The Ultramorphology and Sexual Dimorphism of Antennae and Sensilla in the Pale Grass Blue, Pseudozizeeria maha (Lepidoptera: Lycaenidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collections
2.2. Scanning Electron Microscopy (SEM)
2.3. Terminology and Data Analysis
3. Results
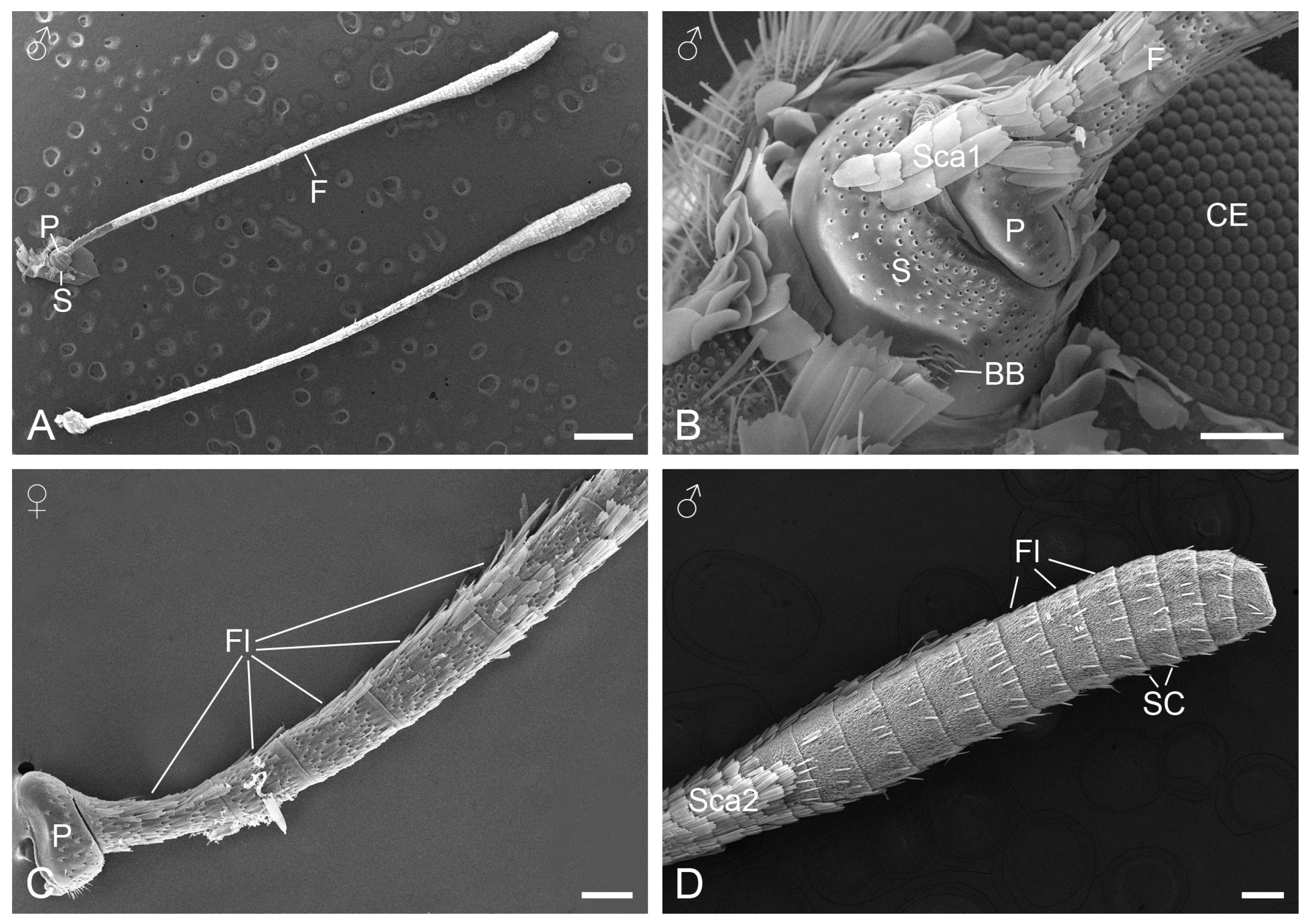
3.1. Antennal Morphology
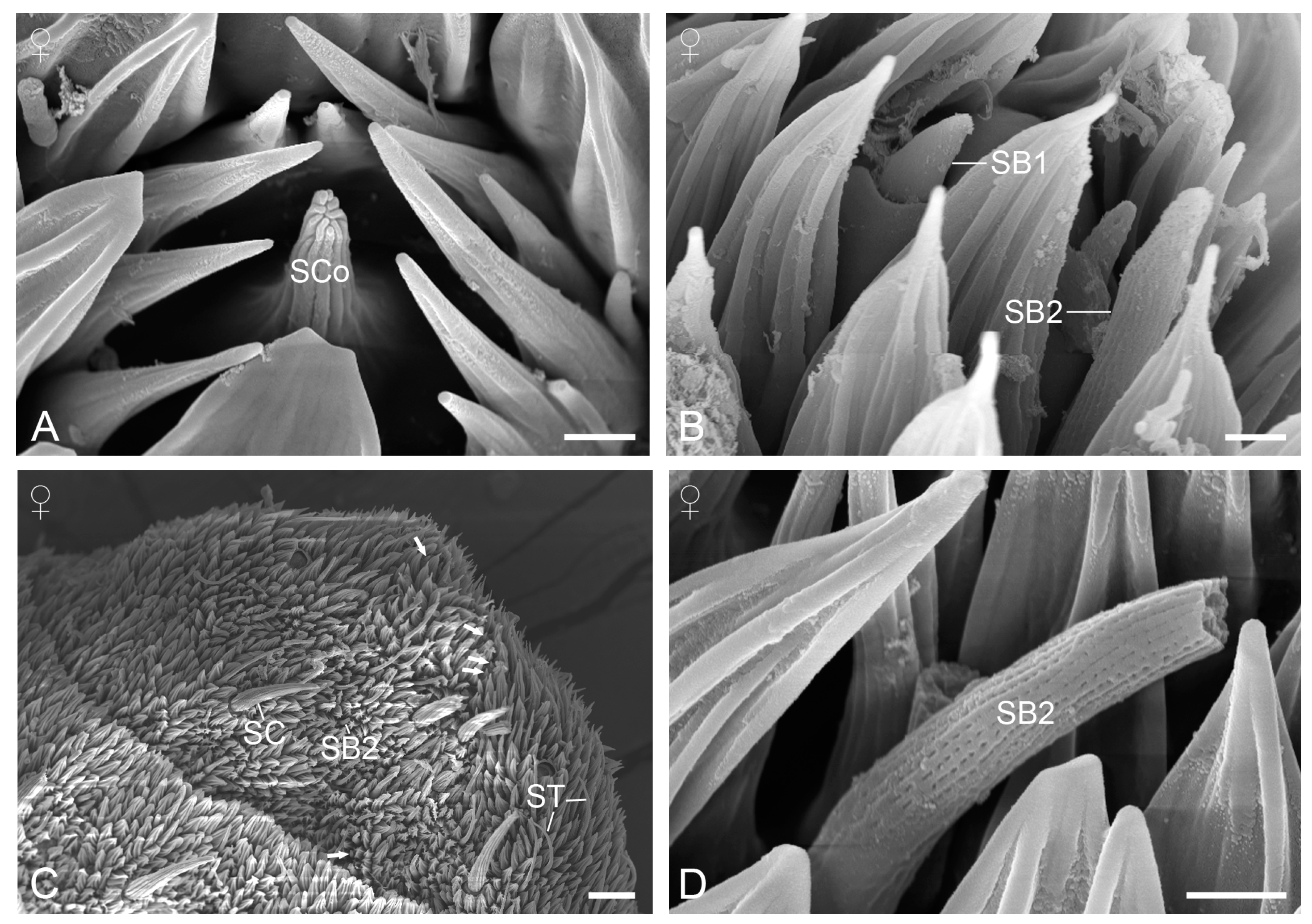
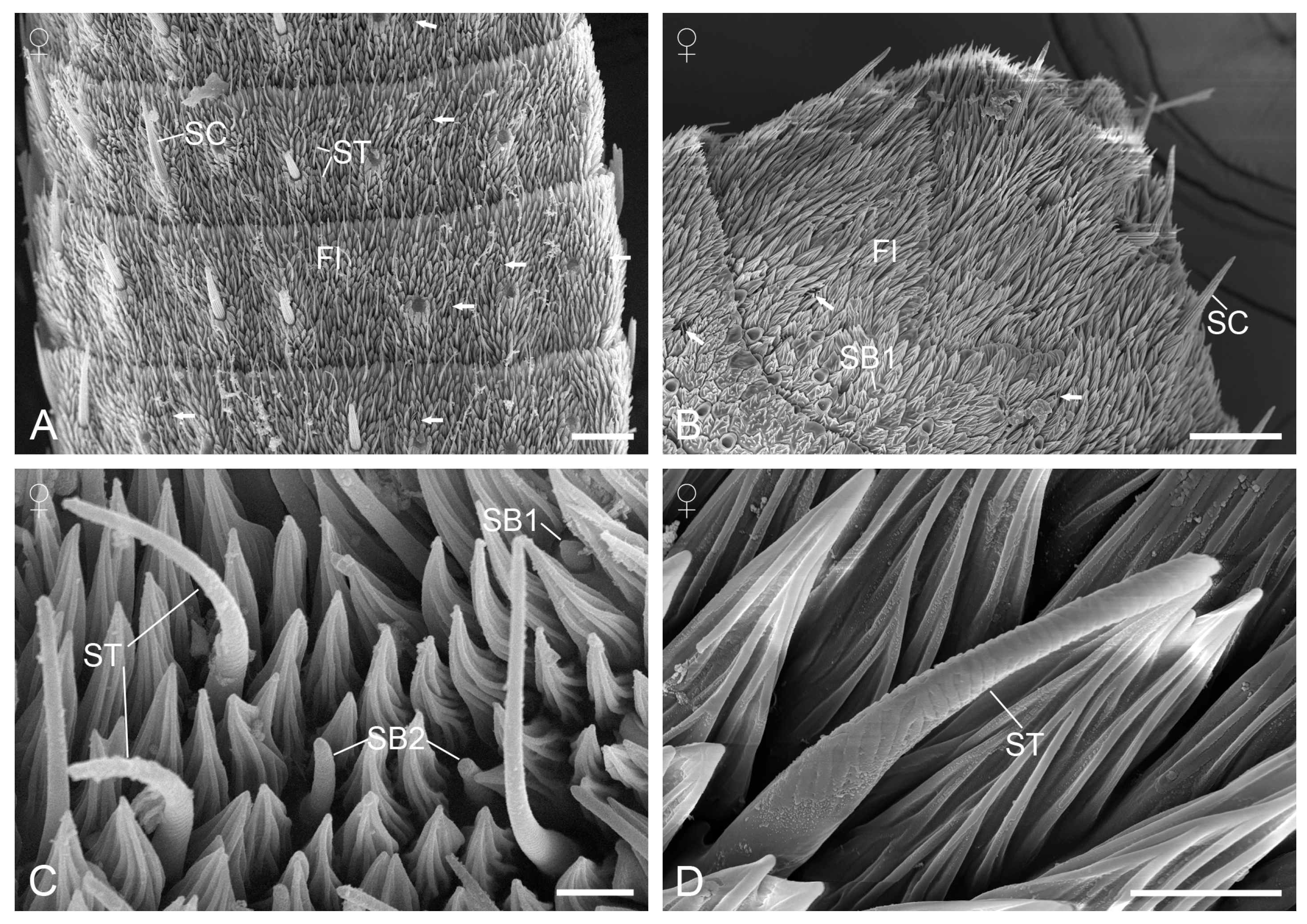
3.2. Sensilla on the Antennae
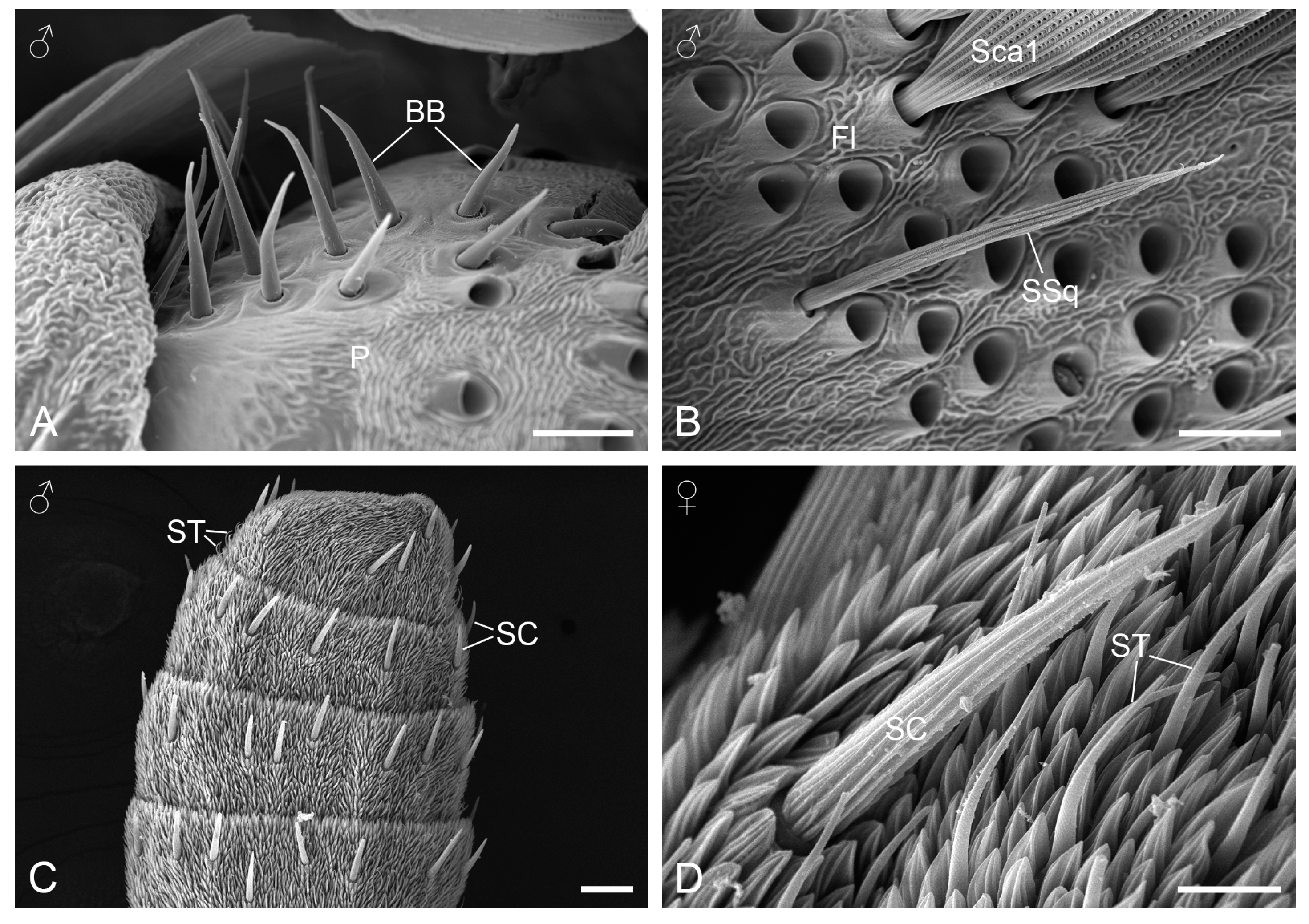
3.2.1. Böhm’s Bristles
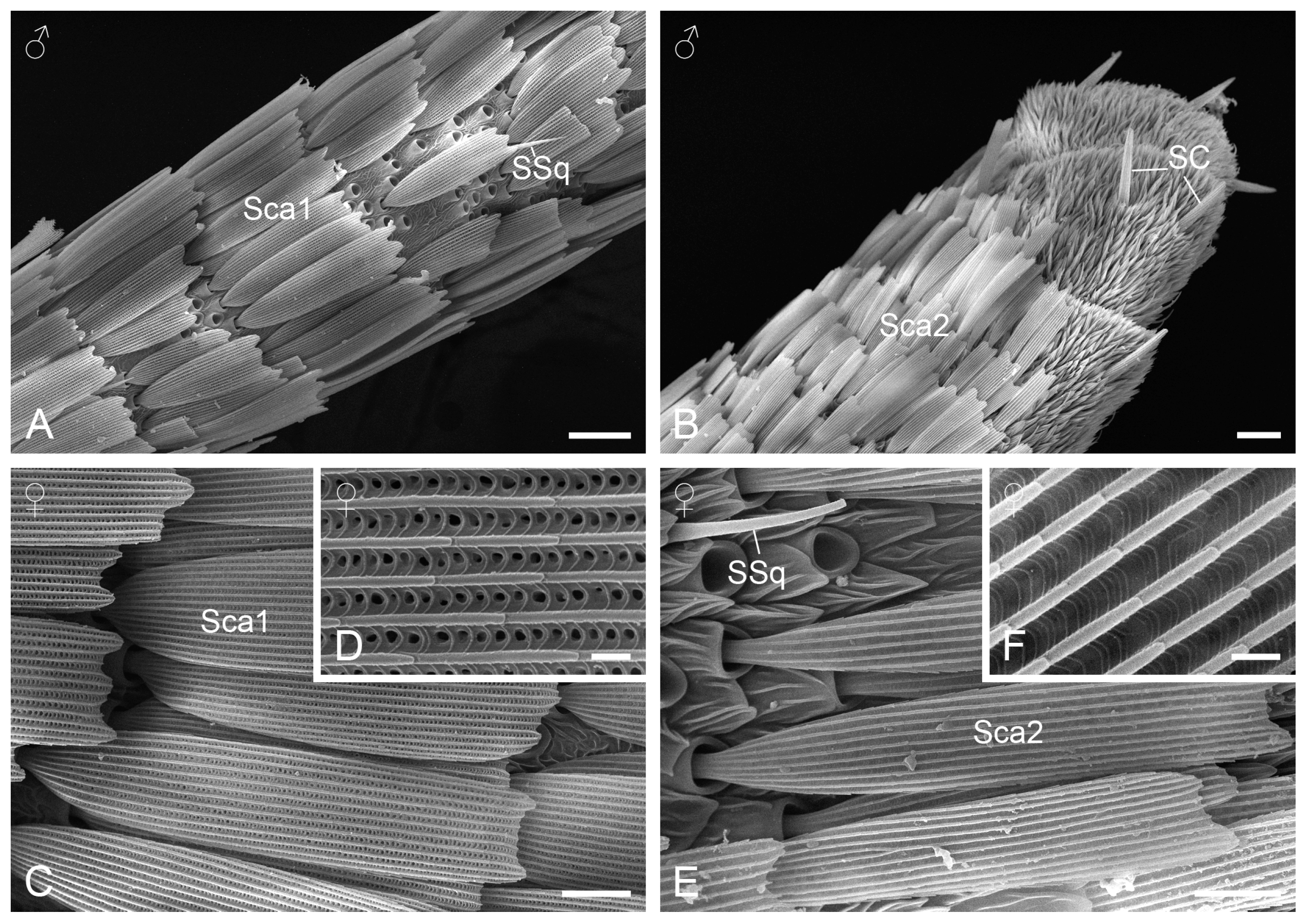
3.2.2. Sensilla Squamiformia
3.2.3. Sensilla Chaetica
3.2.4. Sensilla Trichodea
3.2.5. Sensilla Coeloconica
3.2.6. Sensilla Basiconica
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Shields, V.D. Ultrastructure of insect sensilla. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 4009–4023. [Google Scholar]
- Elgar, M.A.; Zhang, D.; Wang, Q.; Wittwer, B.; Pham, H.T.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Insect antennal morphology: The evolution of diverse solutions to odorant perception. Yale J. Biol. Med. 2018, 91, 457–469. [Google Scholar] [PubMed]
- Faucheux, M.J. Sensory organs on the antennae of Micropterix calthella L. (Lepidoptera, Micropterigidae). Acta Zool. 1997, 78, 1–8. [Google Scholar] [CrossRef]
