Distribution and Biology of Protaetia fieberi (Coleoptera, Scarabaeidae)—Is Protection Status Required?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
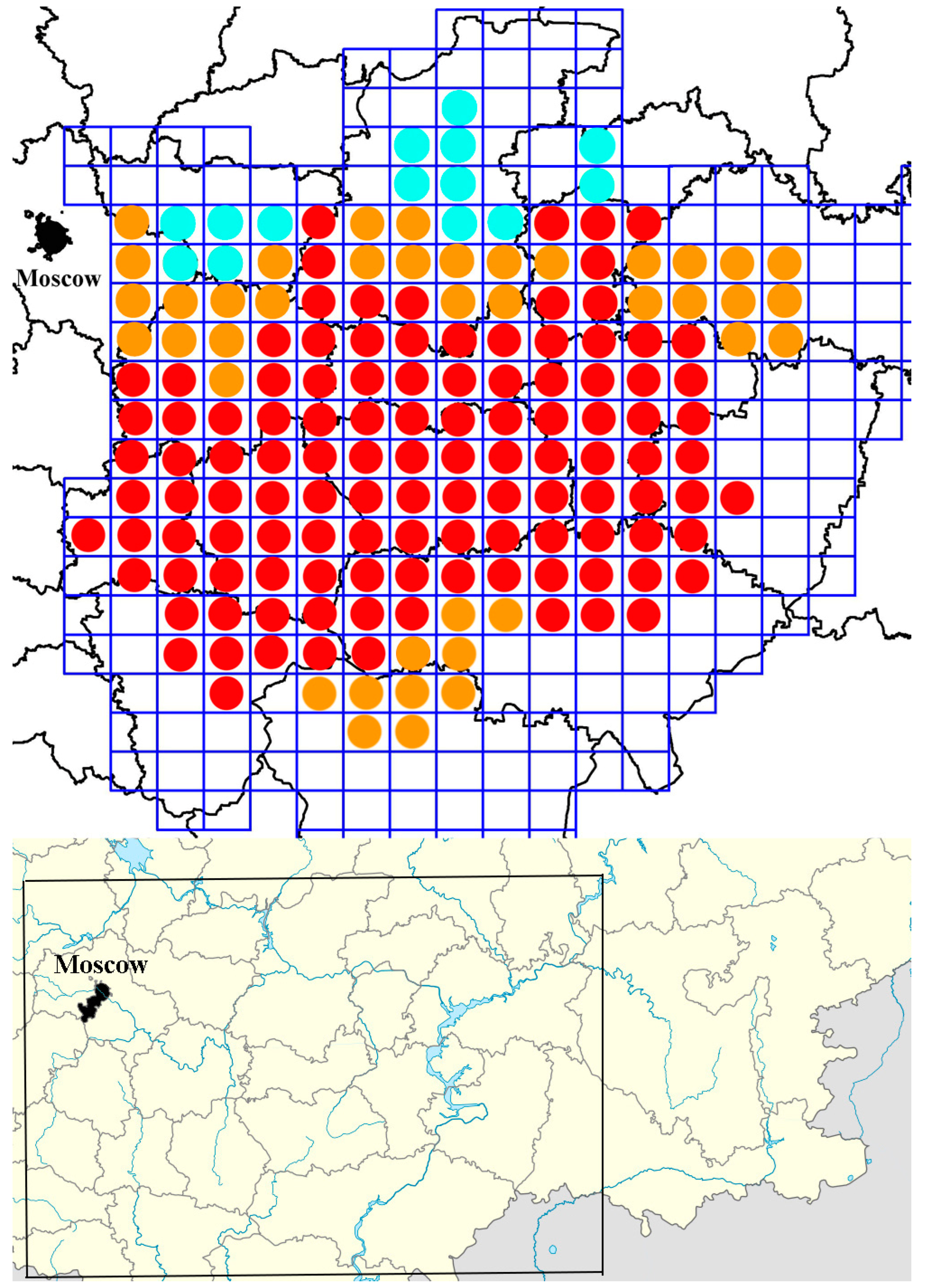
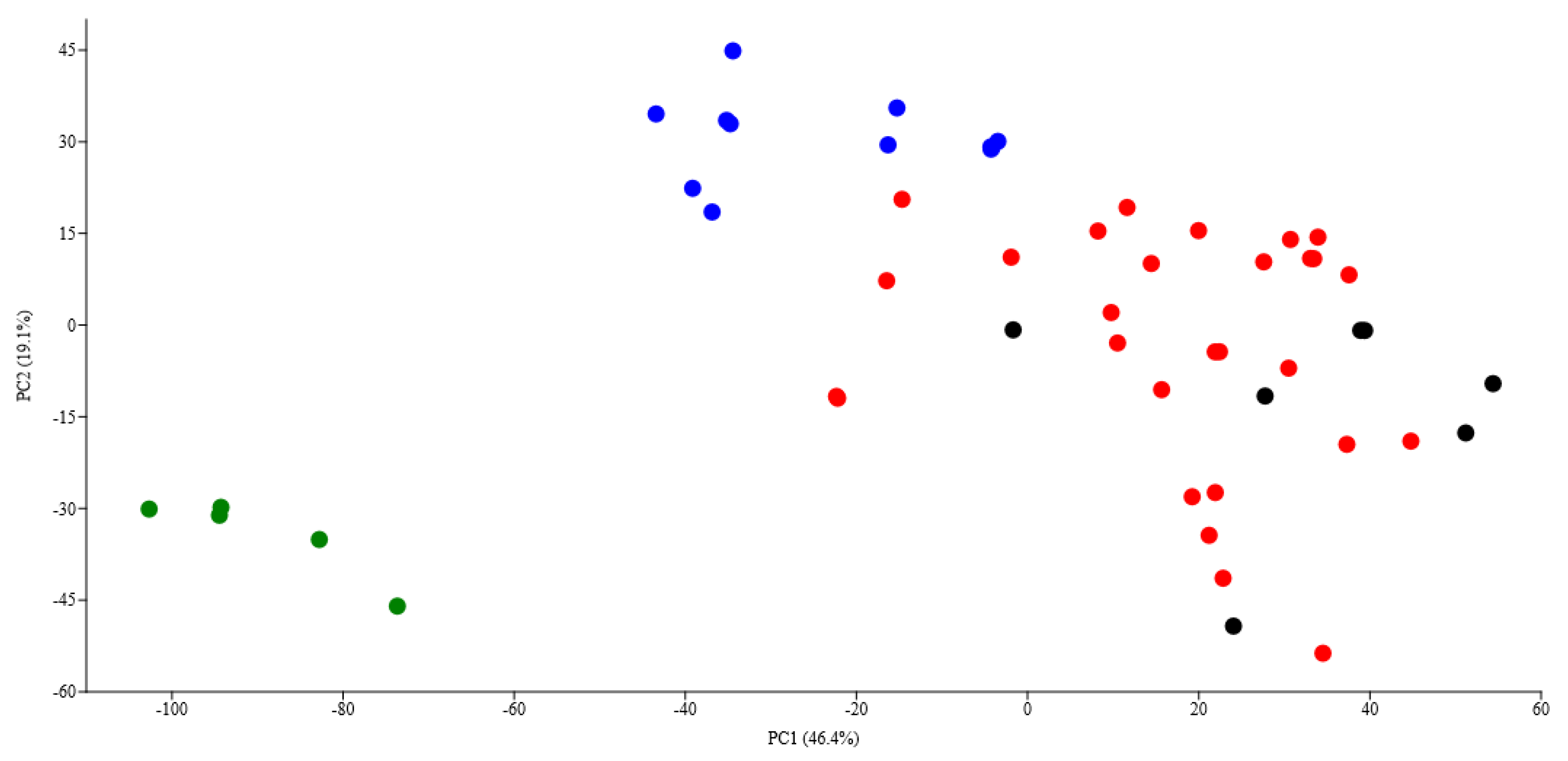
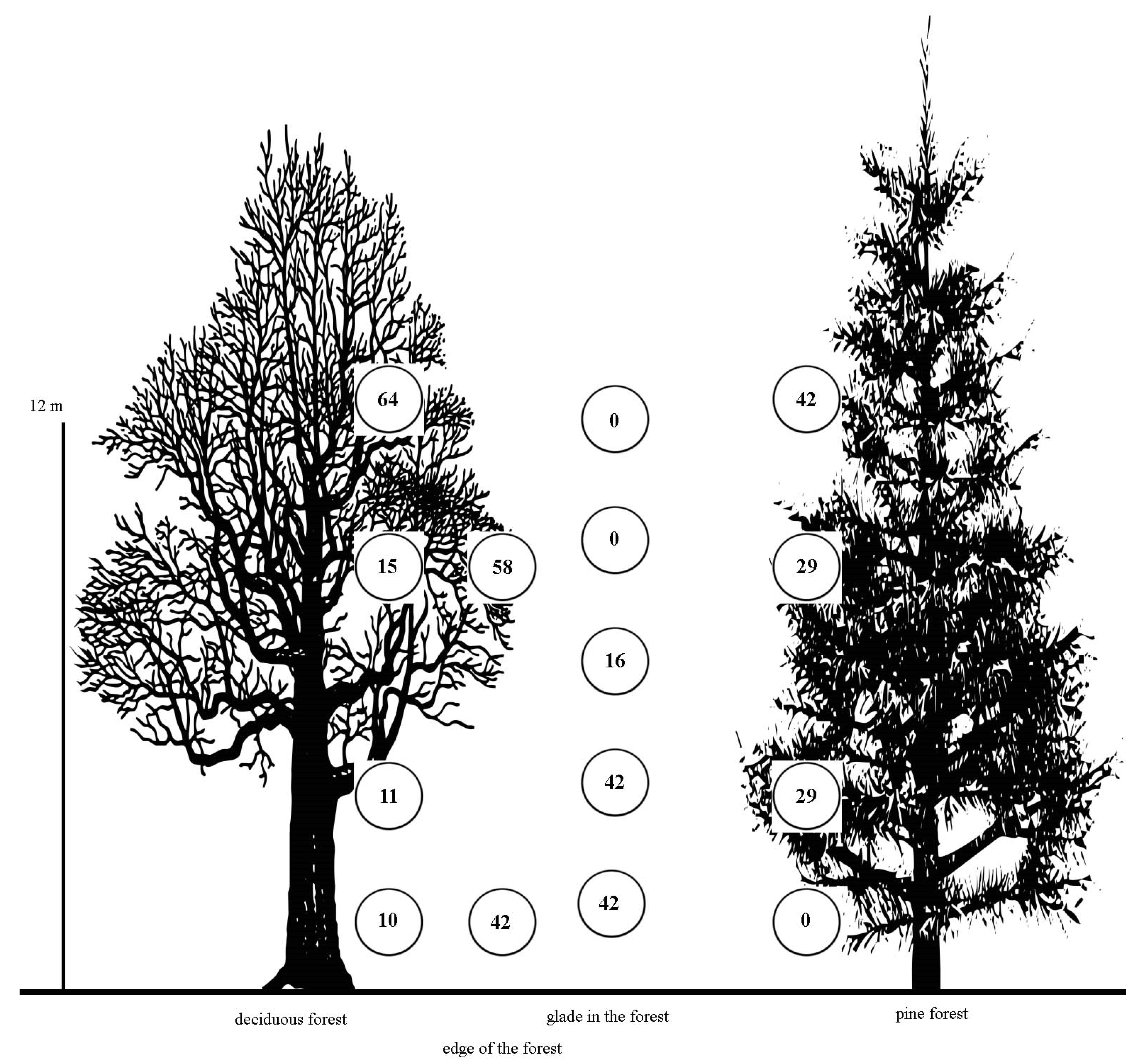
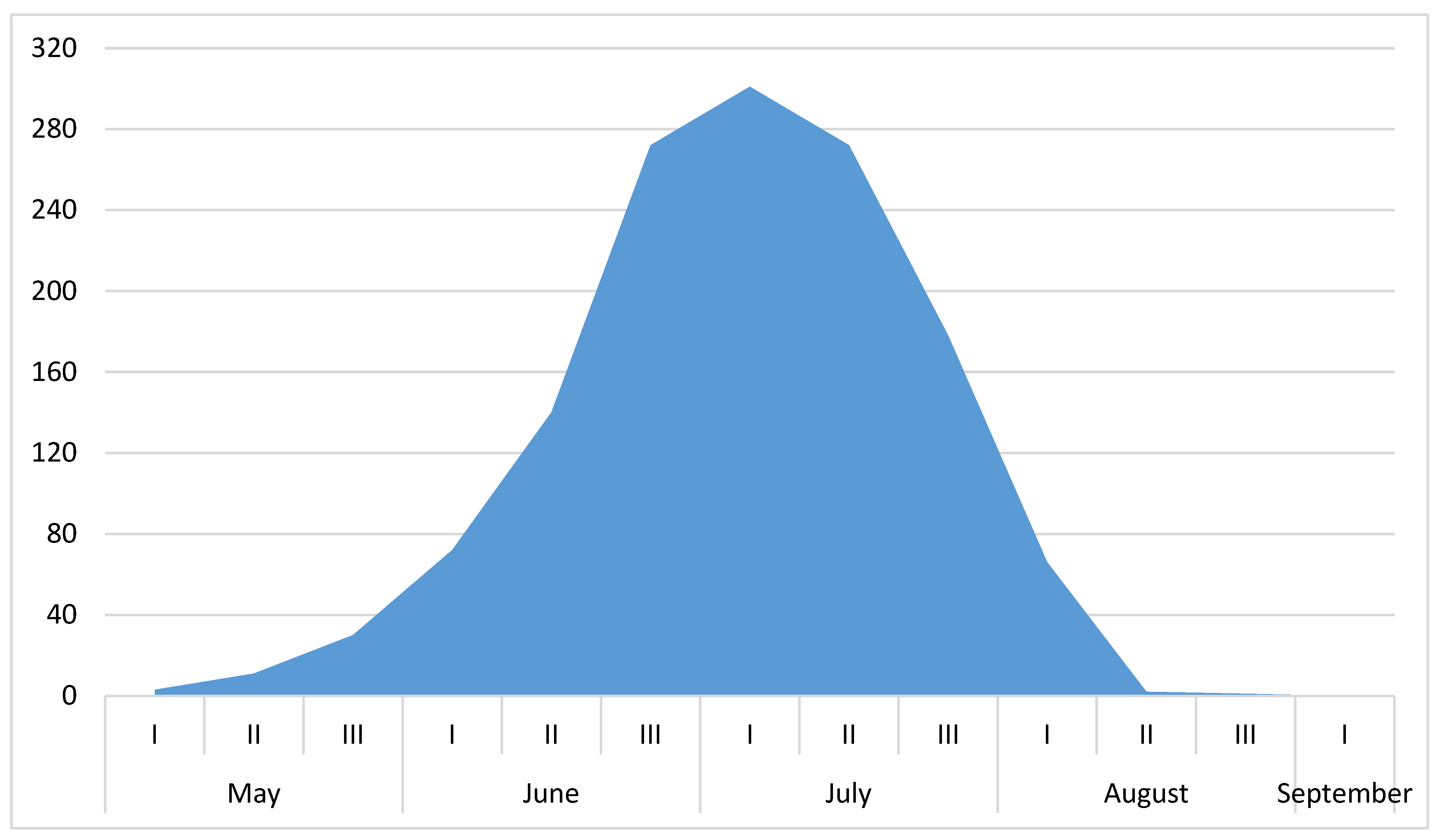
3. Results
- -
- Oak of the 1st tier (average dissimilarity = 6.2, % contribution = 16.2%);
- -
- Birch of the 1st tier (average dissimilarity = 4.1, % contribution = 10.6%);
- -
- Pine of the 1st tier (average dissimilarity = 3.5, % contribution = 9.0%);
- -
- Birch undergrowth (average dissimilarity = 3.1, % contribution = 8.0%);
- -
- Cereals (average dissimilarity = 3.0, % contribution = 7.7%);
- -
- Different grasses (average dissimilarity = 2.8, % contribution = 7.5%).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuemmerle, T.; Radeloff, V.C.; Perzanowski, K.; Hostert, P. Cross-border comparison of land cover and landscape pattern in Eastern Europe using a hybrid classification technique. Remote Sens. Environ. 2006, 103, 449–464. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Eickermann, M.; Junk, J.; Rapisarda, C. Climate Change and Insects. Insects 2023, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Ananyev, V.A.; Pekkoev, A.N.; Grabovik, S.I.; Moshnikov, S.A.; Medvedeva, M.V.; Ruokolainen, A.V.; Kolesnikova, V.M.; Grabeklis, V.V. Biodiversity dynamics in primary mid-taiga spruce forests after total windthrow in the Vodlozersky National Park, Russia. Nat. Conserv. Res. 2023, 8, 75–93. [Google Scholar] [CrossRef]
- Atutova, Z.V. Post-fire restoration of pine forests in the Badary area, Tunkinskiy National Park, Russia. Nat. Conserv. Res. 2023, 8, 22–32. [Google Scholar] [CrossRef]
