Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biting Midge Collection
2.2. Materials
2.2.1. Sorting of Biting Midges
2.2.2. Morphological Identification of Culicoides Species
2.3. Statistical Analysis
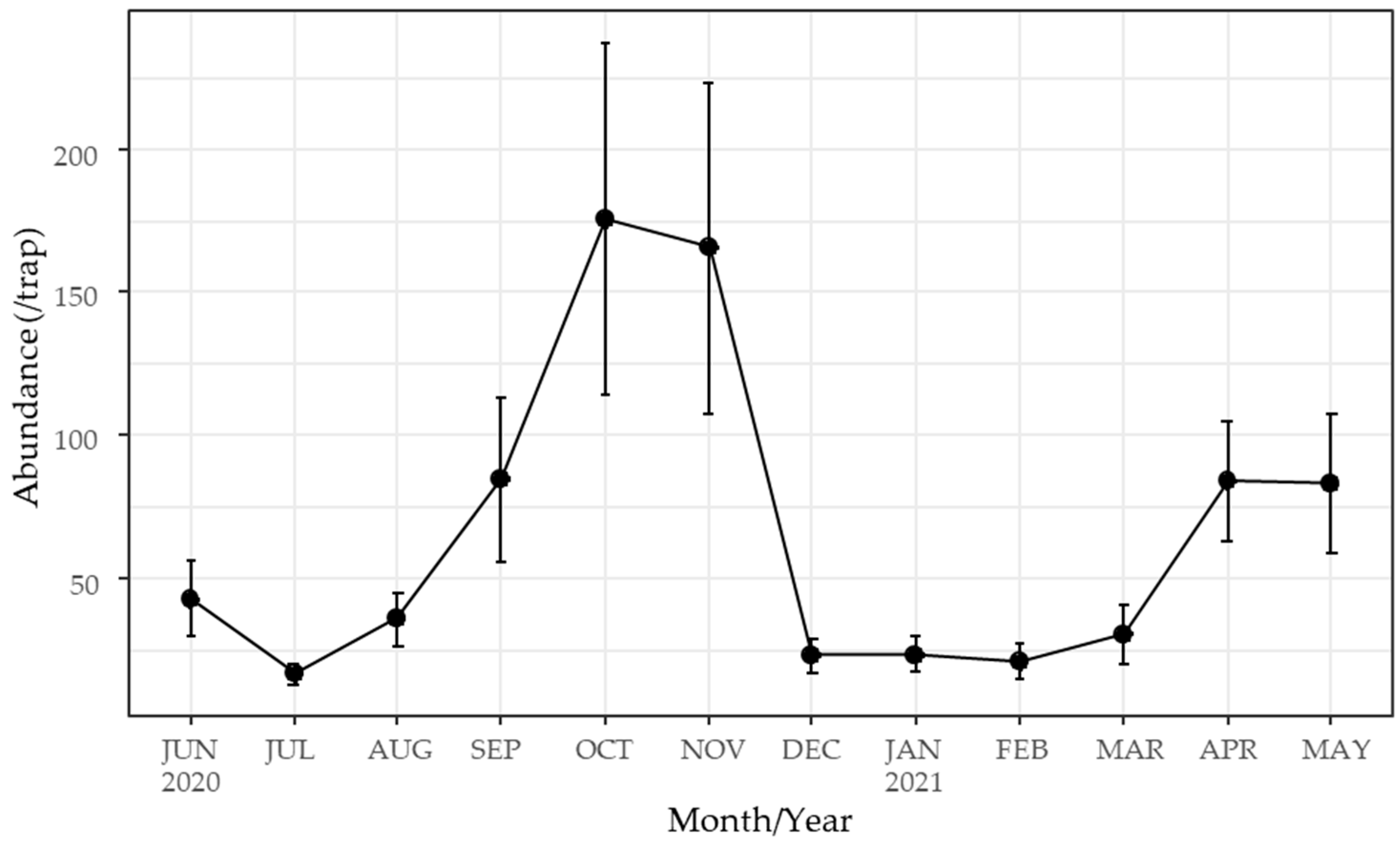
3. Results
3.1. Species Composition of Culicoides Biting Midges
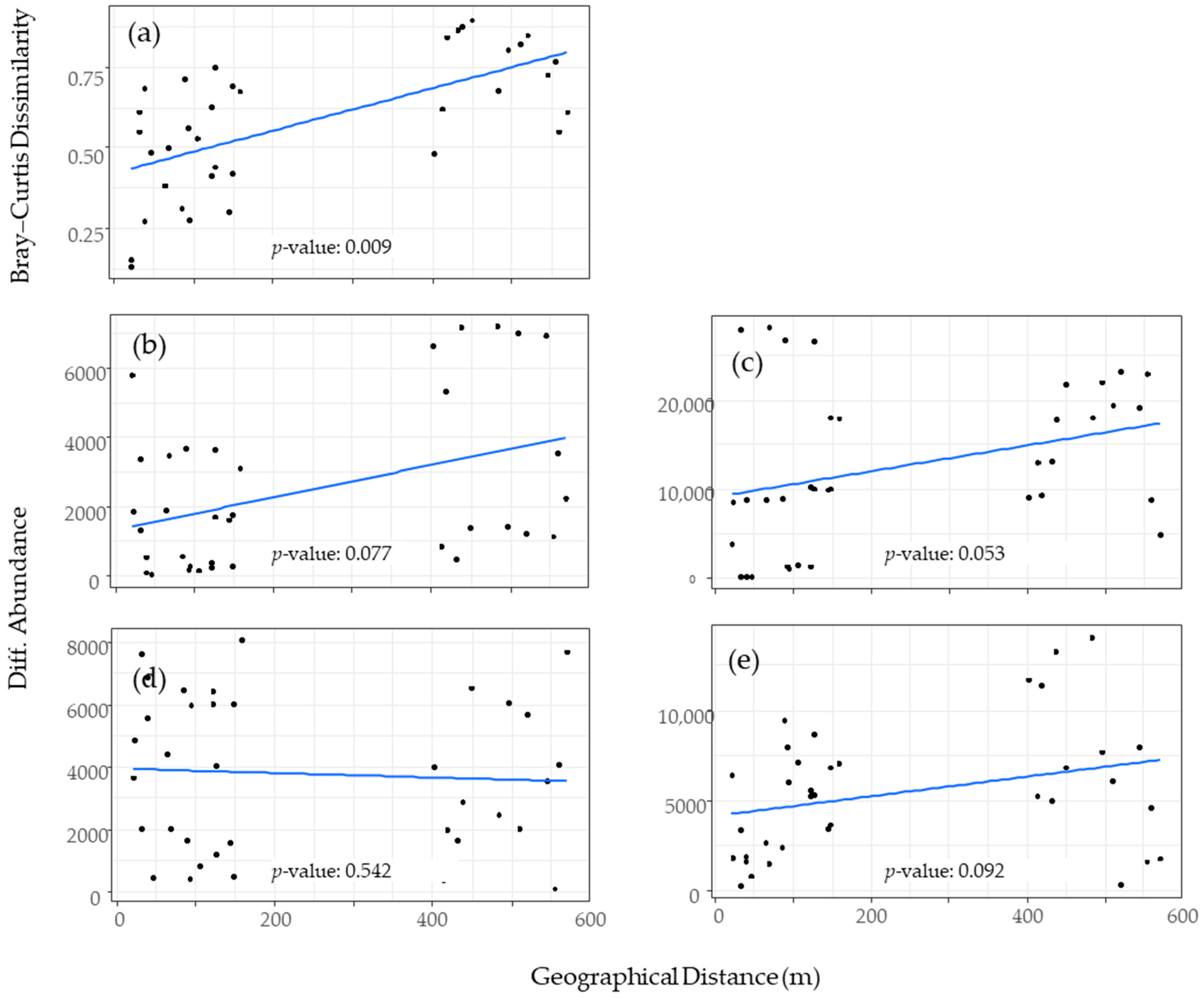
3.2. Factors Influencing Culicoides Biting Midge Assemblage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbers, A.R.; Koenraadt, C.J.; Meiswinkel, R. Mosquitoes and Culicoides biting midges: Vector range and the influence of climate change. Rev. Sci. Tech. 2015, 34, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Borkent, A.; Dominiak, P. Catalog of the Biting Midges of the World (Diptera: Ceratopogonidae); Magnolia Press: Waco, TX, USA, 2020; Volume 4787, pp. 1–377. [Google Scholar]
- Meiswinkel, R.; Venter, G.J.; Nevill, E.M. Vectors: Culicoides spp. In Infectious Diseases of Livestock; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 93–136. [Google Scholar]
- Gopurenko, D.; Bellis, G.; Pengsakul, T.; Siriyasatien, P.; Thepparat, A. DNA barcoding of Culicoides Latreille (Diptera: Ceratopogonidae) from Thailand reveals taxonomic inconsistencies and novel diversity among reported sequences. J. Med. Entomol. 2022, 59, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Jomkumsing, P.; Piraonapicha, K.; Jumpato, W. Integrative taxonomy uncovers a new Culicoides (Diptera: Ceratopogonidae) biting midge species from Thailand. Acta Trop. 2021, 220, 105941. [Google Scholar] [CrossRef] [PubMed]