- Bawin, T.; Collard, F.; De Backer, L.; Yarou, B.B.; Compère, P.; Francis, F.; Verheggen, F.J. Structure and distribution of the sensilla on the antennae of Tuta absoluta (Lepidoptera: Gelechiidae). Micron 2017, 96, 16–28. [Google Scholar] [CrossRef]
- Yan, X.Z.; Deng, C.P.; Xie, J.X.; Wu, L.J.; Sun, X.J.; Hao, C. Distribution patterns and morphology of sensilla on the antennae of Plutella xylostella (L.)—A scanning and transmission electron microscopic study. Micron 2017, 103, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Du, X.; Li, Z.; Wu, J. Ultrastructure of antennal sensilla of three fruit borers (Lepidoptera: Crambidae or Tortricidae). PLoS ONE 2018, 13, e0205604. [Google Scholar] [CrossRef]
- Liu, H.X.; Liu, Z.X.; Zheng, H.X.; Jin, Z.R.; Zhang, J.T.; Zhang, P.Q. Sensilla on the antennae and ovipositor of the carpenterworm, Streltzoviella insularis (Staudinger, 1892) (Lepidoptera, Cossidae). Orient. Insects 2018, 52, 420–433. [Google Scholar] [CrossRef]
- Hu, G.L.; Zhang, C.M.; Wang, Z.Q.; Chen, Q.X.; Lu, J.Q. Sensilla of the antenna and proboscis of Athetis lepigone (Möschler) (Lepidoptera: Noctuidae). J. Morphol. 2021, 282, 733–745. [Google Scholar] [CrossRef]
- Xu, J.; Deng, C.; Lu, W.; Wu, S. Ultrastructure of antennal sensilla in adults of Dioryctria rubella Hampson (Lepidoptera: Pyralidae). Insects 2021, 12, 821. [Google Scholar] [CrossRef]
