Unravelling the Molecular Identity of Bulgarian Jumping Plant Lice of the Family Aphalaridae (Hemiptera: Psylloidea)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. DNA Extraction, Amplification, Sequencing and Alignment
| Subfamily | Species | Locality | Host Plant | Latitude | Longitude | Altitude (m) | Collection Date | Sex | Sofia University Catalog Number | Process ID (BOLD) | Barcode Index Number (BIN) | Accession N (GenBank) | Reference | Figure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aphalarinae | * Aphalara affinis (Zetterstedt, 1828) | Bulgaria, Western Rhodopi Mts., Smolyanski ezera lakes | - | 41.6203 | 24.6771 | 1520 | 15 September 2021 | M | BFUS-I-IG026770 | PSYBG001-23 | BOLD:AFM2800 | PQ109732 | This study | Figure 1j |
| Bulgaria, Western Rhodopi Mts., Smolyanski ezera lakes | - | 41.6203 | 24.6771 | 1520 | 15 September 2021 | M | BFUS-I-IG026975 | PSYBG100-24 | - | PQ100053 | This study | Figure 1j | ||
| Aphalarinae | Aphalara itadori (Shinji, 1938) | Japan | - | - | - | - | - | - | - | - | - | KP113670 | [53] | - |
| United Kingdom | - | - | - | - | - | - | - | - | - | MG988914 | [16]; Percy, pers. comm. | - | ||
| Aphalarinae | Aphalara avicularis Ossiannilsson, 1981 | Bulgaria, Western Stara Planina Mts., Churek | Polygonum aviculare L. | 42.7767 | 23.7157 | 796 | 27 August 2023 | M | BFUS-I-IG026773 | PSYBG004-23 | BOLD:ACY0265 | PQ109737 | This study | Figure 1k |
| Bulgaria, Western Stara Planina Mts., Churek | 42.7767 | 23.7157 | 796 | 27 August 2023 | F | BFUS-I-IG026977 | PSYBG115-24 | - | PQ100054 | This study | Figure 1k | |||
| Aphalarinae | Aphalara freji Burckhardt & Lauterer, 1997 | Bulgaria, Eastern Stara Planina Mts., Bardarevo | - | 42.8983 | 27.6219 | 72 | 27 July 2011 | M | BFUS-I-IG005891 | PSYBG062-23 | BOLD:ACY0265 | PQ109739 | This study | - |
| Aphalarinae | Aphalara maculipennis Löw, 1886 | Bulgaria, Western Stara Planina Mts., Aldomivsko lake | - | 42.8848 | 22.9998 | 660 | 13 May 2022 | F | BFUS-I-IG026960 | PSYBG096-24 | BOLD:AFT1810 | PQ109740 PQ100055 | This study | Figure 1l |
| Aphalarinae | Aphalara nigrimaculosa Gegechkori, 1981 | Bulgaria, Western Rhodopi Mts., Pamporovo, Snezhanka peak | Rumex acetosella L. | 41.6370 | 24.6835 | 1850 | 16 September 2021 | M | BFUS-I-IG026982 | PSYBG097-24 | BOLD:AFT1810 | PQ109742 PQ100056 | This study | Figure 1m |
| Aphalarinae | Aphalara polygoni Foerster, 1848 | Bulgaria, Western Rhodopi Mts., Cigov chark | - | 41.9318 | 24.1292 | 1380 | 18 June 2016 | F | BFUS-I-IG005903 | PSYBG063-23 | BOLD:AFL6229 | PQ109743 | This study | - |
| Aphalarinae | * Colposcenia aliena (Löw, 1881) | Bulgaria, East Danube plain, Poveljanovo district | Tamarix sp. | 43.2122 | 27.6480 | 74 | 6 July 2023 | M | BFUS-I-IG026791 | PSYBG016-23 | BOLD:AFN1581 | PQ109745 | This study | Figure 1d |
| Bulgaria, East Danube plain, Poveljanovo district | 43.2122 | 27.6480 | 74 | 6 July 2023 | M | BFUS-I-IG027010 | PSYBG102-24 | - | PQ100058 | This study | Figure 1d | |||
| Aphalarinae | * Colposcenia bidentata Burckhardt, 1988 | Bulgaria, Eastern Rhodopi Mts., Meden buk | Tamarix sp. | 41.3696 | 26.0545 | 110 | 6 July 2012 | BFUS-I-IG020788 | PSYBG066-23 | BOLD:AFN1583 | PQ109747 | This study | - | |
| Bulgaria, Eastern Rila-Rhodopi Massif, near Arda river | 41.6514 | 25.8687 | 140 | 27 August 2022 | F | BFUS-I-IG027009 | PSYBG103-24 | - | PQ100059 | This study | Figure 1e | |||
| Aphalarinae | Colposcenia osmanica Vondráček, 1953 | Bulgaria, Vlahina Planina Mts., Simitli | Tamarix sp. | 41.8945 | 23.1184 | 290 | 8 May 2022 | M | BFUS-I-IG026785 | PSYBG019-23 | BOLD:AFN1582 | PQ109749 | This study | - |
