An Interspecific Assessment of Bergmann’s Rule in Tenebrionid Beetles (Coleoptera, Tenebrionidae) along an Elevation Gradient
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
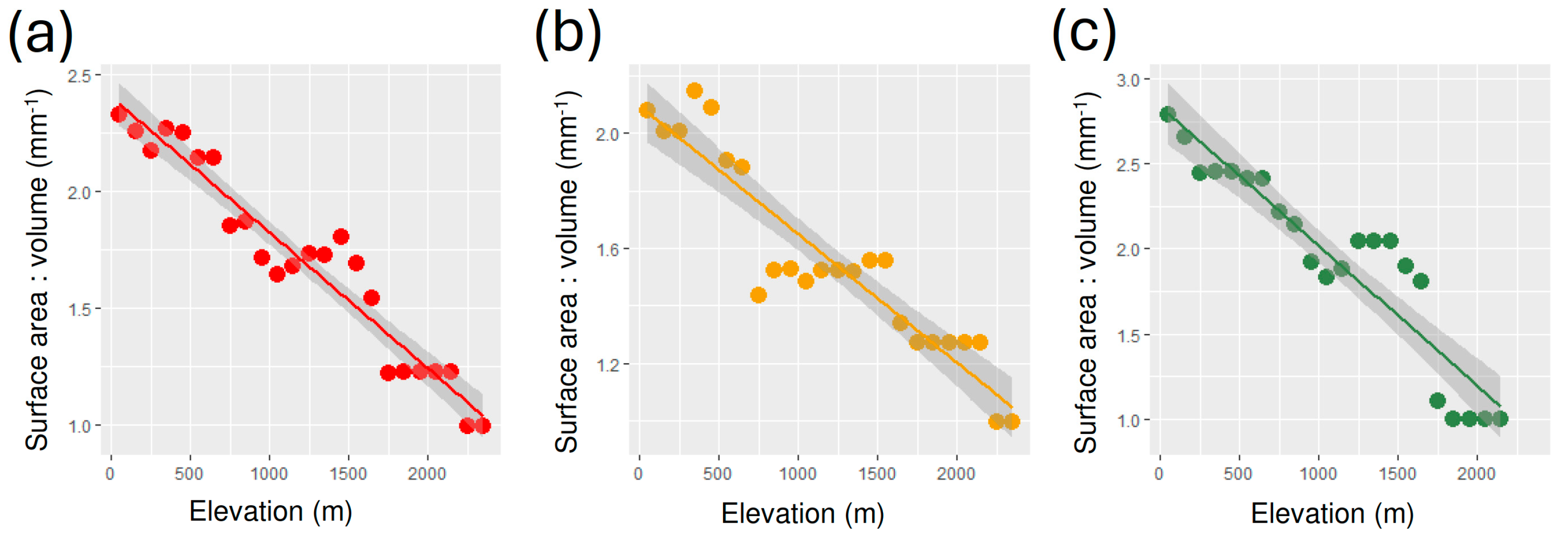
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Stud. 1847, 1, 595–708. [Google Scholar]
- Ashton, K.G. Are ecological and evolutionary rules being dismissed prematurely? Divers. Distrib. 2001, 7, 289–295. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- McNab, B.K. On the ecological significance of Bergmann’s rule. Ecology 1971, 52, 845–854. [Google Scholar] [CrossRef]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Meiri, S.; Thomas, G.H. The geography of body size–challenges of the interspecific approach. Glob. Ecol. Biogeogr. 2007, 16, 689–693. [Google Scholar] [CrossRef]
- Meiri, S. Bergmann’s Rule—What’s in a name? Glob. Ecol. Biogeogr. 2011, 20, 203–207. [Google Scholar] [CrossRef]
- Watt, C.; Mitchell, S.; Salewski, V. Bergmann’s rule; A concept cluster? Oikos 2010, 119, 89–100. [Google Scholar] [CrossRef]
- Olalla-Tárraga, M.Á. “Nullius in Bergmann” or the pluralistic approach to ecogeographical rules: A reply to Watt et al. (2010). Oikos 2011, 120, 1441–1444. [Google Scholar] [CrossRef]
- Watt, C.; Salewski, V. Bergmann’s rule encompasses mechanism: A reply to Olalla-Ta′rraga. Oikos 2011, 120, 1445–1447. [Google Scholar] [CrossRef]
- Bogin, B.; Hermanussen, M.; Scheffler, C. Bergmann’s rule is a “just-so” story of human body size. J. Physiol. Anthropol. 2022, 41, 15. [Google Scholar] [CrossRef]
- McQueen, A.; Klaassen, M.; Tattersall, G.J.; Atkinson, R.; Jessop, R.; Hassell, C.J.; Christie, M.; Victorian Wader Study Group; Australasian Wader Studies Group; Symonds MRE. Thermal adaptation best explains Bergmann’s and Allen’s rules across ecologically diverse shorebirds. Nat. Commun. 2022, 13, 4727. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tu, J.; Yu, J.; Jiang, H. A global assessment of Bergmann’s rule in mammals and birds. Glob. Change Biol. 2023, 29, 5199–5210. [Google Scholar] [CrossRef] [PubMed]
- Salewski, V.; Watt, C. Bergmann’s rule: A biophysiological rule examined in birds. Oikos 2017, 126, 161–172. [Google Scholar] [CrossRef]
- Mayr, E. Geographical character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- James, F.C. Geographic size variation in birds and its relationship to climate. Ecology 1970, 51, 365–390. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef]
- Partridge, L.; Coyne, J.A. Bergmann’s rule in ectotherms: Is it adaptive? Evolution 1997, 51, 632–635. [Google Scholar] [CrossRef]
- Ashton, K.G.; Feldman, C.R. Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 2003, 57, 1151–1163. [Google Scholar]
- Blanckenhorn, W.U.; Demont, M. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef]
- Vinarski, M. On the applicability of Bergmann’s rule to ectotherms: The state of the art. Biol. Bull. Rev. 2014, 4, 232–242. [Google Scholar] [CrossRef]
