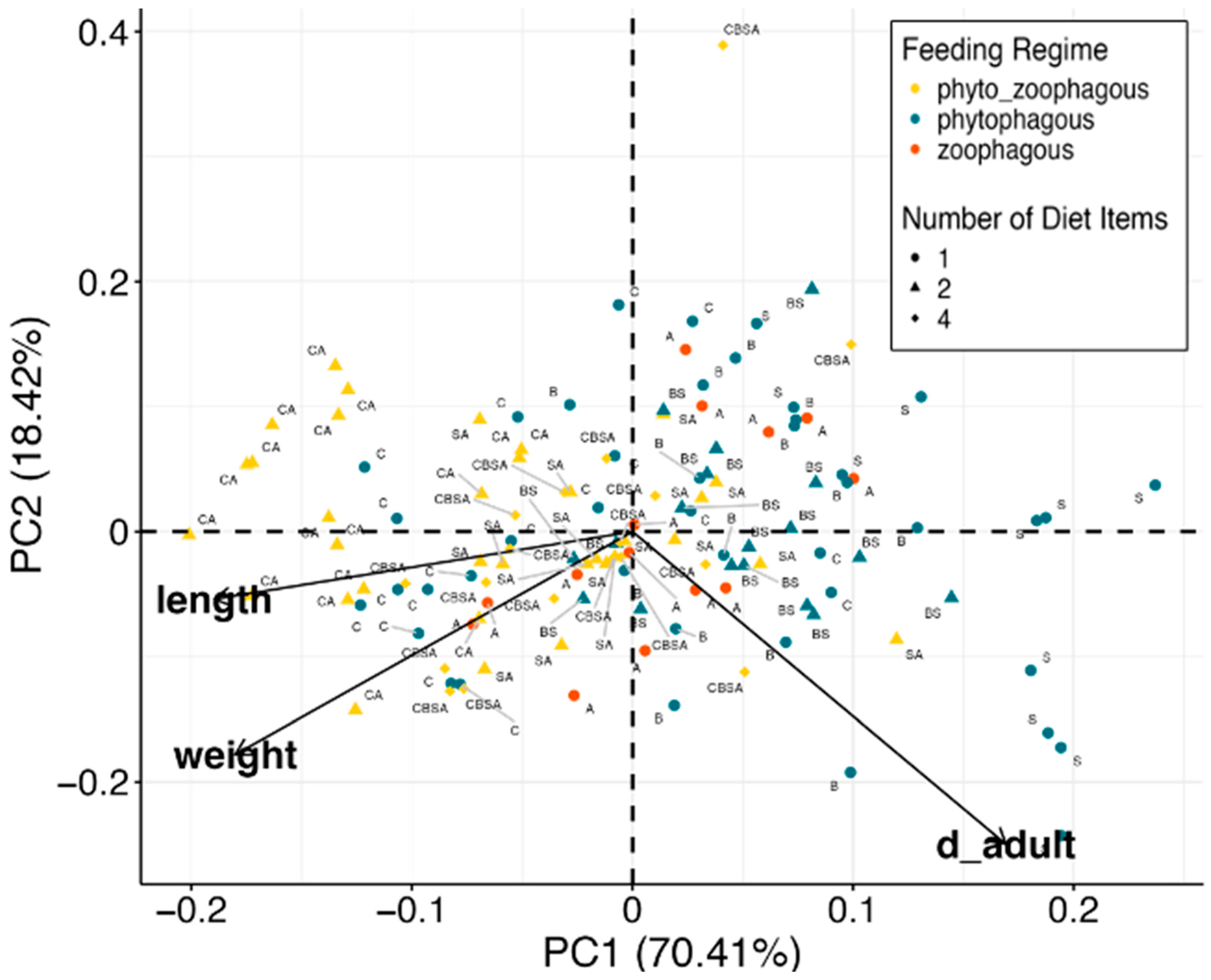
3.4. PCA Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
Conflicts of Interest
References
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Jones, S.A. Nutritional imbalance in an extreme generalist omnivore: Tolerance and recovery through complementary food selection. Anim. Behav. 2006, 71, 1253–1262. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Denno, R.F. Host plants mediate omnivore–herbivore interactions and influence prey suppression. Ecology 2000, 81, 936–947. [Google Scholar]
- Rosenheim, J.A.; Goeriz, R.E.; Thacher, E.F. Omnivore or herbivore? Field observations of foraging by Lygus hesperus (Hemiptera: Miridae). Environ. Entomol. 2004, 33, 1362–1370. [Google Scholar] [CrossRef]
- Hagler, J.R.; Jackson, C.G.; Blackmer, J.L. Diet selection exhibited by juvenile and adult lifestages of the omnivores western tarnished plant bug, Lygus hesperus and tarnished plant bug, Lygus lineolaris. J. Insect Sci. 2010, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.; Whalen, M.; Davenport, T.; Stone, J.; Duffy, J. Physiological effects of diet mixing on consumer fitness: A meta-analysis. Ecology 2013, 94, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.S.; Bernays, E.A. Understanding omnivory needs a behavioral perspective. Ecology 2003, 84, 2532–2537. [Google Scholar] [CrossRef]
- Vankosky, M.A.; VanLaerhoven, S.L. Plant and prey quality interact to influence the foraging behaviour of an omnivorous insect, Dicyphus hesperus. Anim. Behav. 2015, 108, 109–116. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Brent, C.S. Stage-specific effects of population density on the development and fertility of the western tarnished plant bug, Lygus hesperus. J. Insect Sci. 2010, 10, 49. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Cañas, L.A. Visual cues enhance the response of Lygus hesperus (Heteroptera: Miridae) to volatiles from host plants. Environ. Entomol. 2005, 34, 1524–1533. [Google Scholar] [CrossRef]
- Burla, J.P.; Grille, G.; Lorenzo, M.E.; Franco, J.; Bonato, O.; Basso, C. Effect of different diets on the development, mortality, survival, food uptake and fecundity of Tupiocoris cucurbitaceus (Hemiptera: Miridae). Fla. Entomol. 2014, 97, 1816–1824. [Google Scholar] [CrossRef]
- Singer, M.S.; Rodrigues, D.; Stireman, J.O.; Carrière, Y. Roles of Food Quality and Enemy-Free Space in Host Use by a Generalist Insect Herbivore. Ecology 2004, 85, 2747–2753. [Google Scholar] [CrossRef]
- Agustí, N.; Cohen, A.C. Lygus hesperus and L. lineolaris (Hemiptera: Miridae), phytophages, zoophages, or omnivores: Evidence of feeding adaptations suggested by the salivary and midgut digestive enzymes. J. Entomol. Sci. 2000, 35, 176–186. [Google Scholar] [CrossRef]