| Bulgaria, Krumovgrad | 41.5044 | 25.3948 | 255 | 10 October 2023 | M | BFUS-I-IG027006 | PSYBG104-24 | - | PQ100060 | This study | - | |||
| Aphalarinae | Colposcenia traciana (Klimaszewski, 1970) | Bulgaria, Strandzha Mts., Tsarevo | Tamarix sp. | 42.1363 | 27.7992 | 142 | 12 July 2023 | M | BFUS-I-IG026788 | PSYBG022-23 | BOLD:AFL7588 | PQ109752 | This study | Figure 1g |
| Bulgaria, Strandzha Mts., Tsarevo | 42.1363 | 27.7992 | 142 | 12 July 2023 | M | BFUS-I-IG027003 | PSYBG105-24 | - | PQ100061 | This study | Figure 1g | |||
| Aphalarinae | Colposcenia sp. | Bulgaria, Meden buk | - | 41.3696 | 26.0545 | 110 | 6 July 2012 | - | - | - | - | MG989005 MG988706 | [16]; Percy, pers. comm. | - |
| Aphalarinae | Craspedolepta anomola (Crawford, 1914) | Canada | - | - | - | - | - | - | - | - | - | MG989006 MG988707 | [16]; Percy, pers. comm. | - |
| Aphalarinae | Craspedolepta bulgarica Klimaszewski, 1961 | Bulgaria, Western Stara planina Mts., Buhovo | Achillea sp. | 42.7703 | 23.5663 | 743 | 10 June 2023 | M | BFUS-I-IG026794 | PSYBG025-23 | BOLD:AFM9464 | PQ109753 | This study | Figure 1n |
| Aphalarinae | * Craspedolepta conspersa (Löw, 1888) | Czech Republic, South Moravia, Sedlec | Artemisia vulgaris L. | 48.7746 | 16.6998 | 178 | 25 June 2023 | F | BFUS-I-IG026829 | PSYBG026-23 | BOLD:AFM9463 | PQ109754 | This study | - |
| Aphalarinae | Craspedolepta innoxia (Foerster, 1848) | Bulgaria, Western Stara planina Mts., Buhovo | - | 42.7703 | 23.5663 | 743 | 10 June 2023 | F | BFUS-I-IG026795 | PSYBG027-23 | BOLD:AFM9464 | PQ109756 | This study | - |
| Aphalarinae | * Craspedolepta malachitica (Dahlbom, 1851) | Bulgaria, Western Stara Planina Mts., Negovan, lake | Artemisia absinthium L. | 42.7660 | 23.4007 | 513 | 14 July 2022 | F | BFUS-I-IG026799 | PSYBG030-23 | BOLD:AFL5094 | PQ109759 | This study | - |
| Bulgaria, Western Stara Planina Mts., Negovan, lake | 42.7660 | 23.4007 | 513 | 14 July 2022 | F | BFUS-I-IG026992 | PSYBG106-24 | - | PQ100062 | This study | - | |||
| Aphalarinae | Craspedolepta nebulosa (Zetterstedt, 1828) | Bulgaria, Rila-Rhodopi Massif, Rila Mts., Maljovitsa | Chamaenerion angustifolium (L.) Scop. | 42.2083 | 23.3889 | 1750 | 23 July 2021 | F | BFUS-I-IG026801 | PSYBG032-23 | BOLD:AFM9466 | PQ109760 | This study | - |
| Bulgaria, Rila Mts, Maljovitsa hut | 42.1880 | 23.3736 | 2010 | 15 June 2019 | M | BFUS-I-IG026998 | PSYBG107-24 | - | PQ100063 | This study | - | |||
| Aphalarinae | Craspedolepta nervosa (Foerster, 1848) | Bulgaria, Western Stara planina Mts., Buhovo | Achillea sp. | 42.7703 | 23.5663 | 743 | 10 June 2023 | F | BFUS-I-IG026796 | PSYBG036-23 | BOLD:ACY1733 | PQ109765 | This study | - |
| Bulgaria, Western Stara planina Mts., Buhovo | 42.7703 | 23.5663 | 743 | 10 June 2023 | F | BFUS-I-IG026987 | PSYBG108-24 | - | PQ100064 | This study | - | |||
| Aphalarinae | Craspedolepta omissa Wagner, 1944 | Bulgaria, Rila-Rhodopi Massif, Rila Mts., Kartala district | Artemisia vulgaris L. | 42.04232 | 23.36638 | 1464 | 2 August 2020 | M | BFUS-I-IG026806 | PSYBG038-23 | BOLD:AFM9465 | PQ109768 | This study | - |
| Aphalarinae | Craspedolepta pontica Dobreanu & Manolache, 1962 | Bulgaria, Maleshevska Planina Mts., Stara Kresna, tourist shelter | Achillea clypeolata Sibth. & Sm. | 41.7691 | 23.1758 | 560 | 7 May 2022 | M | BFUS-I-IG026808 | PSYBG040-23 | BOLD:AFM1102 | PQ109770 | This study | - |
