Heat Shock Protein 70 Genes Are Involved in the Thermal Tolerance of Hippodamia variegata
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. High-Temperature Treatments
2.3. RNA Extraction and Transcriptome Sequencing
2.4. Quality Control, Analysis, and Functional Annotation
2.5. Differentially Expressed Genes (DEGs) and Functional Enrichment Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Synthesis and Delivery of dsRNA
2.8. Effects of RNA on Survival Rate and Fecundity
2.9. Data Analysis
3. Results
3.1. Transcriptome Sequencing Quality Assessment and Functional Annotation
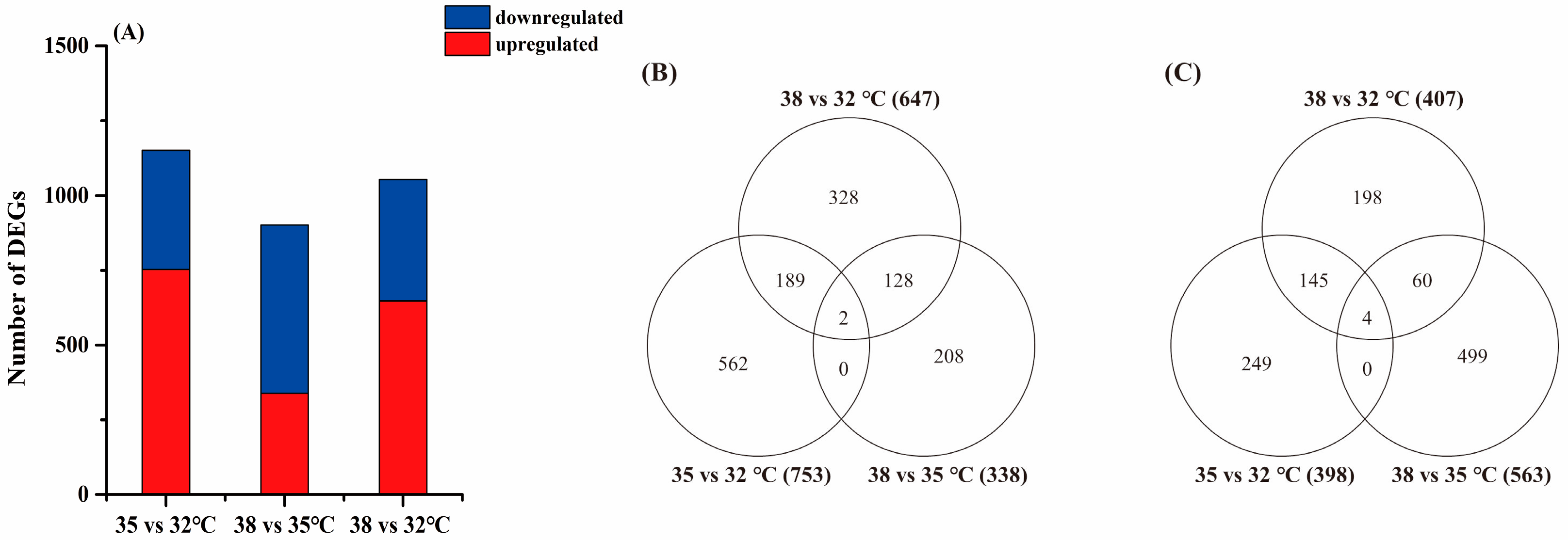
3.2. Differentially Expressed Genes (DEGs)
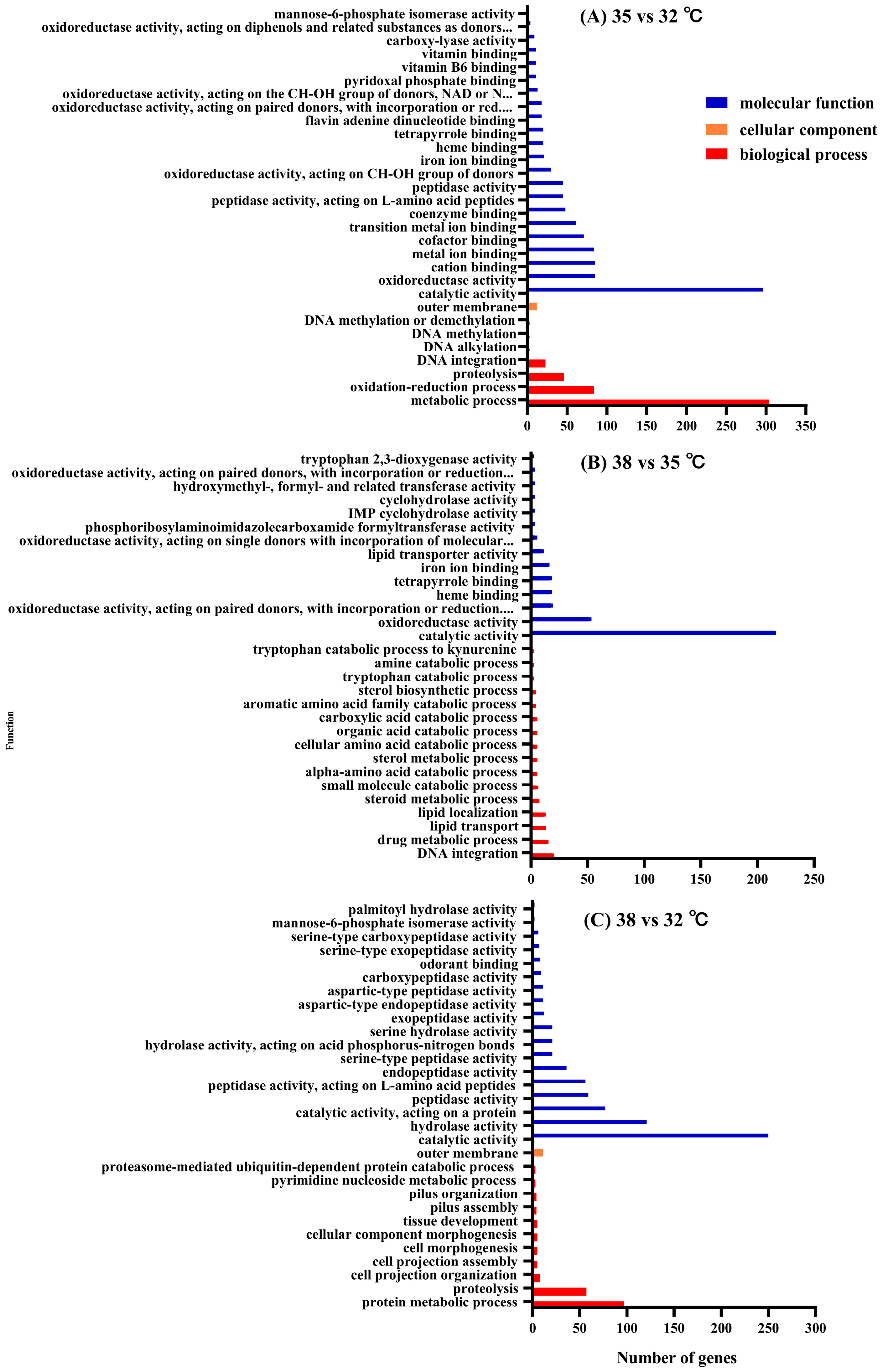
3.3. Identification of Genes Participating in Tolerance to High Temperatures
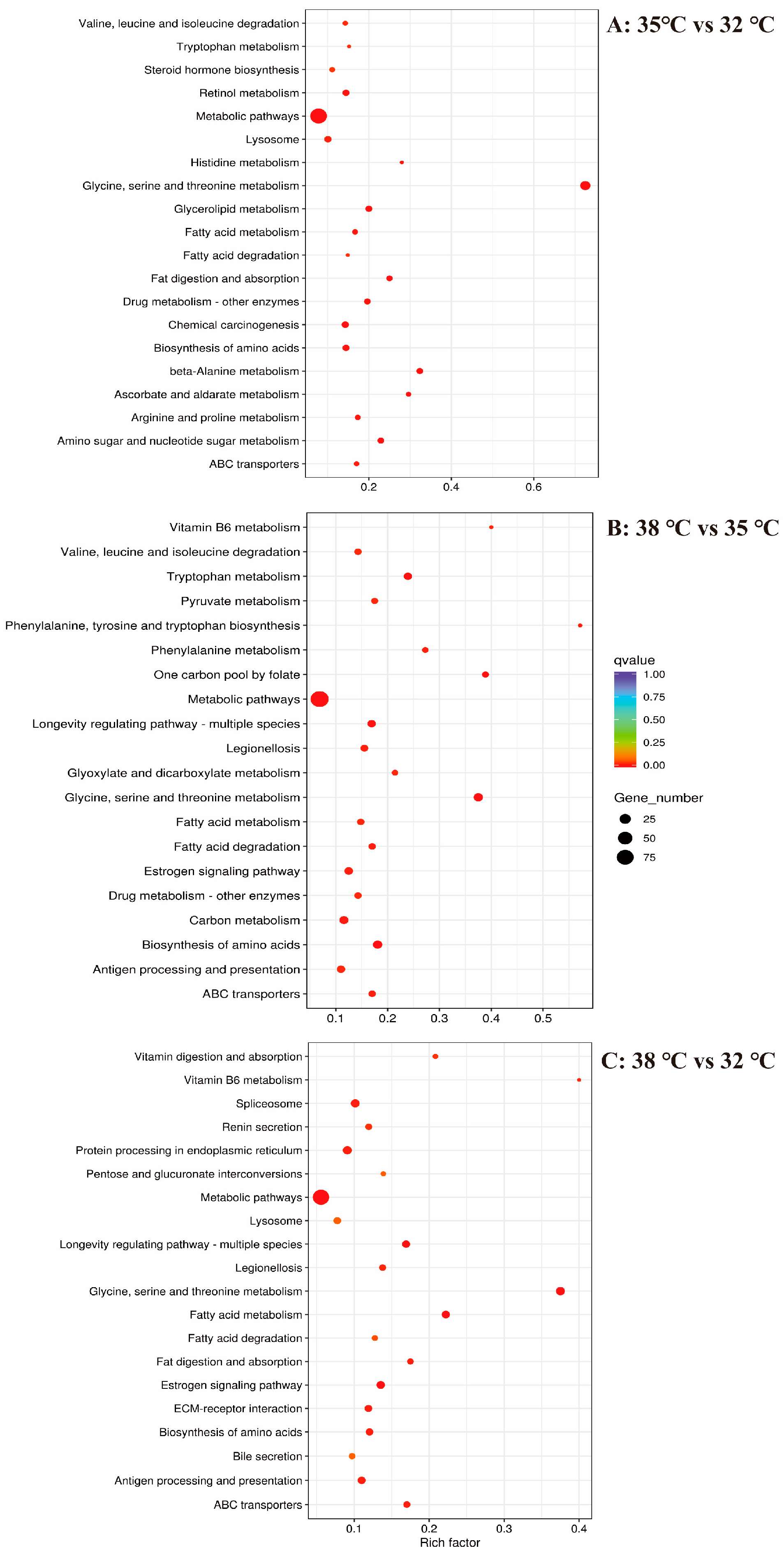
3.4. KEGG Enrichment Analysis of DEGs
3.5. Real-Time Fluorescence Quantitative PCR Validation
3.6. Hsp70 Genes Are Required for H. variegata Survival under High-Temperature Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Angilletta, M.J.; Huey, R.B.; Frazier, M.R. Thermodynamic effects on organismal performance: Is hotter better? Physiol. Biochem. Zool. 2010, 83, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Garrad, R.; Booth, D.T.; Furlong, M.J. The effect of rearing temperature on development, body size, energetics and fecundity of the diamondback moth. Bull. Entomol. Res. 2016, 106, 175–181. [Google Scholar] [CrossRef]
- Ardakani, H.R.; Samih, M.A.; Ravan, S.; Mokhtari, A. Effect of temperature on the development and predatory potential of Exochomus nigripennis (Erichson) (Col.: Coccinellidae) fed on Gossyparia spuria (Modeer) (Hem.: Eriococcidae). Int. J. Trop. Insects 2020, 40, 723–728. [Google Scholar]
- Mathew, A.; Morimoto, R.I. Role of the heat shock response in the life and death of proteins. Ann. N. Y. Acad. Sci. 1998, 851, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Molec. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Ma, C.S.; Zhao, Q.H.; Ma, G.; Yang, H.P. Effects of heat stress on physiological and biochemical mechanisms of insects: A literature review. Acta Ecol. Sin. 2007, 4, 1565–1572. [Google Scholar]