- Thepparat, A.; Bellis, G.; Ketavan, C.; Ruangsittichai, J.; Sumruayphol, S.; Apiwathnasorn, C. Ten species of Culicoides Latreille (Diptera: Ceratopogonidae) newly recorded from Thailand. Zootaxa 2015, 4033, 48–56. [Google Scholar] [CrossRef]
- Thepparat, A.; Tsuruishi, T.; Ketavan, C. Species Diversity and Abundance of Culicoides spp. in Sakaew Province. RRJ.ST 2012, 15, 65–80. [Google Scholar]
- Sunantaraporn, S.; Hortiwakul, T.; Kraivichian, K.; Siriyasatien, P.; Brownell, N. Molecular Identification of Host Blood Meals and Detection of Blood Parasites in Culicoides Latreille (Diptera: Ceratopogonidae) Collected from Phatthalung Province, Southern Thailand. Insects 2022, 13, 912. [Google Scholar] [CrossRef]
- Sunantaraporn, S.; Thepparat, A.; Phumee, A.; Sor-Suwan, S.; Boonserm, R.; Bellis, G.; Siriyasatien, P. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Neglected Trop. Dis. 2021, 15, e0010014. [Google Scholar] [CrossRef]
- King, S.; Rajko-Nenow, P.; Ashby, M.; Frost, L.; Carpenter, S.; Batten, C. Outbreak of African horse sickness in Thailand, 2020. Transbound. Emerg. Dis. 2020, 67, 1764–1767. [Google Scholar] [CrossRef]
- Sariya, L.; Paungpin, W.; Chaiwattanarungruengpaisan, S.; Thongdee, M.; Nakthong, C.; Jitwongwai, A.; Taksinoros, S.; Sutummaporn, K.; Boonmasawai, S.; Kornmatitsuk, B. Molecular detection and characterization of lumpy skin disease viruses from outbreaks in Thailand in 2021. Transbound. Emerg. Dis. 2022, 69, e2145–e2152. [Google Scholar] [CrossRef]