- Guo, J.; Du, Z.; Cui, G.; Wang, Z.; Wang, J.; Zhou, X. Ultrastructure characteristics and sexual dimorphism of antennal sensilla in Tirathaba rufivena (Lepidoptera: Pyralidae). Insects 2022, 13, 797. [Google Scholar] [CrossRef]
- Wang, W.; He, P.; Liu, T.; Jing, X.; Zhang, S. Morphology and distribution of antennal sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae and adults. Diversity 2023, 15, 992. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, K.C.; Oh, H.W.; Park, C.G. Morphology and distribution of antennal sensilla of two tortricid moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 2016, 79, 1069–1081. [Google Scholar] [CrossRef]
- Faucheux, M.J. Antennal sensilla in male and female carpet moth, Trichophaga tapetzella L. (Lepidoptera: Tineidae): A scanning electron microscopic study. In Annales de la Société Entomologique de France (ns); Taylor & Francis: Abingdon, UK, 1989; Volume 25, pp. 83–93. [Google Scholar]
- Myers, J. The structure of the antennae of the florida queen butterfly, Danaus gilippus berenice (Cramer). J. Morph. 1968, 125, 315–328. [Google Scholar] [CrossRef]
- Grula, J.W.; Taylor, O.R. A micromorphological and experimental study of the antennae of the sulfur butterflies, Colias eurytheme and C. philodice (Lepidoptera: Pieridae). J. Kansas Entomol. Soc. 1980, 53, 476–484. [Google Scholar]