- Tchatchoua, D.T.; Hamadou, O.; Maloum, M.; Carlson, J.E.; Palou Madi, O. Influence of environmental conditions on Faidherbia albida parklands in the Sudano Sahelian zone of Cameroon. J. Wildl. Biodivers. 2023, 7, 101–116. [Google Scholar] [CrossRef]
- Trujillo-Arias, N.; Serrano-Cardozo, V.H.; Ramírez-Pinilla, M.P. Role of a campesine reserve zone in the Magdalena Valley (Colombia) in the conservation of endangered tropical rainforests. Nat. Conserv. Res. 2023, 8, 49–63. [Google Scholar] [CrossRef]
- Alexander, K.N.A. Tree biology and saproxylic coleoptera: Issues of definitions and conservation language. Rev. D’écologie 2008, Sup10, 9–13. [Google Scholar] [CrossRef]
- Speight, M.C.D. Saproxylic Invertebrates and Their Conservation; Nature and Environment Series No. 42; Council of Europe: Strasbourg, France, 1989. [Google Scholar]
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forest as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Parisi, F.; Lombardi, F.; Sciarretta, A.; Tognetti, R.; Campanaro, A.; Marchetti, M.; Trematerra, P. Spatial patterns of saproxylic beetles in a relic silver fir forest (Central Italy), relationships with forest structure and biodiversity indicators. For. Ecol. Manag. 2016, 381, 217–234. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification of beetles in deciduous forest communities in the Centre of European Russia. Diversity 2021, 13, 508. [Google Scholar] [CrossRef]
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests—A review. Forest 2018, 11, 423. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J.; Pulkrab, K. How to increase biodiversity of saproxylic beetles in commercial stands through integrated forest management in Central Europe. Forests 2021, 12, 814. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Artaev, O.N.; Esin, M.N. Dataset: Coleoptera (Insecta) collected from beer traps in “Smolny” National Park (Russia). Data 2022, 7, 161. [Google Scholar] [CrossRef]