- Fall, M.; Diarra, M.; Fall, A.G.; Balenghien, T.; Seck, M.T.; Bouyer, J.; Garros, C.; Gimonneau, G.; Allène, X.; Mall, I.; et al. Culicoides (Diptera: Ceratopogonidae) midges, the vectors of African horse sickness virus—A host/vector contact study in the Niayes area of Senegal. Parasit. Vectors 2015, 8, 39. [Google Scholar] [CrossRef]
- Wirth, W.W.; Ratanaworabhan, C.; Blanton, F.S. Synopsis of the genera of Ceratopogonidae (Diptera). Ann. Parasitol. Hum. Comp. 1974, 49, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Wirth, W.W.; Hubert, A.A. The Culicoides of Southeast Asia (Diptera: Ceratopogonidae); Defense Technical Information Center: Fort Belvoir, VA, USA, 1989. [Google Scholar]
- Dyce, A.L.; Bellis, G.A.; Muller, M.J. Pictorial Atlas of Australasian Culicoides Wings (Diptera: Ceratopogonidae); Australian Biological Resources Study: Canberra, Australia, 2007. [Google Scholar]
- Bellis, G.; Dyce, A.L.; Gopurenko, D.; Mitchell, A. Revision of the Immaculatus Group of Culicoides Latreille (Diptera: Ceratopogonidae) from the Australasian Region with description of two new species. Zootaxa 2013, 3680, 15–37. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hijmans, R. Package ‘Geosphere’. Spherical Trigonometry. R Package Version 1.5-19. 2023. Available online: https://github.com/rspatial/geosphere (accessed on 31 August 2024).