| Bulgaria, Maleshevska Planina Mts., road to Stara Kresna | 41.7656 | 23.1659 | 350 | 30 April 2023 | F | BFUS-I-IG026985 | PSYBG109-24 | - | PQ100065 | This study | Figure 1r | |||
| Aphalarinae | Craspedolepta subpunctata (Foerster, 1848) | Bulgaria, Rila Mts, Alen mak hotel | Chamaenerion angustifolium (L.) Scop. | 42.2121 | 23.3870 | 1712 | 14 June 2019 | M | BFUS-I-IG026811 | PSYBG043-23 | BOLD:AAV0240 | PQ109771 | This study | - |
| Bulgaria, Rila Mts., Alen mak hotel | 42.2121 | 23.3870 | 1712 | 14 June 2019 | M | BFUS-I-IG026989 | PSYBG114-24 | - | PQ100066 | This study | - | |||
| Aphalarinae | Lanthanaphalara mira Tuthill, 1959 | Peru | - | - | - | - | - | - | - | - | - | NC038111 | [16]; Percy, pers. comm. | - |
| Aphalarinae | Limataphalara lautereri Burckhardt & Queiroz, 2013 | Brazil | - | - | - | - | - | - | - | - | - | MG988785 MG989094 | [16]; Percy, pers. comm. | - |
| Aphalarinae | Neaphalara fortunae Brown & Hodkinson, 1988 | Costa Rica | - | - | - | - | - | - | - | - | - | MG988801 MG989115 | [16]; Percy, pers. comm. | - |
| Aphalarinae | Rhodochlanis bicolor (Scott, 1880) | Bulgaria, Black Sea coast, Pomorie, salt lake | Salicornia europaea (Moss) Lambinon & Vanderp. | 42.5998 | 27.6261 | 16 | 23 July 2022 | M | BFUS-I-IG026956 | PSYBG099-24 | BOLD:AFT3392 | PQ109781 PQ100069 | This study | Figure 1h |
| Rhinocolinae | Agonoscena atlantica Bastin, Burckhardt & Ouvrard, 2023 | Canary Islands | - | - | - | - | - | - | - | - | - | OR027209 OR067180 | [54] | - |
| Rhinocolinae | * Agonoscena cisti (Puton, 1882) | Albania, Memoraq | Pistacia lentiscus L. | 39.8522 | 20.1470 | 190 | 10 June 2022 | F | BFUS-I-IG027025 | PSYBG098-24 | BOLD:AEB2674 | PQ109723 PQ100051 | This study | - |
| Rhinocolinae | Agonoscena pistaciae Burckhardt & Lauterer, 1989 | Bulgaria, Eastern Rila-Rhodopi Massif, Gaberovo, Gjurgena | Pistacia terebinthus L. | 41.6206 | 25.8851 | 280 | 27 August 2022 | F | BFUS-I-IG026831 | PSYBG067-23 | BOLD:AFM5846 | PQ109726 | This study | - |
| Rhinocolinae | Agonoscena sinuata Bastin, Burckhardt & Ouvrard 2023 | Canary Islands | - | - | - | - | - | - | - | - | - | OR027214 OR067162 | [54,55] | - |
| Rhinocolinae | * Agonoscena targionii (Lichtenstein, 1874) | Bulgaria, Maleshevska Planina Mts., Kresna, Peyo Yavorov station | Pistacia terebinthus L. | 41.7482 | 23.1615 | 217 | 13 August 2022 | M | BFUS-I-IG026813 | PSYBG111-24 | BOLD:AFL7612 | PQ109729 PQ100052 | This study | Figure 1a |
| Rhinocolinae | Apsylla cistellata (Buckton, 1896) | Madagascar | - | - | - | - | - | - | - | - | - | MG988642 MG988918 | [16]; Percy, pers. comm. | - |
| Rhinocolinae | Lisronia echidna Loginova, 1976 | Canary Islands | - | - | - | - | - | - | - | - | - | OR864739 OR067189 | [55] | |
| Rhinocolinae | Megagonoscena gallicola Burckhardt & Lauterer, 1989 | Bulgaria, Maleshevska Planina Mts., Stara Kresna | Pistacia terebinthus L. | 41.7650 | 23.1662 | 360 | 7 May 2022 | M | BFUS-I-IG026820 | PSYBG051-23 | BOLD:AFN1666 | PQ109777 | This study | Figure 1c |
| Bulgaria, Maleshevska Planina Mts., Stara Kresna | 41.7650 | 23.1662 | 360 | 7 May 2022 | F | BFUS-I-IG027016 | PSYBG112-24 | - | PQ100067 | This study | Figure 1c | |||
| Rhinocolinae | Rhinocola aceris (Linnaeus, 1758) | Bulgaria, Western Stara Planina Mts., Churek | Acer sp. | 42.7805 | 23.7137 | 817 | 21 May 2022 | M | BFUS-I-IG026822 | PSYBG055-23 | BOLD:ACK6660 | PQ109778 | This study | - |
| Bulgaria, Transitional region, Lozenska Mts., Lozen | 42.5944 | 23.5081 | 768 | 27 July 2022 | M | BFUS-I-IG026979 | PSYBG113-24 | - | PQ100068 | This study | Figure 1i | |||