- García, M.; Farinós, G.P.; Castañera, P.; Ortego, F. Digestion, growth and reproductive performance of the zoophytophagous rove beetle Philonthus quisquiliarius (Coleoptera: Staphylinidae) fed on animal and plant based diets. J. Insect Physiol. 2012, 58, 1334–1342. [Google Scholar] [CrossRef]
- Royer, P.; Dumont, F.; Provost, C.; Lucas, E. Selecting aggressiveness to improve biological control agents efficiency. J. Pest Sci. 2022, 95, 1589–1596. [Google Scholar] [CrossRef]
- Eubanks, M.D. Predaceous Herbivores and Herbivorous Predators the Biology of Omnivores and the Ecology of Omnivore-Prey Interactions. In Ecology of Predator-Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford Academic: Oxford, UK, 2005. [Google Scholar]
- Cohen, A.C. New oligidic production diet for Lygus hesperus Knight and L. lineolaris (Palisot de Beauvois). J. Entomol. Sci. 2000, 35, 301–310. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Styrsky, J.D.; Denno, R.F. The evolution of omnivory in heteropteran insects. Ecology 2003, 84, 2549–2556. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A. Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J. Econ. Entomol. 2008, 101, 1864–1870. [Google Scholar] [CrossRef]
- Gillespie, D.; Vanlaerhoven, S.; Mcgregor, R.; Chan, S.; Roitberg, B. Plant Feeding in an Omnivorous Mirid, Dicyphus hesperus: Why Plant Context Matters. Psyche 2012, 1, 495805. [Google Scholar] [CrossRef]
- Leman, A.; Ingegno, B.L.; Tavella, L.; Janssen, A.; Messelink, G.J. The omnivorous predator Macrolophus pygmaeus, a good candidate for the control of both greenhouse whitefly and poinsettia thrips on gerbera plants. Insect Sci. 2020, 27, 510–518. [Google Scholar] [CrossRef]
- Urbaneja, A.; Coll, M.; Jaques, J.A.; Serrao, J.E.; Perdikis, D.; Roda, A.L. Special issue on recent advances in zoophytophagous arthropods for agroecosystems sustainability. J. Pest Sci. 2022, 95, 1469–1471. [Google Scholar] [CrossRef]
- Dumont, F.; Solà Cassi, M.; Lemay, M.; Provost, C. Artificial selection of zoophagous lines of the biological control agent Dicyphus hesperus. Entomol. Exp. Appl. 2024. [Google Scholar] [CrossRef]
- Rancourt, B.; Vincent, C.; De Oliveira, D. Circadian activity of Lygus lineolaris (Hemiptera: Miridae) and effectiveness of sampling techniques in strawberry fields. J. Econom. Entomol. 2000, 93, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.G. Four Holarctic Plant Bugs (Hemiptera: Miridae) Associated with Carex utriculata (Cyperaceae) in Montane Grasslands of Valles Caldera, New Mexico: Southernmost US Records and Notes on Seasonality. Proc. Entomol. Soc. Wash. 2011, 113, 203–217. [Google Scholar] [CrossRef]