- Carlsson, M.A.; Schäpers, A.; Nässel, D.R.; Janz, N. Organization of the olfactory system of Nymphalidae butterflies. Chem. Senses 2013, 38, 355–367. [Google Scholar] [CrossRef]
- Abu-shall, A.M.H.; Tawfeek, M.E. Description of the egyptian form of Chilades pandava Horsfield (Lepidoptera: Lycaenidae: Polyommatinae) and ultrastructure of antennal sensilla. J. Entomol. 2015, 12, 67–76. [Google Scholar] [CrossRef][Green Version]
- Limberger, G.M.; Brugnera, R.; da Fonseca, D.B. Antennal morphology and sensilla ultrastructure of Ascia monuste (Linnaeus) (Lepidoptera: Pieridae). Micron 2021, 142, 103000. [Google Scholar] [CrossRef]
- Da Silva, C.B.; Da Silva, K.B.; Da Silva, K.B.; De Freitas, J.D.; Chia, G.S.; Garcia, C.H.; Guzzo, E.C.; Da Costa, J.G.; Feijó, F.M.; Goulart, H.F.; et al. Antennal morphology and ultrastructural sensilla characterization in Caligo illioneus illioneus Cramer (Lepidoptera, Nymphalidae) adults. Zoomorphology 2024, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, L.; Wang, T.; Liao, H.; Tang, C. Morphological characterization of the antenna and scent patch of three Danaus species (Papilionoidea: Nymphalidae, Danainae). Insects 2024, 15, 121. [Google Scholar] [CrossRef]