- Ma, G.; Ma, C.S. The impacts of extreme high temperature on insect populations under climate change: A review. Sci. Sin. Vitae 2016, 46, 556–564. [Google Scholar] [CrossRef][Green Version]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2020, 66, 163–184. [Google Scholar] [CrossRef]
- Oliveira, C.M.D.; Silvatorres, C.S.A.D.; Torres, J.B.; Silva, G.D.S. Estimation of population growth for two species of lady beetles (Coleoptera: Coccinellidae) under different temperatures. Biocontrol Sci. Technol. 2021, 32, 74–89. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yamazaki, K.; Ohashi, K. Northward expansion and climatic factors affecting the distribution limits of Cheilomenes sexmaculata (Coleoptera: Coccinellidae) in Japan. Appl. Entomol. Zool. 2014, 49, 59–66. [Google Scholar] [CrossRef]
- Yue, J.; He, J.; Zhang, R.; He, D.H. Life tables of laboratory population of Hippodamia variegata at different temperatures. Chin. J. Appl. Entomol. 2009, 46, 921–925. [Google Scholar]
- Brown, P.M.J.; Roy, H.E. Reflections on the long-term assessment of ladybird (Coleoptera: Coccinellidae) populations in the Czech Republic and the United Kingdom. Acta Soc. Zool. Bohemicae 2015, 79, 19–27. [Google Scholar]
- Li, X.L.; Luo, Y.L.; Li, H.; Xie, X.; Ma, R.H.; Liu, Y.J.; Wang, P.L.; Lu, Y.H. Regulation and control effects of Suaeda strips on the population occurrence of Hippomidia variegata in cotton fields. Xinjiang Agric. Sci. 2019, 56, 13–22. [Google Scholar]
- Sarkar, N.; Barik, A. Effect of temperature on development and reproduction of Epilachna dodecastigma (Wied.) (Coleoptera: Coccinellidae). Proceed. Zool. Soc. 2017, 70, 150–155. [Google Scholar] [CrossRef]
- Islam, Y.; Güncan, A.; Zhou, X.M.; Naeem, A.; Shah, F.M. Effect of temperature on the life cycle of Harmonia axyridis (Pallas), and its predation rate on the Spodoptera litura (Fabricius) eggs. Sci. Rep. 2022, 12, 15303. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.X.; Meng, B.B.; Guo, P.F.; Wang, P.L. Predation functional response of adult of Adonia variegata (Goeze) to Aphis gossypii Glover at different temperatures. Xinjiang Agric. Sci. 2018, 55, 1314–1318. [Google Scholar]