| Phacopteroninae | Pseudophacopteron sp. | Australia | - | - | - | - | - | - | - | - | - | MG989234 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Anoeconeossa unicornuta Taylor, 1987 | Australia | - | - | - | - | - | - | - | - | - | NC_038108 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Australopsylla sp. | Australia | - | - | - | - | - | - | - | - | - | MG988646 MG988933 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Blastopsylla occidentalis Taylor, 1985 | Australia | - | - | - | - | - | - | - | - | - | NC_038147 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Boreioglycaspis melaleucae Moore, 1964 | Australia | - | - | - | - | - | - | - | - | - | MG988659 MG988952 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Cardiaspina retator Taylor, 1962 | Australia | - | - | - | - | - | - | - | - | - | MG988694 MG988991 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Creiis sp. | Australia | - | - | - | - | - | - | - | - | - | MG988715 MG989015 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Ctenarytaina eucalypti (Maskell, 1890) | Canary Islands | - | - | - | - | - | - | - | - | - | OR068450 OR067182 | [55] | - |
| Spondyliaspidinae | Glycaspis brimblecombei Moore, 1964 | Canary Islands | - | - | - | - | - | - | - | - | - | OR068451 OR067183 | [55] | - |
| Spondyliaspidinae | Lasiopsylla rotundipennis Froggatt, 1900 | Australia | - | - | - | - | - | - | - | - | - | MG988781 MG989090 | [16]; Percy, pers. comm. | - |
| Spondyliaspidinae | Platyobria sp. | Australia | - | - | - | - | - | - | - | - | - | MG988812 MG989131 | [16]; Percy, pers. comm. | - |
| Liviidae, Euphyllurinae | Psyllospsis fraxini (Linnaeus, 1758) | United Kingdom | - | - | - | - | - | - | - | - | - | MG988820 MG989139 | [16]; Percy, pers. comm. | - |
| Psyllidae, Psyllinae | Cacopsylla melanoneura (Foerster, 1848) | Italy | - | - | - | - | - | - | - | - | - | OQ304120 | [56] | - |
| Czech Republic | - | - | - | - | - | - | - | - | - | OR346833 | [57] | - |

| Gene | Primer Set | Primer Sequence′ (5′–3′) | Amplicon Size (bp) | PCR Conditions | Primer References |
|---|---|---|---|---|---|
| COI | LCOP-F | AGAACWAAYCATAAAAYWATTGG | 654 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 50 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | [54] |
| HCO2198R | TAAACTTCAGGGTGACCAAAAAATCA | ||||
| C_LepFolF | ATTCAACCAATCATAAAGATATTGG | 658 | 94 °C for 1 min, 5 cycles of 94 °C for 30 s, 45–50 °C for 40 s, 72 °C for 1 min, 30–35 cycles of 94 °C for 30 s, 51–54 °C for 40 s and 72 °C for 1 min, 72 °C for 10 min | [58] | |
| C_LepFolR | TAAACTTCTGGATGTCCAAAAAATCA | ||||
| LCO1490F | GGTCAACAAATCATAAAGATATTGG | 654 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 51 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | [58] | |
| HCO2198R | TAAACTTCAGGGTGACCAAAAAATCA | ||||
| VpmCOIF2 | TACCTYTGAATTTGCAATTC | 646 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 46 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | [59] | |
| VpmCOIR4 | AATAARTGTTGGTATAARATAGG | ||||
| Cytb | Cytbf | TGAGGNCAAATATCHTTYTGA | 393 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 53 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | [16,49] |
| Cytbr | GCAAATARRAARTATCATTCDG | ||||
| CytbnewF2 | TGATTATGRGGAGGDTTYGC | 330 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 53 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | This study | |
| CytbnewR | GTTGAATATGDATDGGDGTWAC | ||||
| CytbnewF1 | TATGAGGAGGDTTYGCWGTTG | 248 | 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 53 °C 30 s and 72 °C for 1 min; 72 °C for 10 min | This study | |