- Hunger, T.; Steinbrecht, R.A. Functional morphology of a double-walled multiporous olfactory sensillium: The sensillum coeloconicum of Bombyx mori (Insecta, Lepidoptera). Tissue Cell 1998, 30, 14–29. [Google Scholar] [CrossRef]
- Tan, Q.; Yan, X.F.; Wen, J.B.; Li, Z.Y. Phylogenetic relationship of seven Dendrolimus (Lepidoptera: Lasiocampidae) species based on the ultrastructure of male moths’ antennae and antennal sensilla. Microsc. Res. Tech. 2012, 75, 1700–1710. [Google Scholar] [CrossRef]
- Jaffar-Bandjee, M.; Steinmann, T.; Krijnen, G.; Casas, J. Insect pectinate antennae maximize odor capture efficiency at intermediate flight speeds. Proc. Natl. Acad. Sci. USA 2020, 117, 28126–28133. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Dong, Y.; Yang, Z.F. Antennal and proboscis sensilla characteristics of Paranthrene tabaniformis (Lepidoptera: Sesiidae). Microsc. Res. Tech. 2022, 86, 452–464. [Google Scholar] [CrossRef]
- Hambäck, P.A.; Summerville, K.S.; Steffan-Dewenter, I.; Krauss, J.; Englund, G.; Crist, T.O. Habitat specialization, body size, and family identity explain lepidopteran density–area relationships in a cross-continental comparison. Proc. Natl. Acad. Sci. USA 2007, 104, 8368–8373. [Google Scholar] [CrossRef]
- van Nieukerken, E.J.; Kaila, L.; Kitching, I.J.; Kristensen, N.P.; Lees, D.C.; Minet, J.; Mitter, C.; Mutanen, M.; Regier, J.C.; Simonsen, T.J.; et al. Order Lepidoptera Linnaeus, 1758. In Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness; Zhang, Z.Q., Ed.; Zootaxa, Magnolia Press: Auckland, New Zealand, 2011; Volume 3148, pp. 212–221. [Google Scholar]