- Egorov, L.V.; Ruchin, A.B.; Alekseev, S.K.; Lukiyanov, S.V.; Lobachev, E.A.; Esin, M.N.; Artaev, O.N.; Semishin, G.B. Scarabaeoidea (Coleoptera) fauna of the Republic of Mordovia (Russia). Diversity 2023, 15, 745. [Google Scholar] [CrossRef]
- Alimova, L.K.; Umurzakova, M.S.; Zokirova, D.F.; Khamzaev, R.A.; Normuradova, G.; Otakulov, B.; Urazova, R.S.; Khalimov, F.Z. Diversity and features of the fauna of herpetobiont beetles (Carabidae, Tenebrionidae, Elateridae, Scarabaeidae) of the Lower Zeravshan, Uzbekistan. Biosyst. Divers. 2024, 32, 73–82. [Google Scholar] [CrossRef]
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304. [Google Scholar] [CrossRef]
- Jaworski, T.; Plewa, R.; Hilszczański, J.; Szczepkowski, A.; Horak, J. Saproxylic moths reveal complex within-group and group-environment patterns. J. Insect Conserv. 2016, 20, 677–690. [Google Scholar] [CrossRef]
- Plewa, R.; Jaworski, T.; Hilszczański, J.; Horák, J. Investigating the biodiversity of the forest strata: The importance of vertical stratification to the activity and development of saproxylic beetles in managed temperate deciduous forests. For. Ecol. Manag. 2017, 402, 186–193. [Google Scholar] [CrossRef]
- Bouget, C.; Brustel, H.; Brin, A.; Noblecourt, T. Sampling saproxylic beetles with window flight traps: Methodological insights. Rev. D’écologie 2008, suppl. 10, 21–32. [Google Scholar] [CrossRef]
- Wikars, L.O.; Sahlin, E.; Ranius, T. A comparison of three methods to estimate species richness of saproxylic beetles (Coleoptera) in logs and high stumps of Norway spruce. Can. Entomol. 2005, 137, 304–324. [Google Scholar] [CrossRef]