- Rivas, J.; Quiero, A.; Penna, M.; Velásquez, N.A. Body-size variation across environmental gradients in an ectothermic organism: An intraspecific approach to ecogeographic patterns. Herpetologica 2018, 74, 191–198. [Google Scholar] [CrossRef]
- Baranov, V.; Jourdan, J.; Hunter-Moffatt, B.; Noori, S.; Schölderle, S.; Haug, J.T. Global size pattern in a group of important ecological indicators (Diptera, Chironomidae) is driven by latitudinal temperature gradients. Insects 2022, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Lira, A.F.; Andrade, A.R.; Foerster, S.I. Latitudinal trends in scorpion assemblages of Brazilian Atlantic forest: Do the Rapoport’s and Bergmann’s rules apply? In Neotropical Gradients and Their Analysis; Springer: Berlin/Heidelberg, Germany, 2023; pp. 179–203. [Google Scholar]
- Rendoll-Cárcamo, J.; Gañán, M.; Madriz, R.; Convey, P.; Contador, T. Wing reduction and body size variation along a steep elevation gradient: A case study with Magellanic sub-Antarctic mayflies and stoneflies. Front. Ecol. Evol. 2023, 11, 1188889. [Google Scholar] [CrossRef]
- Ramírez-Delgado, V.H.; Sanabria-Urbán, S.; Serrano-Meneses, M.A.; Cueva del Castillo, R. The converse to Bergmann’s rule in bumblebees, a phylogenetic approach. Ecol. Evol. 2016, 6, 6160–6169. [Google Scholar] [CrossRef] [PubMed]
- Baranovská, E.; Knapp, M. Steep converse Bergmann’s cline in a carrion beetle: Between-and within-population variation in body size along an elevational gradient. J. Zool. 2018, 304, 243–251. [Google Scholar] [CrossRef]
- Pallarés, S.; Lai, M.; Abellán, P.; Ribera, I.; Sánchez-Fernández, D. An interspecific test of Bergmann’s rule reveals inconsistent body size patterns across several lineages of water beetles (Coleoptera: Dytiscidae). Ecol. Entomol. 2019, 44, 249–254. [Google Scholar] [CrossRef]
- Gérard, M.; Vanderplanck, M.; Franzen, M.; Kuhlmann, M.; Potts, S.G.; Rasmont, P.; Schweiger, O.; Michez, D. Patterns of size variation in bees at a continental scale: Does Bergmann’s rule apply? Oikos 2018, 127, 1095–1103. [Google Scholar] [CrossRef]
- Benítez, H.A.; Muñoz-Ramírez, C.; Correa, M.; Acuña-Rodríguez, I.S.; Villalobos-Leiva, A.; Contador, T.; Velásquez, N.A.; Suazo, M.J. Breaking the Law: Is it correct to use the converse Bergmann rule in Ceroglossus chilensis? An overview using geometric morphometrics. Insects 2024, 15, 97. [Google Scholar] [CrossRef]
- Mousseau, T.A. Ectotherms follow the converse to Bergmann’s rule. Evolution 1997, 51, 630–632. [Google Scholar] [CrossRef]
- Alcantara, M.J.M.; Fontanilla, A.M.; Ashton, L.A.; Burwell, C.J.; Cao, M.; Han, H.; Huang, H.; Kitching, R.L.; Reshchikov, A.; Shen, X.; et al. Bugs and Bergmann’s rule: A cross-taxon large-scale study reveals idiosyncratic altitudinal and latitudinal body size patterns for different insect taxa. Entomol. Gen. 2024, 44, 715–725. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Gorshkov, V.G.; Li, B.-L. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos 2005, 111, 425–436. [Google Scholar] [CrossRef]