- Nagase, A.; Kurashina, M.; Nomura, M.; MacIvor, J.S. Patterns in urban butterflies and spontaneous plants across a university campus in Japan. Pan-Pac. Entomol. 2018, 94, 195–215. [Google Scholar] [CrossRef]
- Eguchi, E.; Watanabe, K.; Hariyama, T.; Yamamoto, K. A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. J. Comp. Physiol. 1982, 28, 675–682. [Google Scholar] [CrossRef]
- Imafuku, M.; Kitamura, T. Preference of virgin females for male wing color in a sexually dichromatic butterfly, Pseudozizeeria maha (Lycaenidae). J. Lepid. Soc. 2018, 72, 212–217. [Google Scholar] [CrossRef]
- Mizokami, H.; Tomita-Yokotani, K.; Yoshitama, K. Flavonoids in the leaves of Oxalis corniculata and sequestration of the flavonoids in the wing scales of the pale grass blue butterfly, Pseudozizeeria maha. J. Plant Res. 2008, 121, 133–136. [Google Scholar] [CrossRef]
- Watson, G.S.; Watson, J.A.; Cribb, B.W. Diversity of cuticular micro- and nanostructures on insects: Properties, functions, and potential applications. Annu. Rev. Entomol. 2017, 62, 185–205. [Google Scholar] [CrossRef]