| CytbnewR | GTTGAATATGDATDGGDGTWAC |
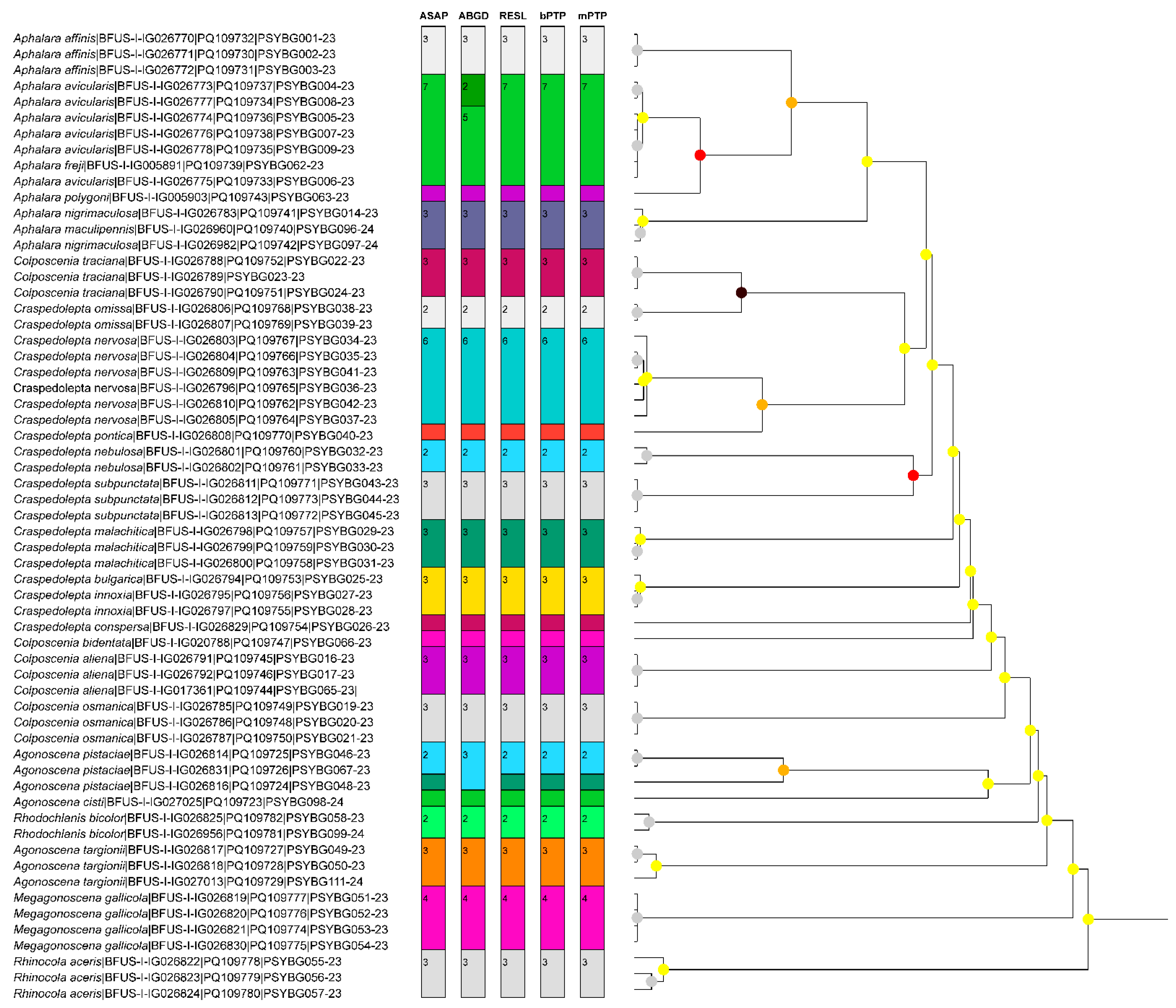
2.3. Species Delimitation Based on Molecular Data
2.4. Phylogenetic Analyses
3. Results
3.1. Molecular Identification of the Bulgarian Aphalarids
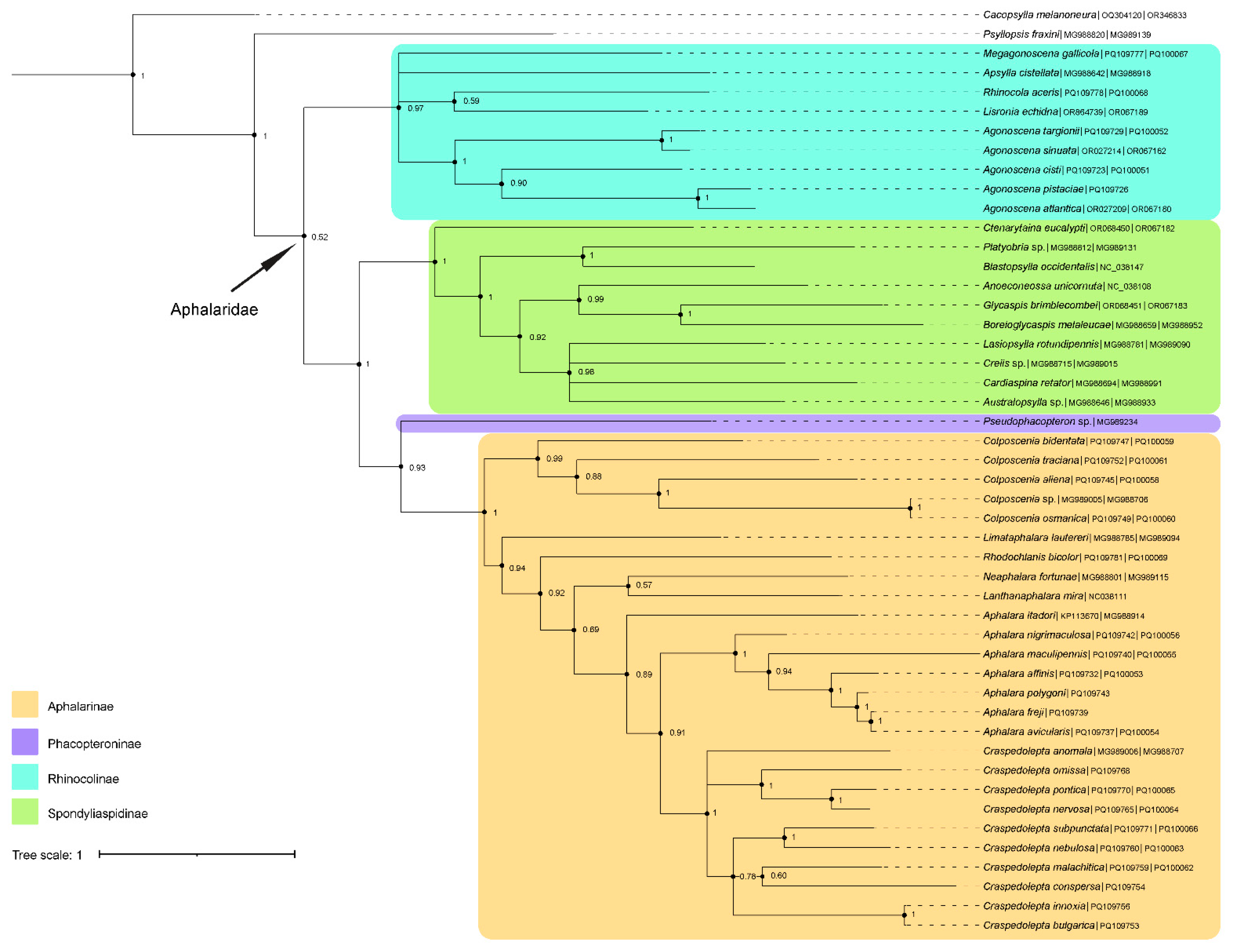
3.2. Phylogeny of Aphalaridae
3.3. Faunistic Data
4. Discussion
4.1. Molecular Identification of Species
4.2. Phylogenetic Relationships within Aphalaridae
4.3. New Data on the Distribution of Aphalarid Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.-T. The Silent Extinction of Species and Taxonomists—An Appeal to Science Policymakers and Legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Martoni, F.; Bulman, S.; Pitman, A.; Taylor, G.; Armstrong, K. DNA Barcoding Highlights Cryptic Diversity in the New Zealand Psylloidea (Hemiptera: Sternorrhyncha). Diversity 2018, 10, 50. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, L.F.; Hu, Y.; Shao, Z.K.; Hong, X.Y.; Hebert, P.D.N.; Xue, X.F. DNA Barcoding Uncovers Cryptic Diversity in Minute Herbivorous Mites (Acari, Eriophyoidea). Mol. Ecol. Resour. 2022, 22, 1986–1998. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Suh, S.-J.; Choi, D.-S. Using DNA Barcoding for Identification of Some Psyllids (Hemiptera: Psylloidea) Intercepted at South Korea Ports-of-Entry. Insecta Mundi 2020, 816, 1–10. [Google Scholar] [CrossRef]
- Sumner-Kalkun, J.; Sjölund, M.; Arnsdorf, Y.; Carnegie, M.; Highet, F.; Ouvrard, D.; Greenslade, A.; Bell, J.; Sigvald, R.; Kenyon, D. A Diagnostic Real-Time PCR Assay for the Rapid Identification of the Tomato-Potato Psyllid, Bactericera cockerelli (Šulc, 1909) and Development of a Psyllid Barcoding Database. PLoS ONE 2020, 15, e0230741. [Google Scholar] [CrossRef]
- Moesges, Z.; Brandis, D.; Ewers, C. Detailed Integrative Taxonomic Analysis Reveals Large-Scale Species Misidentification of Barnacles Based on DNA Barcoding Data. Zool. J. Linn. Soc. 2023, 201, zlad145. [Google Scholar] [CrossRef]
- Cho, G.; Malenovský, I.; Burckhardt, D.; Inoue, H.; Lee, S. DNA Barcoding of Pear Psyllids (Hemiptera: Psylloidea: Psyllidae), a Tale of Continued Misidentifications. Bull. Entomol. Res. 2020, 110, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, D. Psylloid Pests of Temperate and Subtropical Crop and Ornamental Plants (Hemiptera, Psylloidea): A Review. Trends Agric. Sci. Entomol. 1994, 2, 173–186. [Google Scholar]
- De Morais, E.G.F.; Picanço, M.C.; Barreto, R.W.; Silva, N.R.; Campos, M.R. Biological Performance of Diclidophlebia smithi (Hemiptera: Psyllidae), a Potential Biocontrol Agent for the Invasive Weed Miconia calvescens. Biocontrol Sci. Technol. 2010, 20, 107–116. [Google Scholar] [CrossRef]
- Munyaneza, J.E. Psyllids as Vectors of Emerging Bacterial Diseases of Annual Crops. Southwest. Entomol. 2010, 35, 471–477. [Google Scholar] [CrossRef]
- Moreno, A.; Miranda, M.P.; Fereres, A. Psyllids as Major Vectors of Plant Pathogens. Entomol. Gen. 2021, 41, 419–438. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.L.; Percy, D.M. Psyllid Host-Plants (Hemiptera: Psylloidea): Resolving a Semantic Problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D.; Percy, D.M.M. An Updated Classification of the Jumping Plant-Lice (Hemiptera: Psylloidea) Using Molecular and Morphological Evidence. Eur. J. Taxon. 2021, 736, 137–182. [Google Scholar] [CrossRef]
- Ouvrard, D. Psyl’list—The World Psylloidea Database. Available online: http://psyllist.hemiptera.infosyslab.fr/psyllist/ (accessed on 20 June 2024).