- Arzigul, R.; Ding, X.H.; Tursun, A.; Yu, G.Y.; Fu, K.Y.; He, J.; Adili, S.; Guo, W.C. Investigation and diversity of ladybug resources of farmland system in Xinjiang. J. Environ. Entomol. 2021, 43, 292–304. [Google Scholar]
- Yang, Q.; Liu, J.P.; Wyckhuys, K.A.G.; Yang, Y.Z.; Lu, Y.H. Impact of heat stress on the predatory ladybugs Hippodamia variegata and Propylaea quatuordecimpunctata. Insects 2022, 13, 306. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Cai, T.W.; Ren, Z.J.; Liu, Y.; Yuan, M.J.; Cai, Y.F.; Yu, C.; Shu, R.H.; He, S.; Li, J.H.; et al. Decline in symbiont-dependent host detoxification metabolism contributes to increased insecticide susceptibility of insects under high temperature. ISMEJ 2021, 15, 3693–3703. [Google Scholar] [CrossRef]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche J. Entomol. 2011, 2012, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.C.; Su, H.; Li, R.Q.; Li, X.T.; Xu, Y.S.; Dai, X.P.; Zhou, Y.Y.; Wang, H.B. Comparative transcriptome analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) reveals novel insights into heat stress tolerance in insects. BMC Genom. 2017, 18, 974. [Google Scholar] [CrossRef]
- Jiang, F.Z.; Zheng, L.Y.; Guo, J.X.; Zhang, G.R. Effects of temperature stress on insect fertility and its physiological and biochemical mechanisms. J. Environ. Entomol. 2015, 37, 653–663. [Google Scholar]
- Mutz, K.O.; Heilkenbrinker, A.; Lönne, M.; Walter, J.G.; Stahl, F. Transcriptome analysis using next-generation sequencing. Curr. Opin. Biotechnol. 2013, 24, 22–30. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Yuan, M.L. Progress in insect transcriptomics based on the next-generation sequencing technique. Acta Entomol. Sin. 2013, 56, 1489–1508. [Google Scholar]
- Shen, H.Y.; He, H.; Lu, C.D.; Liang, Y.; Wu, H.M.; Zheng, L.Z.; Wang, X.Y.; Liang, G.H. Comparative transcriptome analysis of two populations of Dastarcus helophoroides (Fairmaire) under high temperature stress. Forests 2022, 13, 13. [Google Scholar] [CrossRef]