- Brin, A.; Bouget, C.; Valladares, L.; Brustel, H. Are stumps important for the conservation of saproxylic beetles in managed forests?—Insights from a comparison of assemblages on logs and stumps in oak-dominated forests and pine plantations. Insect Conserv. Divers. 2012, 6, 255–264. [Google Scholar] [CrossRef]
- Foit, J.; Cermák, V.; Kudlácek, T. Spatial distribution of saproxylic beetles on trunks of standing Scots pine trees. Agric. For. Entomol. 2023, 25, 601–611. [Google Scholar] [CrossRef]
- Alinvi, O.; Ball, J.P.; Danell, K.; Hjältén, J.; Pettersson, R.B. Sampling saproxylic beetle assemblages in dead wood logs: Comparing window and eclector traps to traditional bark sieving and a refinement. J. Insect Conserv. 2007, 11, 99–112. [Google Scholar] [CrossRef]
- Nițu, E.; Olenici, N.; Popa, I.; Nae, A.; Biriș, I.A. Soil and saproxylic species (Coleoptera, Collembola, Araneae) in primeval forests from the northern part of South-Easthern Carpathians. Ann. For. Res. 2009, 52, 27–54. [Google Scholar]
- Persiani, A.M.; Audisio, P.; Lunghini, D.; Maggi, O.; Granito, V.M.; Biscaccianti, A.B.; Chiavetta, U.; Marchetti, M. Linking taxonomical and functional biodiversity of saproxylic fungi and beetles in broadleaved forests in southern Italy with varying management histories. Plant Biosyst.–Int. J. Deal. All Asp. Plant Biol. 2010, 144, 250–261. [Google Scholar]
- Boulanger, Y.; Sirois, L. Postfire succession of saproxylic arthropods, with emphasis on Coleoptera, in the north boreal forest of Quebec. Environ. Entomol. 2014, 36, 128–141. [Google Scholar] [CrossRef]
- Peuhu, E.; Thomssen, P.M.; Siitonen, J. Comparison of three trap types in sampling saproxylic beetles living in hollow urban trees. J. Insect. Conserv. 2019, 23, 75–87. [Google Scholar] [CrossRef]