- Winterhalter, W.E.; Mousseau, T.A. The strength of temperature-mediated selection on body size in a wild insect population. J. Orthoptera Res. 2008, 17, 347–351. [Google Scholar] [CrossRef]
- Leather, S.R.; Walters, K.F.A.; Bale, J.S. The Ecology of Insect Overwintering; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Shelomi, M.; Zeuss, D. Bergmann’s and Allen’s rules in native European and Mediterranean Phasmatodea. Front. Ecol. Evol. 2017, 5, 25. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Pace, L. Mountain biodiversity and sustainable development. In Encyclopedia of the UN Sustainable Development Goals. Life on Land; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar]
- Lee, M.A.; Burger, G.; Green, E.R.; Kooij, P.W. Relationships between resource availability and elevation vary between metrics creating gradients of nutritional complexity. Oecologia 2021, 195, 213–223. [Google Scholar] [CrossRef]
- Huston, M.A.; Wolverton, S. Regulation of animal size by eNPP, Bergmann’s rule, and related phenomena. Ecol. Monogr. 2011, 81, 349–405. [Google Scholar] [CrossRef]
- Blasi, C. Fitoclimatologia del Lazio; Università “La Sapienza”-Roma, Regione Lazio: Roma, Italy, 1994; pp. 1–58. [Google Scholar]
- Salvati, L.; Perini, L.; Bajocco, S.; Sabbi, A. Climate aridity and land use change: A regional-scale analysis. Geogr. Res. 2012, 50, 193–203. [Google Scholar] [CrossRef]
- Fattorini, S. The role of vegetation in elevational diversity patterns of tenebrionid beetles in Central Italy. Diversity 2024, 16, 110. [Google Scholar] [CrossRef]
- Fattorini, S. Regional insect inventories require long time, extensive spatial sampling and good will. PLoS ONE 2013, 8, e62118. [Google Scholar] [CrossRef]
- Fattorini, S. Disentangling the effects of available area, mid-domain constraints, and species environmental tolerance on the altitudinal distribution of tenebrionid beetles in a Mediterranean area. Biodivers. Conserv. 2014, 23, 2545–2560. [Google Scholar] [CrossRef]
- Fattorini, S.; Salvati, L. Tenebrionid beetles as proxy indicators of climate aridity in a Mediterranean area. Ecol. Indic. 2014, 38, 256–261. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Strona, G.; Pace, L.; Biondi, M. Elevational patterns of generic diversity in the tenebrionid beetles (Coleoptera Tenebrionidae) of Latium (Central Italy). Diversity 2020, 12, 47. [Google Scholar] [CrossRef]
- Pons, J.; Bruvo, B.; Petitpierre, E.; Plohl, M.; Ugarkovic, D.; Juan, C. Complex structural features of satellite DNA sequences in the genus Pimelia (Coleoptera: Tenebrionidae): Random differential amplification from a common ‘satellite DNA library’. Heredity 2004, 9, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Soldati, F.; Soldati, L. Species delimitation using morphological and molecular tools in the Asida (Polasida) jurinei Solier, 1836 species complex. Preliminary results. (Coleoptera: Tenebrionidae: Tentyrinae). Cah. Sci. Muséum Lyon 2006, 10, 111–116. [Google Scholar] [CrossRef]