- Oksanen, J.F.; Guillaume, B.; Michael, F.; Roeland, K.; Pierre, L.; Dan, M.; Peter, R.M.; O’Hara, R.B.; Gavin, L.S.; Peter, S.; et al. Package ‘ Vegan‘. Community Ecology. R Package Version 2.7-0. 2024. Available online: https://github.com/vegandevs/vegan (accessed on 29 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Muller, M.; Murray, M.; Edwards, J. Blood-Sucking Midges and Mosquitoes Feeding on Mammals at Beatrice Hill, N.T. Aust. J. Zool. 1981, 29, 573–588. [Google Scholar] [CrossRef]
- Liebenberg, D.; Piketh, S.; Labuschagne, K.; Venter, G.; Greyling, T.; Mienie, C.; de Waal, T.; Hamburg, H. Culicoides species composition and environmental factors influencing African horse sickness distribution at three sites in Namibia. Acta Tropica 2016, 163, 70–79. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides Biting Midges: Their Role as Arbovirus Vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Sedda, L.; Brown, H.E.; Purse, B.V.; Burgin, L.; Gloster, J.; Rogers, D.J. A new algorithm quantifies the roles of wind and midge flight activity in the bluetongue epizootic in northwest Europe. Proc. Biol. Sci. 2012, 279, 2354–2362. [Google Scholar] [CrossRef]
- Mullen, G.R.; Murphree, C.S. Chapter 13—Biting Midges (Ceratopogonidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 213–236. [Google Scholar]
- Edwards, P. Seasonal changes in Larval populations of Culicoides subimmaculatus Lee&Reye (Diptera: Ceratopogonidae), with observations on the influence of tides on larval ecology. Mar. Freshw. Res. 1989, 40, 69–78. [Google Scholar] [CrossRef]
- Conte, A.; Goffredo, M.; Ippoliti, C.; Meiswinkel, R. Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus Complex in Italy. Vet. Parasitol. 2007, 150, 333–344. [Google Scholar] [CrossRef]
- Kettle, D.S. The biting habits of Culicoides furens (Poey) and C. barbosai Wirth&Blanton. III. Seasonal cycle, with a note on the relative importance of ten factors that might influence the biting rate. Bull. Entomol. Res. 1972, 61, 565–576. [Google Scholar] [CrossRef]
- Lillie, T.H.; Kline, D.L.; Hall, D.W. Diel and seasonal activity of Culicoides spp. (Diptera: Ceratopogonidae) near yankeetown, Florida, monitored with a vehicle-mounted insect trap. J. Med. Entomol. 1987, 24, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R. Seasonal fluctuations of Culicoides species (Diptera: Ceratopogonidae) in Kenya. Bull. Entomol. Res. 1977, 67, 217–233. [Google Scholar] [CrossRef]
- Braverman, Y.; Phelps, R.J. Species composition and blood-meal identification in samples of Culicoides (Diptera: Ceratopogonidae) collected near Salisbury, Zimbabwe in 1976–77. J. Soc. S. Afr. 1981, 44, 315–323. [Google Scholar]
- Standfast, H.A.; Dyce, A.L. Attacks on cattle by mosquitoes and biting midges. Aust. Vet. J. 1968, 44, 585–586. [Google Scholar] [CrossRef]
- Akiba, K. Studies on the leucocytozoon found in the chicken, in Japan. II. On the transmission of L. caulleryi by Culicoides arakawae. Jpn. J. Vet. Sci. 1960, 22, 309–317. [Google Scholar] [CrossRef]
- Jomkumsing, P.; Surapinit, A.; Saengpara, T.; Pramual, P. Genetic variation, DNA barcoding and blood meal identification of Culicoides Latreille biting midges (Diptera: Ceratopogonidae) in Thailand. Acta Trop. 2021, 217, 105866. [Google Scholar] [CrossRef]