- Bardiani, M.; Tini, M.; Carpaneto, G.M.; Audisio, P.; Bussola, E.; Campanaro, A.; Cini, A.; Maurizi, E.; Mason, F.; Peverieri, G.S.; et al. Effects of trap baits and height on stag beetle and flower chafer monitoring: Ecological and conservation implications. J. Insect Conserv. 2017, 21, 157–168. [Google Scholar] [CrossRef]
- Touroult, J.; Witté, I. Beer, wine, or fruit juice: Which is best? A case study of bait efficiency to sample saproxylic beetles (Coleoptera) in an oak woodland. Coleopt. Bull. 2020, 74, 763–771. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. On the distribution of Coleoptera in forests and open areas (center of the European part of Russia): A study using beer traps. J. Wildl. Biodivers. 2024, 8, 171–191. [Google Scholar] [CrossRef]
- Geography of Russia. Encyclopaedic Dictionary; Gorkina, A.P., Ed.; The Great Russian Encyclopedia: Moscow, Russia, 1998; 800p. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Shokhin, I.V. Contribution to the fauna of lamellicorn beetles (Coleoptera, Scarabaeoidea) of Southern Russia, with some nomenclatural changes in the family Scarabaeidae. Cauc. Entomol. Bull. 2007, 3, 105–185. (In Russian) [Google Scholar] [CrossRef]
- Shokhin, I.V. Contribution to the fauna of lamellicorn beetles (Coleoptera: Scarabaeoidea) of Southern Russia. Addition 1. Cauc. Entomol. Bull. 2016, 12, 75–79. (In Russian) [Google Scholar] [CrossRef]
- Shapovalov, A.M.; Nemkov, V.A.; Rusakov, A.V. Protected coleopterans (Insecta, Coleoptera) of Orenburg region. Proc. Orenbg. Branch Russ. Entomol. Soc. 2011, 1, 49–79. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Vertical distribution of beetles (Coleoptera) in Pine Forests in Central European Russia. Diversity 2022, 14, 622. [Google Scholar] [CrossRef]
- Tauzin, P. Chorologie et éco-éthologie de Protaetia (Potosia) fieberi Kraatz 1880 en France (Coleoptera, Cetoniinae, Cetoniini). Cetoniimania 2007, 3, 115–146. [Google Scholar]