- Trichas, A. The genus Dendarus Latreille, 1829 (Coleoptera, Tenebrionidae: Dendarini) in Greece (A systematic account of the genus with description of a new species and four new systematic combinations). In Advances in Arachnology and Developmental Biology; Makarov, S.E., Dimitrijević, R.N., Eds.; SASA, Belgrade and UNESCO MAB Serbia: Belgrade, Serbia, 2008; pp. 417–462. [Google Scholar]
- Ferrer, J. Contribución al conocimiento de los Asidini iberobaleares. Segunda nota. Las Alphasida (Glabrasida) del grupo Tricostatae Escalera 1922 (Coleoptera, Tenebrionidae, Pimeliinae). Boletín Soc. Entomológica Aragonesa 2008, 43, 61–73. [Google Scholar]
- Condamine, F.L.; Soldati, L.; Rasplus, J.Y.; Kergoat, G.J. New insights on systematics and phylogenetics of Mediterranean Blaps species (Coleoptera: Tenebrionidae: Blaptini), assessed through morphology and dense taxon sampling. Syst. Entomol. 2011, 36, 340–361. [Google Scholar] [CrossRef]
- Ferrer, J. Contribución al conocimiento del género Phylan Dejean, 1821, y descripción de una specie nueva del género Heliopates Dejean, 1834 (Coleoptera, Tenebrionidae, Pedinini). Boletín Soc. Entomológica Aragonesa 2011, 49, 75–82. [Google Scholar]
- Iwan, D.; Löbl, I. Catalogue of Palaearctic Coleoptera. Vol. 5. Revised and Updated Second Edition. Tenebrionoidea; Brill: Leiden, The Netherlands, 2020; pp. 1–946. [Google Scholar]
- Ferrer, J. Revisión del género Leptoderis Billberg, 1820 y comentarios sobre el origen, composición, anatomía y necrofagia de la tribu Elenophorini (Coleoptera, Tenebrionidae). Boletín Soc. Entomológica Aragonesa 2015, 57, 19–38. [Google Scholar]
- Ferrer, J. Contribución al conocimiento del género Pachychila Eschscholtz, 1831 y descripción de dos especies nuevas de la Península Ibérica y una de Italia (Coleoptera, Tenebrionidae, Pimeliinae). Boletín Soc. Entomológica Aragonesa 2018, 62, 35–54. [Google Scholar]
- Carpaneto, G.M.; Chiari, S.; Audisio, P.A.; Leo, P.; Liberto, A.; Jansson, N.; Zauli, A. Biological and distributional overview of the genus Eledonoprius (Coleoptera: Tenebrionidae): Rare fungus-feeding beetles of European old-growth forests. Eur. J. Entomol. 2013, 110, 173–176. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef]
- Fattorini, S.; Lo Monaco, R.; Di Giulio, A.; Ulrich, W. Latitudinal trends in body length distributions of European darkling beetles (Tenebrionidae). Acta Oecol. 2013, 53, 88–94. [Google Scholar] [CrossRef]
- Fattorini, S. Darkling beetle communities in two geologically contrasting biotopes: Testing biodiversity patterns by microsite comparisons. Biol. J. Linn. Soc. 2009, 98, 787–793. [Google Scholar] [CrossRef]