- Zhu, Y.C.; West, S.; Snodgrass, G.; Luttrell, R. Variability in resistance-related enzyme activities in field populations of the tarnished plant bug, Lygus lineolaris. Pestic. Biochem. Physiol. 2011, 99, 265–273. [Google Scholar] [CrossRef]
- George, J.; Glover, J.P.; Gore, J.; Crow, W.D.; Reddy, G.V.P. Biology, Ecology, and Pest Management of the Tarnished Plant Bug, Lygus lineolaris (Palisot de Beauvois) in Southern Row Crops. Insects 2021, 12, 807. [Google Scholar] [CrossRef]
- Snodgrass, G.L.; Scott, W.P.; Smith, J.W. Host plants and seasonal distribution of the tarnished plant bug (Hemiptera: Miridae) in the Delta of Arkansas, Louisiana, and Mississippi. Environ. Entomol. 1984, 13, 110–116. [Google Scholar] [CrossRef]
- Young, O.P. Host plants of the tarnished plant bug, Lygus lineolaris (Heteroptera: Miridae). Ann. Entomol. Soc. Am. 1986, 79, 747–762. [Google Scholar] [CrossRef]
- Al-Ghamdi, K.M.; Stewart, R.K.; Boivin, G. Synchrony between populations of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois)(Hemiptera: Miridae), and its egg parasitoids in southwestern Quebec. Can. Entomol. 1995, 127, 457–472. [Google Scholar] [CrossRef]
- Solà Cassi, M.; Dumont, F.; Provost, C.; Lucas, E. Lygus lineolaris: A pest preying on other pests. In Proceedings of the International Symposium Ecology of Aphidophaga XV, Lleida, Spain, 19–23 September 2022. [Google Scholar]
- Ugine, T.A.; Krasnoff, S.B.; Grebenok, R.J.; Behmer, S.T.; Losey, J.E. Prey nutrient content creates omnivores out of predators. Ecol. Lett. 2019, 2, 275–283. [Google Scholar] [CrossRef]
- Cárcamo, H.A.; Meers, S.B.; Herle, C.E. Managing cabbage seedpod weevils (Coleoptera: Curculionidae) in canola (Brassicaceae)—Are Lygus (Hemiptera: Miridae) affected? Can. Entomol. 2019, 151, 85–93. [Google Scholar] [CrossRef]
- Dumont, F.; Provost, C. Combining the use of trap crops and insecticide sprays to control the tarnished plant bug (Hemiptera: Miridae) in strawberry (Rosaceae) fields. Can. Entomol. 2019, 151, 251–259. [Google Scholar] [CrossRef]
- Bianchi, F.M.; Marsaro Júnior, A.L.; Grazia, J.; Pereira, P.R.V.S.; Panizzi, A.R. Diversity of Stink Bugs (Pentatomidae) Associated with Canola: Looking for Potential Pests. Neotrop. Entomol. 2019, 48, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Denno, R.F. The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 1999, 80, 1253–1266. [Google Scholar] [CrossRef]
- Thompson, R.M.; Hemberg, M.; Starzomski, B.M.; Shurin, J.B. Trophic levels and trophic tangles: The prevalence of omnivory in real food webs. Ecology 2007, 88, 612–617. [Google Scholar] [CrossRef]