- Sinigla, M.; Farkas, E. Role of old wood-pastures in the preservation of lichen diversity. Folia Musei Hist. -Nat. Bakony. 2020, 37, 7–18. [Google Scholar]
- László, N. A Protaetia (Potosia) fieberi (Kraatz, 1880) életmódja és elterjedési adatai Magyarországon (Coleoptera, Scarabaeidae: Cetoniinae). Folia Hist. Nat. Musei Matraensi 2008, 32, 175–178. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V. Overview of insect species included in the Red Data Book of Russian Federation in the Mordovia State Nature Reserve. Nat. Conserv. Res. 2017, 2 (Suppl. S1), 2–9. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Sazhnev, A.S.; Polumordvinov, O.A.; Ishin, R.N. Present distribution of Protaetia fieberi (Kraatz, 1880) (Insecta, Coleoptera, Scarabaeidae) in the European part of Russia. Biharean Biol. 2019, 13, 12–16. [Google Scholar]
- Ruchin, A.B.; Khapugin, A.A. Red data book invertebrates in a protected area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Nikitsky, N.B.; Shokhin, I.V. Protaetia fieberi (Kraatz, 1880). In Red Data Book of Russian Federation; Tome “Animals”: Moscow, Russia, 2021; p. 172. [Google Scholar]
- Egorov, L.V.; Alekseev, S.K.; Ruchin, A.B.; Sazhnev, A.S.; Artaev, O.N.; Esin, M.N.; Lobachev, E.A.; Lukiyanov, S.V.; Semenov, A.V.; Lukyanova, Y.A.; et al. Biodiversity of Coleoptera (Insecta) in the Middle and Lower Volga Regions (Russia). Diversity 2022, 14, 1128. [Google Scholar] [CrossRef]
- Egorov, L.V.; Isaev, A.V. Coleoptera of the State Nature Reserve “Bolshaya Kokshaga” (based on the results of collections by fermental crown traps in 2021). Proc. Mordovia State Nat. Reserve 2024, 34, 161–172. [Google Scholar] [CrossRef]