- Dürr, V.; Berendes, V.; Strube-Bloss, M. Sensorimotor ecology of the insect antenna: Active sampling by a multimodal sensory organ. Adv. Insect Physiol. 2022, 63, 1–105. [Google Scholar]
- Yuan, X.; Gao, K.; Yuan, F.; Zhang, Y. Ultrastructure of antennal sensilla of four skipper butterflies in Parnara sp. and Pelopidas sp. (Lepidoptera, Hesperiidae). Zookeys 2014, 399, 17–27. [Google Scholar]
- Pophof, B. Olfactory responses recorded from sensilla coeloconica of the silkmoth Bombyx mori. Physiol. Entomol. 1997, 22, 239–248. [Google Scholar] [CrossRef]
- Odendaal, F.J.; Ehrlich, P.R.; Thomas, F.C. Structure and function of the antennae of Euphydryas editha (Lepidoptera: Nymphalidae). J. Morphol. 1985, 184, 3–22. [Google Scholar] [CrossRef]
| Sensilla Types/Subtypes | Length (μm) | Basal Width (μm) | ||||
|---|---|---|---|---|---|---|
| Female | Male | t-Test | Female | Male | t-Test | |
| Böhm’s bristles on scape | 13.75 ± 0.38 (11) | 14.46 ± 0.42 (11) | NS | 2.13 ± 0.04 (14) | 2.12 ± 0.07 (14) | NS |
| Böhm’s bristles on pedicel | 14.07 ± 0.42 (8) | 13.37 ± 0.43 (8) | NS | 2.01 ± 0.04 (10) | 1.99 ± 0.08 (10) | NS |
| Sensilla squamiformia | 32.65 ± 0.59 (7) | 33.52 ± 0.96 (7) | NS | 1.76 ± 0.02 (7) | 1.84 ± 0.05 (7) | NS |
| Sensilla chaetica | 33.54 ± 2.25 (8) | 32.62 ± 1.57 (8) | NS | 5.13 ± 0.15 (8) | 5.35 ± 0.14 (8) | NS |
| Sensilla trichodea | 13.73 ± 0.49 (7) | 16.69 ± 1.46 (7) | NS | 1.53 ± 0.03 (7) | 1.55 ± 0.06 (7) | NS |