- Percy, D.M.; Crampton-Platt, A.; Sveinsson, S.; Lemmon, A.R.; Lemmon, E.M.; Ouvrard, D.; Burckhardt, D. Resolving the Psyllid Tree of Life: Phylogenomic Analyses of the Superfamily Psylloidea (Hemiptera). Syst. Entomol. 2018, 43, 762–776. [Google Scholar] [CrossRef]
- Cho, G.; Malenovský, I.; Lee, S. Higher-level Molecular Phylogeny of Jumping Plant Lice (Hemiptera: Sternorrhyncha: Psylloidea). Syst. Entomol. 2019, 44, 638–651. [Google Scholar] [CrossRef]
- Wang, W.; Dong, Z.; Du, Z.; Wu, P. Genome-Scale Approach to Reconstructing the Phylogenetic Tree of Psyllids (Superfamily Psylloidea) with Account of Systematic Bias. Mol. Phylogenet. Evol. 2023, 189, 107924. [Google Scholar] [CrossRef]
- Burckhardt, D. Ctenarytaina eucalypti (Maskell) (Hemiptera, Psylloidea) Neu Für Mitteleuropa Mit Bemerkungen Zur Blattflohfauna von Eucalyptus. Mitt. Ent. Ges. 1998, 48, 59–67. [Google Scholar]
- Valente, C.; Manta, A.; Vaz, A. First Record of the Australian Psyllid Ctenarytaina spatulata Taylor (Homoptera: Psyllidae) in Europe. J. Appl. Entomol. 2004, 128, 369–370. [Google Scholar] [CrossRef]
- Valente, C.; Hodkinson, I. First Record of the Red Gum Lerp Psyllid, Glycaspis brimblecombei Moore (Hemiptera: Psyllidae), in Europe. J. Appl. Entomol. 2008, 133, 315–317. [Google Scholar] [CrossRef]
- Mifsud, D. The Jumping Plant-Lice (Hemiptera: Pysilloidea) of the Maltese Islands. Bull. Entomol. Soc. 2020, 11, 103–117. [Google Scholar] [CrossRef]
- Burckhardt, D.; Lauterer, P. Systematics and Biology of the Rhinocolinae (Homoptera: Psylloidea). J. Nat. Hist. 1989, 23, 643–712. [Google Scholar] [CrossRef]
- Burckhardt, D.; Basset, Y. The Jumping Plant-Lice (Hemiptera, Psylloidea) Associated with Schinus (Anacardiaceae): Systematics, Biogeography and Host Plant Relationships. J. Nat. Hist. 2000, 34, 57–155. [Google Scholar] [CrossRef]
- Burckhardt, D.; Queiroz, D.L. Phylogenetic Relationships within the Subfamily Aphalarinae Including a Revision of Limataphalara (Hemiptera: Psylloidea: Aphalaridae). Acta Musei Morav. Sci. Biol. 2013, 98, 35–56. [Google Scholar]
- Joakimov, D. Po Faunata Na Hemiptera va Balgaria (On the Fauna of Hemiptera of Bulgaria). Nar. Umotvor. Nauk. Knizhnina. 1909, 25, 1–34. [Google Scholar]
- Klimaszewski, S. Psyllidologische Notizen XII–XIV (Homoptera). Ann. Zool. 1965, 23, 195–209. [Google Scholar]
- Harizanov, A.; Lauterer, P. Beitrag Zur Fauna Der Blattflöhe (Homoptera-Psylloidea) in Bulgarien. Landwirtsch. Hochsch. Wassil Kolar. 1968, 17, 139–145. [Google Scholar]
- Klimaszewski, S. Psyllidologische Notizen XVIII—XX (Homoptera). Ann. Zool. 1970, 27, 417–428. [Google Scholar]
- Głowacka, E.; Harizanov, A. The Jumping Plant Lice (Homoptera, Psylloidea) from Western Rodope Mountains (Bulgaria). Acta Biol. 1983, 12, 62–69. [Google Scholar]
- Głowacka, E. Jumpin Plant-Lice (Homaptera: Psylloidea) of the Pirin Mountains (Bulgaria). Acta Biol. 1989, 13, 14–19. [Google Scholar]
- Burckhardt, D.; Lauterer, P. Systematics and Biology of the Aphalara exilis (Weber & Mohr) Species Assemblage (Hemiptera: Psyllidae). Entomol. Scand. 1997, 28, 271–305. [Google Scholar] [CrossRef]
- Lauterer, P.; Malenovský, I. New Distributional and Biological Data on European Psylloidea (Hemiptera, Sternorrhyncha), with Special Reference to the Fauna of the Czech Republic and Slovakia. Entomol. Basiliensia 2002, 24, 161–177. [Google Scholar]
- Labina, E.S. The Jumping-Lice (Homoptera, Psyllinea) Fauna of the Republic of Altai. Entomol. Rev. 2008, 88, 277–285. [Google Scholar] [CrossRef]
- Pramatarova, M.; Malenovský, I.; Gjonov, I. Notes on Jumping Plant-Lice (Hemiptera, Psylloidea) from the Sarnena Gora Mountains. In Fauna of Sarnena Sredna Gora Mts, Part 2 ZooNotes, Supplement 10; Georgiev, D., Bechev, D., Yancheva, V., Eds.; Plovdiv University Press “Paisii Hilendarski”: Plovdiv, Bulgaria, 2021; pp. 28–37. [Google Scholar] [CrossRef]
- Pramatarova, M.; Malenovský, I.; Gjonov, I. Psyllids (Hemiptera: Psylloidea) in the Entomological Collection of the National Museum of Natural History, Bulgarian Academy of Sciences. Hist. Nat. Bulg. 2023, 45, 261–269. [Google Scholar] [CrossRef]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 1992, 26, 1–347. [Google Scholar]
- Loginova, M. Jumping Plant Lice of the Tribe Stigmaphalarini Vondr. (Psylloidea, Aphalaridae) from Arid Regions of Palaearctic. Rev. Entomoogie URSS 1974, LIII, 150–170. [Google Scholar]
- Burckhardt, D. Angaben Zur Psyllidenfauna Der Nordosttürkei (Homoptera: Psylloidea). Mitteilungen Entomol. Gesellschafi 1988, 38, 31–44. [Google Scholar]