- Ma, W.H.; Li, X.Y.; Shen, J.S.; Du, Y.L.; Xu, K.; Jiang, Y.S. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotox. Environ. Safe. 2019, 184, 109599. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Z.; Huang, C.L.; Jiang, L.; Cheng, T.C.; Feng, T.S.; Xia, Q.Y. Transcriptome analysis of the response of silkworm to drastic changes in ambient temperature. Appl. Microbiol. Biotechnol. 2018, 102, 10161–10170. [Google Scholar] [CrossRef]
- Ashraf, H.J.; Aguila, L.C.R.; Ahmed, S.; Haq, I.U.; Ali, H.; Ilyas, M.; Gu, S.Y.; Wang, L.D. Comparative transcriptome analysis of Tamarixia radiata (Hymenoptera: Eulophidae) reveals differentially expressed genes upon heat shock. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100940. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.P.; Yang, Y.Z.; Lu, Y.H. Transcriptome analysis of Propylaea quatuordecimpunctata L. (Coleoptera: Coccinellidae) under high temperature stress. Agriculture 2022, 12, 1088. [Google Scholar] [CrossRef]
- Ren, S.X.; Wang, X.M.; Pang, H.; Peng, Z.Q.; Zeng, T. Colored Pictorial Handbook of Ladybird Beetles in China; Science Press: Beijing, China, 2009. [Google Scholar]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 2, 644–652. [Google Scholar] [CrossRef]
- Pertea, G.; Huang, X.Q.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Liu, T.H.; Khashaveh, A.; Yi, C.Q.; Liu, X.X.; Zhang, Y.J. Identification and evaluation of suitable reference genes for RT-qPCR analysis in Hippodamia variegata (Coleoptera: Coccinellidae) under different biotic and abiotic conditions. Front. Physiol. 2021, 12, 669510. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.Y.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Michel, D.; Fiaboe, K.K.M.; Kekeunou, S.; Nanga, S.; Kuate, A.F.; Tonnang, H.E.Z.; Gnanvossou, D.; Hanna, R. Temperature-based phenology model to predict the development, survival, and reproduction of the oriental fruit fly Bactrocera dorsalis. J. Therm. Biol. 2021, 97, 102877. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, X.H.; Yan, X.F.; Li, G.; Luo, C.Y.; He, Y. Effects of short-term high temperatures on survival and reproduction of Trabala vishnou gigantina Yang (Lepidoptera: Lasiocampidae). Pak. J. Zool. 2022, 54, 145–151. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Quan, N.; Palfreyman, R.W.; Chan, L.C.L.; Reid, S.; Nielsen, L.K. Transcriptome sequencing of and microarray development for a Helicoverpa zea cell line to investigate in vitro insect cell–baculovirus interactions. PLoS ONE 2012, 7, e36324. [Google Scholar]
- Lv, J.H.; Huang, Z.E.; Shi, Y.; Kang, Y.L. Influences of different temperatures on the growth and reproduction of Tribolium castaneum. J. Chin. Cereals Oils Assoc. 2020, 35, 132–136. [Google Scholar]
- King, A.M.; Macrae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Lu, M.X.; Hua, J.; Cui, Y.D.; Du, Y.Z. Five small heat shock protein genes from Chilo suppressalis: Characteristics of gene, genomic organization, structural analysis, and transcription profiles. Cell Stress Chaperones 2014, 19, 91–104. [Google Scholar] [CrossRef]
- Lu, M.X.; Li, H.B.; Zheng, Y.T.; Shi, L.; Du, Y.Z. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. J. Therm. Biol. 2016, 57, 110–118. [Google Scholar] [CrossRef]
- Zhang, B.; Leonard, S.P.; Li, Y.Y.; Moran, N.A. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. USA 2019, 116, 24712–24718. [Google Scholar] [CrossRef]
- Tang, X.T.; Sun, M.; Lu, M.X.; Du, Y.Z. Expression patterns of five heat shock proteins in Sesamia inferens (Lepidoptera: Noctuidae) during heat stress. J. Asia-Pac. Entomol. 2015, 18, 529–533. [Google Scholar] [CrossRef]
- Chen, N.; Tan, J.Y.; Wang, Y.; Qi, M.H.; Peng, J.N.; Chen, D.X.; Liu, S.; Li, M.Y. A heat shock protein 70 protects the green peach aphid (Myzus persicae) against high-temperature stress. J. Asia-Pac. Entomol. 2022, 25, 101992. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Lee, G.J.; Vierling, E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein1. Plant Physiol. 2000, 122, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.K.; Saibil, H.R. ATP-Driven molecular chaperone machines. Biopolymers 2013, 99, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Boutet, I.; Tanguy, A.; Rousseau, S.; Auffret, M.; Moraga, D. Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 2003, 8, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Deane, E.E.; Woo, N.Y.S. Cloning and characterization of the hsp70 multigene family from silver sea bream: Modulated gene expression between warm and cold temperature acclimation. Biochem. Biophys. Res. Commun. 2005, 330, 776–783. [Google Scholar] [CrossRef]
- Garbuz, D.G.; Yushenova, I.A.; Zatsepina, O.G.; Przhiboro, A.A.; Bettencourt, B.R.; Evgen’ev, M.B. Organization and evolution of hsp70 clusters strikingly differ in two species of Stratiomyidae (Diptera) inhabiting thermally contrasting environments. BMC Evol. Biol. 2011, 11, 74. [Google Scholar] [CrossRef]
- Kourtidis, A.; Drosopoulou, E.; Nikolaidis, N.; Hatzi, V.I.; Chintiroglou, C.C.; Scouras, Z.G. Identifcation of several cytoplasmic HSP70 genes from the Mediterranean mussel (Mytilus galloprovincialis) and their long-term evolution in Mollusca and Metazoa. J. Mol. Evol. 2006, 62, 446–459. [Google Scholar] [CrossRef]
- Yu, E.M.; Yoshinaga, T.; Jalufka, F.L.; Ehsan, H.; Kaneko, G. The complex evolution of the metazoan HSP70 gene family. Sci. Rep. 2021, 11, 17794. [Google Scholar] [CrossRef]
- Jin, J.S.; Zhao, M.; Wang, Y.; Zhou, Z.S.; Wan, F.H.; Guo, J.Y. Induced thermotolerance and expression of three key Hsp genes (Hsp70, Hsp21, and sHsp21) and their roles in the high temperature tolerance of Agasicles hygrophila. Front. Physiol. 2020, 14, 1593. [Google Scholar] [CrossRef]
- Huang, L.H.; Bing, C.; Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 2007, 53, 1199–1205. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.Y.; Pei, H.Y.; Fan, D. Cloning a peroxidase cDNA sequence from the Oriental Armyworm, Mythimna separata Walker and its induction to different temperature Stress. Chin. J. Biol. Control. 2019, 35, 44–52. [Google Scholar]