- Aubry, O.; Cormier, D.; Chouinard, G.; Lucas, E. Influence of plant, animal and mixed resources on development of the zoophytophagous plant bug Campylomma verbasci (Hemiptera: Miridae). Biocontrol. Sci. Technol. 2015, 25, 1426–1442. [Google Scholar] [CrossRef]
- Janssen, A.; Willemse, E.; Van Der Hammen, T. Poor host plant quality causes omnivore to consume predator eggs. J. Anim. Ecol. 2003, 72, 478–483. [Google Scholar] [CrossRef]
- Lindquist, R.K.; Sorensen, E.L. Interrelationships among aphids, tarnished plant bugs, and alfalfas. J. Econ. Entomol. 1970, 63, 192–195. [Google Scholar] [CrossRef]
- Wheeler, G.S.; Slansky, F., Jr.; Yu, S.J. Food consumption, utilization and detoxification enzyme activity of larvae of three polyphagous noctuid moth species when fed the botanical insecticide rotenone. Entomol. Exp. Appl. 2001, 98, 225–239. [Google Scholar] [CrossRef]
- Woelke, J.B.; Bouw, M.; Cusumano, A.; Messelink, G.J. Lygus rugulipennis on chrysanthemum: Supplemental prey effects and an evaluation of trap plants. J. Appl. Entomol. 2023, 147, 157–166. [Google Scholar] [CrossRef]
- Oberhauser, K.; Howard, E.; Batalden, R. Monarch Butterfly Monitoring in North America: Overview of Initiatives and Protocols; Commission for Environmental Cooperation, Communications Department: Montreal, QC, Canada, 2009. [Google Scholar]
- Dolezal, A.G.; Carrillo-Tripp, J.; Judd, T.M.; Allen Miller, W.; Bonning, B.C.; Toth, A.L. Interacting stressors matter: Diet quality and virus infection in honeybee health. R. Soc. Open Sci. 2019, 6, 181803. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Bouagga, S.; Zhang, N.X.; Moerkens, R.; Messelink, G.; Jaques, J.A.; Flors, V.; Broufas, B.; Urbaneja, A.; Pappas, M.L. Induction of plant defenses: The added value of zoophytophagous predators. J. Pest Sci. 2022, 95, 1501–1517. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kobayashi, C.; Thaler, J.S. Influence of Prey Availability and Induced Host-Plant Resistance on Omnivory by Western Flower Thrips. Ecology 1999, 80, 518–523. [Google Scholar] [CrossRef]
- Thomine, E.; Jeavons, E.; Rusch, A.; Bearez, P.; Desneux, N. Effect of crop diversity on predation activity and population dynamics of the mirid predator Nesidiocoris tenuis. J. Pest Sci. 2020, 93, 1255–1265. [Google Scholar] [CrossRef]
- Esquivel, J.F.; Mowery, S.V. Host plants of the tarnished plant bug (Heteroptera: Miridae) in central Texas. Environ. Entomol. 2007, 36, 725–730. [Google Scholar] [CrossRef]