| Trap ID | Latitude/ Longitude | Animals | Animal Data Used for Analysis |
|---|---|---|---|
| Trap1 | 13.8706° N 99.7783° E | 3 cows 1 | Cattle, 3 |
| Trap2 | 13.8706° N 99.7786° E | 100 free-range chickens 2 5 game fowls 3 | Avians, 105 |
| Trap3 | 13.8708° N 99.7789° E | 100 pigs 4 (No pigs in AUG–OCT 2020) 20 free-range chickens 2 | Pigs, 100 (0, AUG–OCT 2020) Avians, 20 |
| Trap4 | 13.8714° N 99.7783° E | 20 chickens 2 3 game fowls 3 | Avians, 23 |
| Trap5 | 13.8717° N 99.7786° E | 100 ducks 5 | Avians, 100 |
| Trap6 | 13.8719° N 99.7789° E | 5 birds 6 20 game fowls 3 | Avians, 25 |
| Trap7 | 13.8719° N 99.7786° E | 20 chickens 2 5 dairy cattle 1 (No cattle in and after MAR 2021) | Avians, 20 Cattle, 5 (0, MAR–MAY 2021) |
| Trap8 | 13.8750° N 99.7808° E | 3 parrots 7 40 cows | Avians, 3 Cattle, 40 |
| Trap9 | 13.8750° N 99.7810° E | 3 cows | Cattle, 3 |
| Scientific Name | Subgenus | ID | Identified Abundance | % | Extrapolated Abundance | % | Rank |
|---|---|---|---|---|---|---|---|
| Culicoides actoni Smith | Avaritia | Sp01 | 210 | 0.16 | 320 | 0.14 | 12 |
| Culicoides brevipalpis Delfinado | Avaritia | Sp02 | 114 | 0.09 | 158 | 0.07 | 16 |
| Culicoides brevitarsis Kieffer | Avaritia | Sp03 | 166 | 0.13 | 211 | 0.09 | 14 |
| Culicoides flavipunctatus Kitaoka | Avaritia | Sp04 | 6 | 0.00 | 15 | 0.01 | 24 |
| Culicoides fulvus Sen and Das Gupta | Avaritia | Sp05 | 259 | 0.20 | 386 | 0.17 | 11 |
| Culicoides imicola Kieffer | Avaritia | Sp06 | 10,721 | 8.20 | 15,703 | 6.95 | 4 |
| Culicoides jacobsoni Macfie | Avaritia | Sp07 | 21 | 0.02 | 22 | 0.01 | 23 |
| Culicoides minimus Wirth and Hubert | Avaritia | Sp08 | 5 | <0.001 | 5 | <0.001 | 27 |
| Culicoides obscurus Tokunaga and Murachi | Avaritia | Sp09 | 2 | <0.001 | 2 | <0.001 | 28 |
| Culicoides orientalis Macfie | Avaritia | Sp10 | 52 | 0.04 | 65 | 0.03 | 20 |
| Culicoides tainanus Kieffer | Avaritia | Sp11 | 118 | 0.09 | 194 | 0.09 | 15 |
| Culicoides wadai Kitaoka | Avaritia | Sp12 | 1 | <0.001 | 1 | <0.001 | 31 |
| Culicoides clavipalpis Mukerji | Clavipalpis group | Sp13 | 109 | 0.08 | 111 | 0.05 | 17 |
| Culicoides arenicola Howarth | Clavipalpis group | Sp14 | 2 | <0.001 | 2 | <0.001 | 29 |
| Culicoides huffi Causey | Clavipalpis group | Sp15 | 1548 | 1.18 | 1756 | 0.78 | 5 |
| Culicoides innoxius Sen and Das Gupta | Hoffmania | Sp16 | 743 | 0.57 | 993 | 0.44 | 7 |
| Culicoides peregrinus Kieffer | Hoffmania | Sp17 | 36,950 | 28.28 | 97,098 | 42.99 | 1 |
| Culicoides arakawae Arakawa | Meijerehelea | Sp18 | 30,857 | 23.61 | 45,996 | 20.36 | 3 |
| Culicoides guttifer Meijere | Meijerehelea | Sp19 | 500 | 0.38 | 576 | 0.25 | 10 |
| Culicoides histrio Johannsen | Meijerehelea | Sp20 | 8 | 0.01 | 8 | <0.001 | 25 |
| Culicoides homotomus Kieffer | Monoculicoides | Sp21 | 1235 | 0.95 | 1582 | 0.70 | 6 |
| Culicoides pampangensis Delfinado | Ornatus group | Sp22 | 44 | 0.03 | 44 | 0.02 | 21 |
| Culicoides oxystoma Kieffer | Remmia | Sp23 | 43,570 | 33.34 | 55,579 | 24.61 | 2 |
| Culicoides shortti Smith and Swaminath | Shortti group | Sp24 | 93 | 0.07 | 103 | 0.05 | 18 |
| Culicoides albibasis Wirth and Hubert | Trithecoides | Sp25 | 2 | <0.001 | 2 | <0.001 | 30 |
| Culicoides anophelis Edwards | Trithecoides | Sp26 | 460 | 0.35 | 803 | 0.36 | 9 |