- Hódar, J.A. The use of regression equations for estimation of arthropod biomass in ecological studies. Acta Oecol. 1996, 17, 421–433. [Google Scholar]
- Kuschka, V. Der Einfluss der Körpergestalt auf die Massen-Körperlängen-Relation von Arthropoden. Zool. Anz. 1994, 233, 265–281. [Google Scholar]
- Michon, G.P. Spheroids & Scalene Ellipsoids. Available online: https://www.numericana.com/answer/ellipsoid.htm#thomsen (accessed on 15 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 March 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fattorini, S.; Lo Monaco, R.; Di Giulio, A.; Ulrich, W. Climatic correlates of body size in European tenebrionid beetles (Coleoptera: Tenebrionidae). Org. Divers. Evol. 2014, 14, 215–224. [Google Scholar] [CrossRef]
- Fattorini, S.; Sciotti, A.; Tratzi, P.; Di Giulio, A. Species distribution, ecology, abundance, body size and phylogeny originate interrelated rarity patterns at regional scale. J. Zool. Syst. Evol. Res. 2013, 51, 279–286. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. The strategy of body size in mammalian carnivores. Am. Midl. Nat. 1968, 80, 299–315. [Google Scholar] [CrossRef]
- Geist, V. Bergmann’s rule is invalid. Can. J. Zool. 1987, 65, 1035–1038. [Google Scholar] [CrossRef]
- Dajoz, R. Les Insectes et la Forêt: Rôle et Diversité des Insectes dans le Milieu Forestier, 2nd ed.; Lavoisier: Paris, France, 2007; pp. 1–648. [Google Scholar]
- Vermunt, B.; Cuddington, K.; Sobek-Swant, S.; Crosthwaite, J.C.; Lyons, D.B.; Sinclair, B.J. Temperatures experienced by wood-boring beetles in the under-bark microclimate. For. Ecol. Manag. 2012, 269, 149–157. [Google Scholar] [CrossRef]
- Lindman, L.; Öckinger, E.; Ranius, T. Microclimate in hollow trees and how it affects an inhabiting beetle species, Osmoderma eremita. Ecol. Entomol. 2023, 48, 112–126. [Google Scholar] [CrossRef]
- Cushman, J.H.; Lawton, J.H.; Manly, B.F.J. Latitudinal patterns in European ant assemblages: Variation in species richness and body size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Olalla-Tárraga, M.Á.; Hawkins, B.A. Bergmann’s rule and the geography of mammal body size in the Western Hemisphere. Glob. Ecol. Biogeogr. 2008, 17, 274–283. [Google Scholar] [CrossRef]
- Olson, V.A.; Davies, R.G.; Orme, C.D.L.; Thomas, G.H.; Meiri, S.; Blackburn, T.M.; Gaston, K.J.; Owens, I.P.F.; Bennett, P.M. Global biogeography and ecology of body size in birds. Ecol. Lett. 2009, 12, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, S.K.; Pabst, D.A.; McLellan, W.A.; Thorne, L.H. Do bigger bodies require bigger radiators? Insights into thermal ecology from closely related marine mammal species and implications for ecogeographic rules. J. Biogeogr. 2020, 47, 1193–1206. [Google Scholar] [CrossRef]

| Measure | Group | Intercept | Slope | R2 | F | p |
|---|---|---|---|---|---|---|