- Dumont, F.; Provost, C. Using Autumnal Trap Crops to Manage Tarnished Plant Bugs (Lygus lineolaris). Insects 2022, 13, 441. [Google Scholar] [CrossRef]
- Swezey, S.L.; Nieto, D.J.; Bryer, J.A. Control of Western Tarnished Plant Bug Lygus hesperus Knight (Hemiptera: Miridae) in California Organic Strawberries Using Alfalfa Trap Crops and Tractor-Mounted Vacuums. Environ. Entomol. 2007, 36, 1457–1465. [Google Scholar] [CrossRef]
- Lucas, E.; Rosenheim, J.A. Influence of extraguild prey density on intraguild predation by heteropteran predators: A review of the evidence and a case study. Biol. Control 2011, 59, 61–67. [Google Scholar] [CrossRef]


| Feeding Regime | Diet Source [20 *] | Number of Diet Items |
|---|---|---|
| Phyto-Zoophagous [59] | Canola + Buckwheat + Aphids + Strawberry (CBSA) | 4 [20] |
| Canola + Aphids (CA) | 2 [59] | |
| Strawberry + Aphids (SA) | ||
| Phytophagous [80] | Buckwheat + Strawberry (BS) | |
| Canola (C) | 1 [80] | |
| Buckwheat (B) | ||
| Strawberry (S) | ||
| Zoophagous [20] | Aphids (A) | |
| Spider mites ** (M) |
| Fitness Measure | Diet (df = 8) | Feeding Regime (df = 2) | Number of Diet Items (df = 2) | |||
|---|---|---|---|---|---|---|
| Statistic | p-Value | Statistic | p-Value | Statistic | p-Value | |
| Nymph survivorship | X2 = 65.65 | *** | X2 = 6.54 | * | X2 = 11.84 | * |
| Nymph survival curves | LR = 46.14 | *** | LR = 3.51 | 0.2 | LR = 9.05 | * |
| Nymph development time | X2 = 57.40 | ** | X2 = 17.22 | ** | X2 = 5.70 | 0.06 |
| Adult body weight | X2 = 55.70 | ** | X2 = 24.73 | *** | X2 = 11.01 | * |
| Adult body length | X2 = 69.94 | ** | X2 = 23.84 | ** | X2 = 12.00 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solà Cassi, M.; Dumont, F.; Provost, C.; Lucas, E. Enhancing Biological Control Efficacy: Insights into the Feeding Behavior and Fitness of the Omnivorous Pest Lygus lineolaris. Insects 2024, 15, 665. https://doi.org/10.3390/insects15090665
Solà Cassi M, Dumont F, Provost C, Lucas E. Enhancing Biological Control Efficacy: Insights into the Feeding Behavior and Fitness of the Omnivorous Pest Lygus lineolaris. Insects. 2024; 15(9):665. https://doi.org/10.3390/insects15090665
Chicago/Turabian StyleSolà Cassi, Mireia, François Dumont, Caroline Provost, and Eric Lucas. 2024. "Enhancing Biological Control Efficacy: Insights into the Feeding Behavior and Fitness of the Omnivorous Pest Lygus lineolaris" Insects 15, no. 9: 665. https://doi.org/10.3390/insects15090665
APA StyleSolà Cassi, M., Dumont, F., Provost, C., & Lucas, E. (2024). Enhancing Biological Control Efficacy: Insights into the Feeding Behavior and Fitness of the Omnivorous Pest Lygus lineolaris. Insects, 15(9), 665. https://doi.org/10.3390/insects15090665