- Durak, R.; Dampc, J.; Kula-Maximenko, M.; Mołoń, M.; Durak, T. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.N.; Liu, W.X.; Wan, F.H.; Lv, Z.C.; Guo, J.Y. The role of cytochrome P450 4C1 and carbonic anhydrase 3 in response to temperature stress in Bemisia tabaci. Insects 2021, 12, 1071. [Google Scholar] [CrossRef] [PubMed]




| Gene | Right Primer (5′–3′) | Reverse Primer (5′–3′) | E | R2 | Purpose |
|---|---|---|---|---|---|
| P450-01 | AGGCATTCACCACGATCCAG | AAACTCTTGGCCCTTCTCCG | 1.9648 | 99.97% | qRT-PCR |
| P450-02 | TGGACTGCTCCAGAGGATTG | TCATGAGGCAGCTCTTTCCA | 1.9687 | 99.45% | |
| P450-03 | CAAAGTTGGATTGGCAGCCC | TTTTTCTGTCGCAATGCTGC | 1.9435 | 99.64% | |
| P450-04 | TGCTGAAGGAAGTTGATGCTCT | ACGCCTGTCATTTCTGCAGA | 1.9760 | 99.91% | |
| P450-05 | GATGCCGACGCCTATTAGCT | AGAGACCCAAACGCCGAATT | 2.0024 | 99.77% | |
| P450-06 | TCTGAAGCTTGATGAGTCACCA | TGGCTTTTGTGGCACTCGTA | 1.9430 | 99.73% | |
| Hsp70-01 | AACCCTGACGAAGCAGTAGC | TTGGTCATGACTCCACCTGC | 1.9592 | 99.97% | |
| Hsp70-02 | CAACCTTGAAGGGCCAATGC | AGCGATGAATCCCAGCAACA | 1.9778 | 99.91% | |
| Hsp68 | TGGTGGCTGAAGCAGAGAAG | GCTCAACTTGCTGCCACAAT | 1.9743 | 99.73% | |
| EF1α | AGCCAACATTACCACTGA | GTATCCACGACGCAATTC | 1.9966 | 99.43% | |
| ds-Hsp70-01 | TGGCACAGTGATGACAGCAT | ACCCCAAGTTATGTGGCGTT | -- | -- | RNAi |
| ds-Hsp68 | ATGCGAAACGTCTCATCGGAA | GTTCTTAGTCGACGCAGGCT | -- | -- | |
| ds-GFP | TGGTCCCAATTCTCGTGGAAC | CTTGAAGTTGACCTTGATGCC | -- | -- |
| ID | Raw Reads | Clean Reads | Error Rate | Q20 | Q30 | GC Content |
|---|---|---|---|---|---|---|
| 32 °C-1 | 25,908,610 | 24,488,975 | 0.03% | 97.93% | 94.03% | 41.06% |
| 32 °C-2 | 28,820,184 | 27,389,182 | 0.03% | 97.89% | 93.98% | 41.21% |
| 32 °C-3 | 29,288,207 | 27,600,521 | 0.03% | 97.85% | 93.88% | 40.61% |
| 35 °C-1 | 28,403,718 | 26,967,327 | 0.02% | 98.06% | 94.35% | 41.22% |
| 35 °C-2 | 27,939,525 | 26,536,254 | 0.02% | 98.09% | 94.39% | 41.30% |
| 35 °C-3 | 29,349,401 | 27,911,084 | 0.02% | 98.08% | 94.36% | 41.26% |
| 38 °C-1 | 21,953,796 | 20,974,286 | 0.03% | 97.91% | 94.02% | 41.11% |
| 38 °C-2 | 28,418,287 | 27,094,748 | 0.02% | 98.03% | 94.28% | 41.44% |
| 38 °C-3 | 28,719,964 | 27,425,348 | 0.02% | 98.05% | 94.29% | 40.70% |
| 32 °C-1 | 25,908,610 | 24,488,975 | 0.03% | 97.93% | 94.03% | 41.06% |