| Body length (mm) | All | 9.578 ± 0.159 | 0.0008 ± 0.0001 | 0.689 | 48.7 | 5.315 × 10−7 |
| Geophilous | 11.108 ± 0.309 | −0.0003 ± 0.0002 | 0.098 | 2.4 | 0.136 | |
| Geophilous (<1100 m) | 11.399 ± 0.471 | −0.0005 ± 0.0007 | 0.043 | 0.4 | 0.541 | |
| Geophilous (>1100 m) | 7.972 ± 0.396 | 0.0013 ± 0.0002 | 0.769 | 36.5 | 8.393 × 10−5 | |
| Xylophilous | 6.307 ± 0.468 | 0.0040 ± 0.0004 | 0.869 | 132.2 | 2.875 × 10−10 | |
| Body mass—fresh (mg) | All | 167.988 ± 8.109 | −0.038 ± 0.006 | 0.653 | 41.4 | 1.798 × 10−6 |
| Geophilous | 210.425 ± 16.665 | −0.078 ± 0.012 | 0.655 | 41.8 | 1.666 × 10−6 | |
| Geophilous (<1100 m) | 247.387 ± 26.337 | −0.130 ± 0.042 | 0.522 | 9.8 | 0.012 | |
| Geophilous (>1100 m) | 44.723 ± 5.610 | 0.013 ± 0.003 | 0.609 | 17.1 | 0.002 | |
| Xylophilous | 62.444 ± 15.261 | 0.086 ± 0.012 | 0.718 | 50.9 | 6.497 × 10−7 | |
| Body mass—dry (mg) | All | 47.550 ± 1.820 | −0.005 ± 0.001 | 0.443 | 17.5 | 0.0004 |
| Geophilous | 59.470 ± 3.952 | −0.016 ± 0.003 | 0.601 | 33.1 | 8.687 × 10−6 | |
| Geophilous (<1100 m) | 66.658 ± 6.174 | −0.025 ± 0.010 | 0.428 | 6.7 | 0.029 | |
| Geophilous (>1100 m) | 18.663 ± 2.153 | 0.006 ± 0.001 | 0.678 | 23.1 | 0.0005 | |
| Xylophilous | 18.038 ± 3.997 | 0.029 ± 0.003 | 0.810 | 85.3 | 1.181 × 10−8 | |
| Body volume (mm3) | All | 175.287 ± 9.735 | −0.019 ± 0.007 | 0.247 | 7.2 | 0.013 |
| Geophilous | 247.812 ± 18.539 | −0.063 ± 0.013 | 0.505 | 22.4 | 0.0001 | |
| Geophilous (<1100 m) | 281.646 ± 28.567 | −0.105 ± 0.045 | 0.376 | 5.4 | 0.045 | |
| Geophilous (>1100 m) | 55.261 ± 12.196 | 0.041 ± 0.007 | 0.770 | 36.9 | 8.054 × 10−5 | |
| Xylophilous | 62.561 ± 11.732 | 0.066 ± 0.009 | 0.719 | 51.2 | 6.275 × 10−7 |
| Group | Intercept | Slope | R2 | F | p |
|---|---|---|---|---|---|
| All | 2.405 ± 0.046 | −5.821 × 10−4 ± 3.344 × 10−5 | 0.932 | 303.1 | 2.371 × 10−14 |
| Geophilous | 2.094 ± 0.052 | −4.455 × 10−4 ± 3.754 × 10−5 | 0.865 | 140.8 | 4.933 × 10−11 |
| Xylohiplous | 2.840 ± 0.091 | −8.223 × 10−4 ± 7.146 × 10−5 | 0.869 | 132.4 | 2.837 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S. An Interspecific Assessment of Bergmann’s Rule in Tenebrionid Beetles (Coleoptera, Tenebrionidae) along an Elevation Gradient. Insects 2024, 15, 673. https://doi.org/10.3390/insects15090673
Fattorini S. An Interspecific Assessment of Bergmann’s Rule in Tenebrionid Beetles (Coleoptera, Tenebrionidae) along an Elevation Gradient. Insects. 2024; 15(9):673. https://doi.org/10.3390/insects15090673
Chicago/Turabian StyleFattorini, Simone. 2024. "An Interspecific Assessment of Bergmann’s Rule in Tenebrionid Beetles (Coleoptera, Tenebrionidae) along an Elevation Gradient" Insects 15, no. 9: 673. https://doi.org/10.3390/insects15090673
APA StyleFattorini, S. (2024). An Interspecific Assessment of Bergmann’s Rule in Tenebrionid Beetles (Coleoptera, Tenebrionidae) along an Elevation Gradient. Insects, 15(9), 673. https://doi.org/10.3390/insects15090673