- Loginova, M.M. A Revision of the Species of the Genera Aphalara Frst. and Craspedolepta Enderl. (Homoptera, Psylloidea) in the Fauna of the USSR. I. Rev. Entomoogie URSS 1961, XL, 602–623. [Google Scholar]
- Loginova, M. Revision of the Species of the Genera Aphalara Frsr. and Craspedolepta Enderl. (Homoptera, Psylloidea) in the Fauna of the USSR. II. Rev. Entomoogie URSS 1963, XLII, 621–648. [Google Scholar]
- Dobreanu, E.; Manolache, C. Insecta, Homoptera, Psylloidea. In Fauna Republicii Populare Romane; Botnariuc, N., Ed.; Academia Română: Bucharest, Romania, 1962; pp. 1–376. [Google Scholar]
- Conci, C.; Tamanini, L. Craspedolepta conspersa, Nuova per l’Italia, Da Artemisia vulgaris (Insecta: Homoptera: Psylloidea). Stud. Trentini Sci. Nat. Acta Biol. 1983, 60, 77–85. [Google Scholar]
- Burckhardt, D.; Lauterer, P. Faunistic and Taxonomic Notes on Jumping Plant-Lice from the Alps (Hemiptera: Psylloidea: Aphalarinae). Contrib. Nat. Hist. 2009, 12, 341–348. [Google Scholar] [CrossRef]
- Conci, C.; Tamanini, L. Rhodochlanis salicorniae Klim. Nuovo per l’Italia, R. Hodkinsoni n. Sp. Di Puglia, Da Suaeda vera e Considerazioni Sul Genere. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. 1984, 125, 61–80. [Google Scholar]
- Burckhardt, D. Les Psylles (Insecta, Homoptera, Psylloidea) de l’Algerie. Arch. Sci. 1989, 42, 367–424. [Google Scholar] [CrossRef]
- Lashkari, M.; Burckhardt, D.; Gushki, R.S. Molecular and Morphometric Identification of Pistachio Psyllids with Niche Modeling of Agonoscena pistaciae (Hemiptera: Aphalaridae). Bull. Entomol. Res. 2019, 110, 1–11. [Google Scholar] [CrossRef]
- Percy, D.M. Making the Most of Your Host: The Metrosideros-Feeding Psyllids (Hemiptera, Psylloidea) of the Hawaiian Islands. Zookeys 2017, 649, 1–163. [Google Scholar] [CrossRef]
- Timmermans, M.J.T.N.; Dodsworth, S.; Culverwell, C.L.; Bocak, L.; Ahrens, D.; Littlewood, D.T.J.; Pons, J.; Vogler, A.P. Why Barcode? High-Throughput Multiplex Sequencing of Mitochondrial Genomes for Molecular Systematics. Nucleic Acids Res. 2010, 38, e197. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Andersen, J.C.; Bourchier, R.S.; Grevstad, F.S.; Van Driesche, R.; Mills, N.J. Development and Verification of SNP Arrays to Monitor Hybridization between Two Host-Associated Strains of Knotweed Psyllid, Aphalara itadori. Biol. Control. 2016, 93, 49–55. [Google Scholar] [CrossRef]
- Bastin, S.; Percy, D.M.; Siverio, F. Establishing Reliable DNA Barcoding Primers for Jumping Plant Lice (Psylloidea, Hemiptera). BMC Res. Notes 2023, 16, 322. [Google Scholar] [CrossRef] [PubMed]
- Bastin, S.; Reyes-Betancort, J.A.; Siverio, F.; Percy, D.M. Origins of the Central Macaronesian Psyllid Lineages (Hemiptera; Psylloidea) with Characterization of a New Island Radiation on Endemic Convolvulus floridus (Convolvulaceae) in the Canary Islands. PLoS ONE 2024, 19, e0297062. [Google Scholar] [CrossRef] [PubMed]
- Corretto, E.; Trenti, M.; Serbina, L.; Howie, J.; Dittmer, J.; Kerschbamer, C.; Candian, V.; Tedeschi, R.; Janik, K.; Schuler, H. Multiple Factors Driving the Acquisition Efficiency of Apple Proliferation Phytoplasma in Cacopsylla melanoneura. J. Pest Sci. 2023, 97, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Šafářová, D.; Zrníková, E.; Holušová, K.; Ouředníčková, J.; Starý, M.; Navrátil, M. Molecular Characterization of Mitogenome of Cacopsylla picta and Cacopsylla melanoneura, Two Vector Species of ‘Candidatus Phytoplasma Mali’. Agronomy 2023, 13, 2210. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef]
- Oettl, S.; Schlink, K. Molecular Identification of Two Vector Species, Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psyllidae), of Apple Proliferation Disease and Further Common Psyllids of Northern Italy. J. Econ. Entomol. 2015, 108, 2174–2183. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble Species by Automatic Partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian Phylogenetic and Phylodynamic Data Integration Using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A General Species Delimitation Method with Applications to Phylogenetic Placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-Rate Poisson Tree Processes for Single-Locus Species Delimitation under Maximum Likelihood and Markov Chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.; Wright, A.; Senfeld, T.; Calcott, B. Partitionfinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.; Maddison, D. Mesquite: A Modular System for Evolutionary Analysis. Version 3.81. 2023. Available online: http://www.mesquiteproject.org (accessed on 19 January 2024).