| 32 °C-2 | 28,820,184 | 27,389,182 | 0.03% | 97.89% | 93.98% | 41.21% |
| Database | Number of Unigenes | Percentage (%) |
|---|---|---|
| Annotated in NR | 23,616 | 34.45 |
| Annotated in NT | 7212 | 10.52 |
| Annotated in KO | 2747 | 4.01 |
| Annotated in SwissProt | 15,761 | 22.99 |
| Annotated in Pfam | 21,268 | 31.03 |
| Annotated in GO | 11,604 | 16.93 |
| Annotated in COG/KOG | 14,801 | 21.59 |
| Annotated in all databases | 762 | 1.11 |
| Annotated in at least one database | 28,651 | 41.80 |
| Total unigenes | 68,546 | 100 |
| Tem | Group | ds-Hsp70-01 | ds-Hsp68 | ||||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| 32 °C | Control | 1.01 ± 0.05 | 0.99 ± 0.05 | 0.99 ± 0.03 | 1.01 ± 0.05 | 0.99 ± 0.05 | 0.99 ± 0.03 |
| ds-target genes | 0.62 ± 0.06 ** | 0.54 ± 0.03 ** | 0.65 ± 0.03 ** | 0.67 ± 0.06 ** | 0.63 ± 0.06 ** | 0.67 ± 0.08 * | |
| 35 °C | Control | 1.01 ± 0.07 | 1.00 ± 0.08 | 1.00 ± 0.01 | 1.01 ± 0.07 | 1.00 ± 0.08 | 1.00 ± 0.01 |
| ds-target genes | 0.51 ± 0.05 ** | 0.51 ± 0.04 ** | 0.65 ± 0.02 ** | 0.53 ± 0.05 ** | 0.51 ± 0.02 ** | 0.54 ± 0.04 ** | |
| 38 °C | Control | 1.04 ± 0.04 | 1.01 ± 0.05 | 1.02 ± 0.04 | 1.04 ± 0.04 | 1.01 ± 0.05 | 1.02 ± 0.04 |
| ds-target genes | 0.51 ± 0.03 ** | 0.42 ± 0.01 ** | 0.52 ± 0.03 ** | 0.58 ± 0.01 ** | 0.56 ± 0.01 ** | 0.60 ± 0.01 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Lu, Y. Heat Shock Protein 70 Genes Are Involved in the Thermal Tolerance of Hippodamia variegata. Insects 2024, 15, 678. https://doi.org/10.3390/insects15090678
Yang Q, Lu Y. Heat Shock Protein 70 Genes Are Involved in the Thermal Tolerance of Hippodamia variegata. Insects. 2024; 15(9):678. https://doi.org/10.3390/insects15090678
Chicago/Turabian StyleYang, Qing, and Yanhui Lu. 2024. "Heat Shock Protein 70 Genes Are Involved in the Thermal Tolerance of Hippodamia variegata" Insects 15, no. 9: 678. https://doi.org/10.3390/insects15090678
APA StyleYang, Q., & Lu, Y. (2024). Heat Shock Protein 70 Genes Are Involved in the Thermal Tolerance of Hippodamia variegata. Insects, 15(9), 678. https://doi.org/10.3390/insects15090678