| Sensilla basiconica 1 | 2.49 ± 0.35 (4) | 2.55 ± 0.11 (4) | NS | 1.46 ± 0.19 (4) | 1.11 ± 0.04 (4) | NS |
| Sensilla basiconica 2 | 6.43 ± 0.70 (7) | 6.82 ± 0.57 (7) | NS | 1.08 ± 0.05 (8) | 1.08 ± 0.06 (8) | NS |
| Sensilla coeloconica | 2.34 ± 0.17 (4) | − | − | 1.15 ± 0.03 (4) | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-X.; Han, Y.; Li, Y.-F. The Ultramorphology and Sexual Dimorphism of Antennae and Sensilla in the Pale Grass Blue, Pseudozizeeria maha (Lepidoptera: Lycaenidae). Insects 2024, 15, 698. https://doi.org/10.3390/insects15090698
Chen Q-X, Han Y, Li Y-F. The Ultramorphology and Sexual Dimorphism of Antennae and Sensilla in the Pale Grass Blue, Pseudozizeeria maha (Lepidoptera: Lycaenidae). Insects. 2024; 15(9):698. https://doi.org/10.3390/insects15090698
Chicago/Turabian StyleChen, Qing-Xiao, Ying Han, and Ya-Fei Li. 2024. "The Ultramorphology and Sexual Dimorphism of Antennae and Sensilla in the Pale Grass Blue, Pseudozizeeria maha (Lepidoptera: Lycaenidae)" Insects 15, no. 9: 698. https://doi.org/10.3390/insects15090698
APA StyleChen, Q.-X., Han, Y., & Li, Y.-F. (2024). The Ultramorphology and Sexual Dimorphism of Antennae and Sensilla in the Pale Grass Blue, Pseudozizeeria maha (Lepidoptera: Lycaenidae). Insects, 15(9), 698. https://doi.org/10.3390/insects15090698