- Medvedev, S.I. Scarabaeidae: Subfamily. Cetoninae, Valginae; Fauna of the USSR NS No. 90. Coleoptera; Leningrad: Moscow, Russia, 1964; Volume 10, p. 375. (In Russian) [Google Scholar]
- Dedyukhin, S.V. The data on the Red book Coleoptera and the species recommended to a new red list in the Udmurt Republic. Ser. Biol. 2006, 10, 129–140. [Google Scholar]
- Recalde Irurzun, J.I.; San Martín Moreno, A.F. Aproximación a la fauna de escarabajos saproxílicos (Coleoptera) del Parque Natural del Señorío de Bertiz (Navarra). Heteropterus Rev. De Entomol. 2015, 15, 43–57. [Google Scholar]
- Olenici, N.; Fodor, E. The diversity of saproxylic beetles’ from the Natural Reserve Voievodeasa forest, North-Eastern Romania. Ann. For. Res. 2021, 64, 31–60. [Google Scholar] [CrossRef]
- Jäger, O.; Brunk, I.; Lorenz, J. Zur Insekten- und Spinnenfauna der Kleinraschützer Heide bei Großenhain in Sachsen—Allgemeiner Teil und Käfer (Coleoptera). Sächsische Entomol. Z. 2015, 8, 30–67. [Google Scholar]
- Trach, V.A.; Gontarenko, A.V. The beetles of superfamily Scarabaeoidea (Coleoptera: Scarabaeoidea: Lucanidae; Glaphyridae; Scarabaeidae: Melolonthinae, Sericinae, Rutelinae, Dynastinae, Cetoniinae) of the Odessa Region. Kharkov Entomol. Soc. Gaz. 2010, XVIII, 87–94. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification and seasonal dynamics of Coleoptera in open biotopes of forest ecosystems (Centre of European Russia). Forests 2022, 13, 1014. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Edge Effects in the Distribution of Coleoptera in the Forests of the Center of the European Part of Russia. Insects 2023, 14, 371. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for the study of the species diversity of Coleoptera in open biotopes. Insects 2023, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Villani, M.; Pezzi, G. Interessanti ritrovamenti entomologici in Emilia-Romagna e zone limitrofe (Insecta Orthoptera, Dermaptera, Coleoptera). Quad. Di Studi E Not. Di Stor. Nat. Della Romagna 2016, 43, 101–115. [Google Scholar]