| Culicoides flavescens Macfie | Trithecoides | Sp27 | 595 | 0.46 | 916 | 0.41 | 8 |
| Culicoides gewertzi Causey | Trithecoides | Sp28 | 9 | 0.01 | 31 | 0.01 | 22 |
| Culicoides parahumeralis Wirth and Hubert | Trithecoides | Sp29 | 64 | 0.05 | 92 | 0.04 | 19 |
| Culicoides palpifer Das Gupta and Ghosh | Trithecoides | Sp30 | 160 | 0.12 | 239 | 0.11 | 13 |
| Culicoides rugulithecus Wirth and Hubert | Trithecoides | Sp31 | 6 | <0.001 | 8 | <0.001 | 26 |
| Unidentified Culicoides spp. | 2040 | 1.56 | 2863 | 1.27 | |||
| TOTAL | 130,670 | 100 | 224,153 | 100 |
| Df | Sum of Squares | R2 | F | Pr (>F) | ||
|---|---|---|---|---|---|---|
| DSIN | 1 | 0.590 | 0.019 | 2.431 | 0.024 | * |
| DCOS | 1 | 0.564 | 0.018 | 2.324 | 0.027 | * |
| Temperature | 1 | 0.162 | 0.005 | 0.669 | 0.692 | |
| Rainfall | 1 | 0.656 | 0.022 | 2.702 | 0.016 | * |
| Humidity | 1 | 1.159 | 0.038 | 4.773 | 0.000 | *** |
| Wind speed | 1 | 0.433 | 0.014 | 1.785 | 0.073 | . |
| Cattle | 1 | 1.390 | 0.046 | 5.721 | 0.000 | *** |
| Pigs | 1 | 0.269 | 0.009 | 1.107 | 0.328 | |
| Avians | 1 | 0.720 | 0.024 | 2.966 | 0.006 | ** |
| Residual | 98 | 23.809 | 0.800 | |||
| Total | 107 | 29.757 | 1.000 |
| Culicoides sp. | Total | C. imicola | C. peregrinus | C. arakawae | C. oxystoma | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Environmental Factors | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value |
| Temperature | 0.257 | <0.001 | 0.146 | <0.001 | 0.184 | <0.001 | 0.273 | <0.001 | 0.403 | <0.001 |
| Rainfall | 0.229 | <0.001 | −0.374 | <0.001 | 0.429 | <0.001 | 0.169 | <0.001 | −0.173 | <0.001 |
| Humidity | 0.347 | <0.001 | 0.487 | <0.001 | 0.213 | <0.001 | 0.348 | <0.001 | 0.495 | <0.001 |
| Wind speed | −0.256 | <0.001 | −0.241 | <0.001 | −0.473 | <0.001 | −0.572 | <0.001 | −0.052 | <0.001 |
| Culicoides sp. | Total | C. imicola | C. peregrinus | C. arakawae | C. oxystoma | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value | Coef. | p-Value |
| Cattle | 0.176 | <0.001 | 0.447 | <0.001 | 0.022 | <0.001 | −0.001 | 0.727 | 0.363 | <0.001 |
| Pigs | −0.100 | <0.001 | −0.274 | <0.001 | −0.412 | <0.001 | −0.208 | <0.001 | 0.096 | <0.001 |
| Avians | −0.392 | <0.001 | −1.047 | <0.001 | −1.800 | <0.001 | 0.021 | <0.001 | 0.040 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepparat, A.; Kamata, N.; Siriyasatien, P.; Prempree, W.; Dasuntad, K.; Chittsamart, B.; Sanguansub, S. Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand. Insects 2024, 15, 701. https://doi.org/10.3390/insects15090701
Thepparat A, Kamata N, Siriyasatien P, Prempree W, Dasuntad K, Chittsamart B, Sanguansub S. Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand. Insects. 2024; 15(9):701. https://doi.org/10.3390/insects15090701
Chicago/Turabian StyleThepparat, Arunrat, Naoto Kamata, Padet Siriyasatien, Waranya Prempree, Kannika Dasuntad, Boonruam Chittsamart, and Sunisa Sanguansub. 2024. "Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand" Insects 15, no. 9: 701. https://doi.org/10.3390/insects15090701
APA StyleThepparat, A., Kamata, N., Siriyasatien, P., Prempree, W., Dasuntad, K., Chittsamart, B., & Sanguansub, S. (2024). Seasonal Abundance and Diversity of Culicoides Biting Midges in Livestock Sheds in Kanchanaburi Province, Thailand. Insects, 15(9), 701. https://doi.org/10.3390/insects15090701