- Trifinopoulos, J.; Nguyen, L.; Haeseler, A.; Minh, B. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annota-tion. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Burckhardt, D.; Serbina, L.; Malenovský, I.; De Queiroz, D.L.; Aléné, D.C.; Cho, G.; Percy, D.M. Phylogeny and Classification of Jumping Plant Lice of the Subfamily Liviinae (Hemiptera: Psylloidea: Liviidae) Based on Molecular and Morphological Data. Zool. J. Linn. Soc. 2023, 201, 387–421. [Google Scholar] [CrossRef]
- Halbert, S.E.; Burckhardt, D. The Psyllids (Hemiptera: Psylloidea) of Florida: Newly Established and Rarely Collected Taxa and Checklist. Insecta Mundi 2020, 788, 1–88. [Google Scholar] [CrossRef]
- Dowle, E.J.; Morgan-Richards, M.; Trewick, S.A. Morphological Differentiation despite Gene Flow in an Endangered Grasshopper. BMC Evol. Biol. 2014, 14, 19–23. [Google Scholar] [CrossRef]
- Kurina, O.; Õunap, E.; Põldmaa, K. Two New Neuratelia rondani (Diptera, Mycetophilidae) Species from Western Palaearctic: A Case of Limited Congruence between Morphology and DNA Sequence Data. Zookeys 2015, 129, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Lauterer, P. A Contribution to the Knowledge of the Psyllid Fauna of Czechoslovakia II. Acta Musei Morav. Sci. Biol. 1965, 50, 171–190. [Google Scholar]
- Burckhardt, D. Vorläufiges Verzeichnis Der Blattflöhe Mitteleuropas Mit Wirtspflanzenangaben (Insecta, Hemiptera, Psylloidea). Beiträge Zikadenkd. 2002, 5, 1–9. [Google Scholar]
- Bastin, S.; Burckhardt, D.; Reyes-Betancort, J.A.; Hernández-Suárez, E.; Ouvrard, D. A Review of the Jumping Plant-Lice (Hemiptera: Psylloidea) of the Canary Islands, with Descriptions of Two New Genera and Sixteen New Species. Zootaxa 2023, 5313, 1–98. [Google Scholar] [CrossRef]
- Percy, D.M.; Cronk, Q. Salix Transect of Europe: Patterns in the Distribution of Willow-Feeding Psyllids (Hemiptera: Psylloidea) from Greece to Arctic Norway. Biodivers. Data J. 2020, 8, e53788. [Google Scholar] [CrossRef]
- Pramatarova, M. Taxonomic, Faunistic, and Ecological Studies of the Superfamily Psylloidea in Bulgaria. Master’s Thesis, Sofia University “St. Kliment Ohridski”, Sofia, Bulgaria, 2021. [Google Scholar]
- Malenovský, I.; Kment, P.; Sychra, J. True Bugs, Leafhoppers, Planthoppers and Psyllids (Hemiptera: Heteroptera, Auchenorrhyncha, Psylloidea) of the Environs of Přebuz in the Krušné Hory Mountains (Czech Republic). Klapalekiana 2014, 50, 181–234. [Google Scholar]
- Malenovský, I.; Lauterer, P.; Burckhardt, D.; Labina, E.S.; Burckhardt, D. Jumping Plant-Lice (Hemiptera: Psylloidea) of Afghanistan. Acta Entomol. Musei Natl. Pragae 2012, 52, 1–22. [Google Scholar]
- Burckhardt, D.; Önuçar, A. A Review of Turkish Jumping Plant-Lice (Homoptera, Psylloidea). Rev. Suisse Zool. 1993, 100, 547–574. [Google Scholar] [CrossRef]
- Luker, S. A Pistachio Psyllid Agonoscene targionii (Hemiptera: Aphalaridae) New to Britain. Bull. Nat. Hist. Mus. Entomol. 2015, 28, 1–5. [Google Scholar]
- Vondráček, K. Mery-Psylloidea [Jumping Plant Lice—Psylloidea]. In Fauna ČSR, Svazek 9. [Fauna of Czechoslovakia, Vol. 9]; Československá akademie věd: Praha, Czech Republic, 1957; pp. 1–430. [Google Scholar]
- Lauterer, P. Psyllodea. Psylloidea. In Enumeratio Insectorum Bohemoslovakiae. Check List Tschechoslowakische Insektenfauna; Dlabola, J., Ed. Acta Faun. Entomol. Mus. Natl. Pragae 1977, 15 (Suppl. 4), 97–100. [Google Scholar]
- Malenovský, I.; Lauterer, P. Psylloidea (Mery). In Red List of Threatened Species of the Czech Republic. Invertebrates; Hejda, R., Farkač, J., Chobot, K., Eds. Příroda 2017, 36, 161–165. [Google Scholar]
- Oswald, T. Erstfunde von Bactericera perrisii Puton, 1876 Und Craspedolepta conspersa (Löw, 1888) Aus Österreich (Hemiptera: Psylloidea). Cicadina 2024, 23, 23–27. [Google Scholar] [CrossRef]
- Pramatarova, M.; Mifsud, D.; Gjonov, I. First Record of the Pistachio Psyllid Agonoscena cisti (Puton, 1882) (Hemiptera, Psylloidea) in the Maltese Islands (Central Mediterranean). Check List 2024, 20, 991–995. [Google Scholar] [CrossRef]
- Masonick, P.; Weirauch, C. Integrative Species Delimitation in Nearctic Ambush Bugs (Heteroptera: Reduviidae: Phymatinae): Insights from Molecules, Geometric Morphometrics and Ecological Associations. Syst. Entomol. 2020, 45, 205–223. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramatarova, M.; Burckhardt, D.; Malenovský, I.; Gjonov, I.; Schuler, H.; Štarhová Serbina, L. Unravelling the Molecular Identity of Bulgarian Jumping Plant Lice of the Family Aphalaridae (Hemiptera: Psylloidea). Insects 2024, 15, 683. https://doi.org/10.3390/insects15090683
Pramatarova M, Burckhardt D, Malenovský I, Gjonov I, Schuler H, Štarhová Serbina L. Unravelling the Molecular Identity of Bulgarian Jumping Plant Lice of the Family Aphalaridae (Hemiptera: Psylloidea). Insects. 2024; 15(9):683. https://doi.org/10.3390/insects15090683
Chicago/Turabian StylePramatarova, Monika, Daniel Burckhardt, Igor Malenovský, Ilia Gjonov, Hannes Schuler, and Liliya Štarhová Serbina. 2024. "Unravelling the Molecular Identity of Bulgarian Jumping Plant Lice of the Family Aphalaridae (Hemiptera: Psylloidea)" Insects 15, no. 9: 683. https://doi.org/10.3390/insects15090683
APA StylePramatarova, M., Burckhardt, D., Malenovský, I., Gjonov, I., Schuler, H., & Štarhová Serbina, L. (2024). Unravelling the Molecular Identity of Bulgarian Jumping Plant Lice of the Family Aphalaridae (Hemiptera: Psylloidea). Insects, 15(9), 683. https://doi.org/10.3390/insects15090683