- Rößner, E. Die Hirschkäfer und Blatthornkäfer Ostdeutschlands (Coleoptera: Scarabaeoidea); Verein der Freunde & Förderer des Naturkundemuseums Erfurt e. V.: Erfurt, Germany, 2012; p. 508. [Google Scholar]
- Egorov, L.V.; Ruchin, A.B. New data on beetles (Insecta: Coleoptera) of the Tambov Region. Sci. Proc. Prisursky State Nat. Reserve 2023, 38, 118–130. [Google Scholar]
- Egorov, L.; Ruchin, A.; Esin, M.; Artaev, O. Biodiversity of Coleoptera (Insecta) in Mordovia State Nature Reserve (Russia) using fermental traps. Biodivers. Data J. 2022, 10, e96989. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for studying the species diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef]
- Egorov, L.V. Rare Beetles (Insecta: Coleoptera) of Chuvashia (by the Materials of 2023). Nat. Sci. Res. Chuvashia Adjac. Reg. 2024, 10, 63–74. (In Russian) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, L.V.; Ruchin, A.B.; Khapugin, A.A. Distribution and Biology of Protaetia fieberi (Coleoptera, Scarabaeidae)—Is Protection Status Required? Insects 2024, 15, 695. https://doi.org/10.3390/insects15090695
Egorov LV, Ruchin AB, Khapugin AA. Distribution and Biology of Protaetia fieberi (Coleoptera, Scarabaeidae)—Is Protection Status Required? Insects. 2024; 15(9):695. https://doi.org/10.3390/insects15090695
Chicago/Turabian StyleEgorov, Leonid V., Alexander B. Ruchin, and Anatoliy A. Khapugin. 2024. "Distribution and Biology of Protaetia fieberi (Coleoptera, Scarabaeidae)—Is Protection Status Required?" Insects 15, no. 9: 695. https://doi.org/10.3390/insects15090695







