Transcriptome-Wide Evaluation Characterization of microRNAs and Assessment of Their Functional Roles as Regulators of Diapause in Ostrinia furnacalis Larvae (Lepidoptera: Crambidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Materials
2.2. Deep Sequencing
2.3. Sequencing Data Processing
2.4. Differentially Expressed miRNA Analyses
2.5. Target Prediction and Functional Annotation
2.6. qPCR Validation
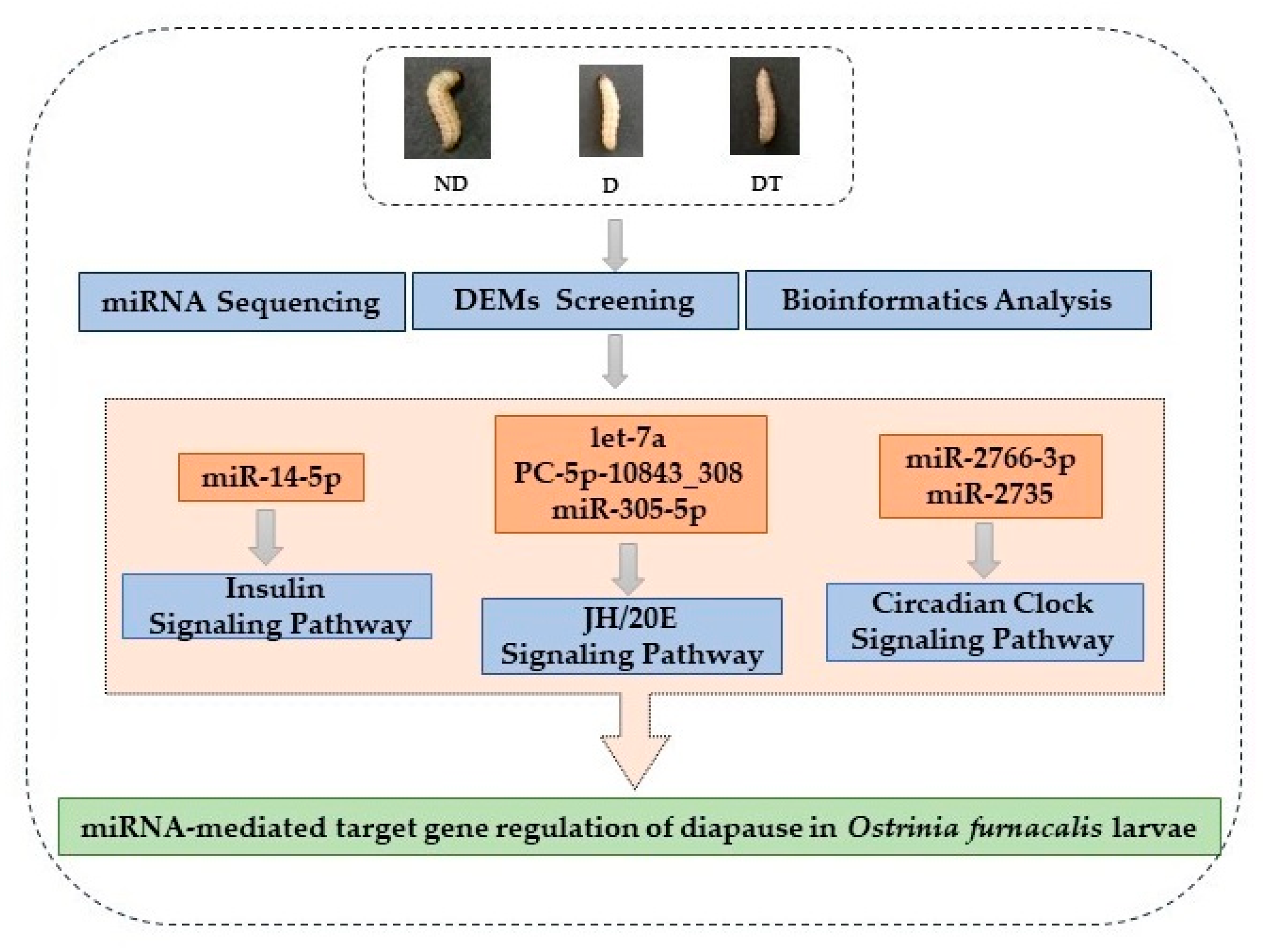
3. Results
3.1. Analyses of sRNA Data
3.2. miRNA Expression Profiles
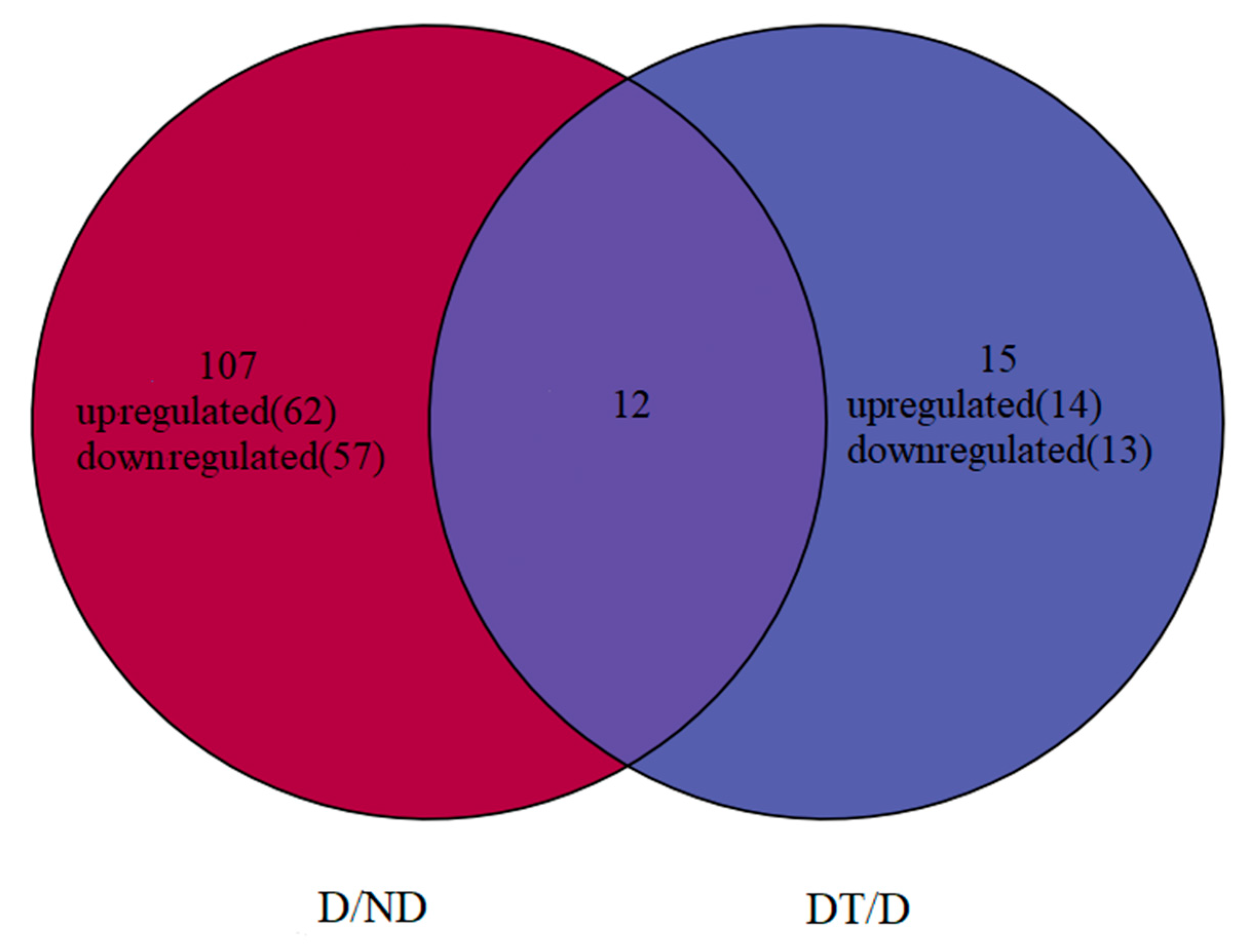
3.3. DEMs Identification
3.4. Predictive Identification and Functional Annotation of DEM Targets
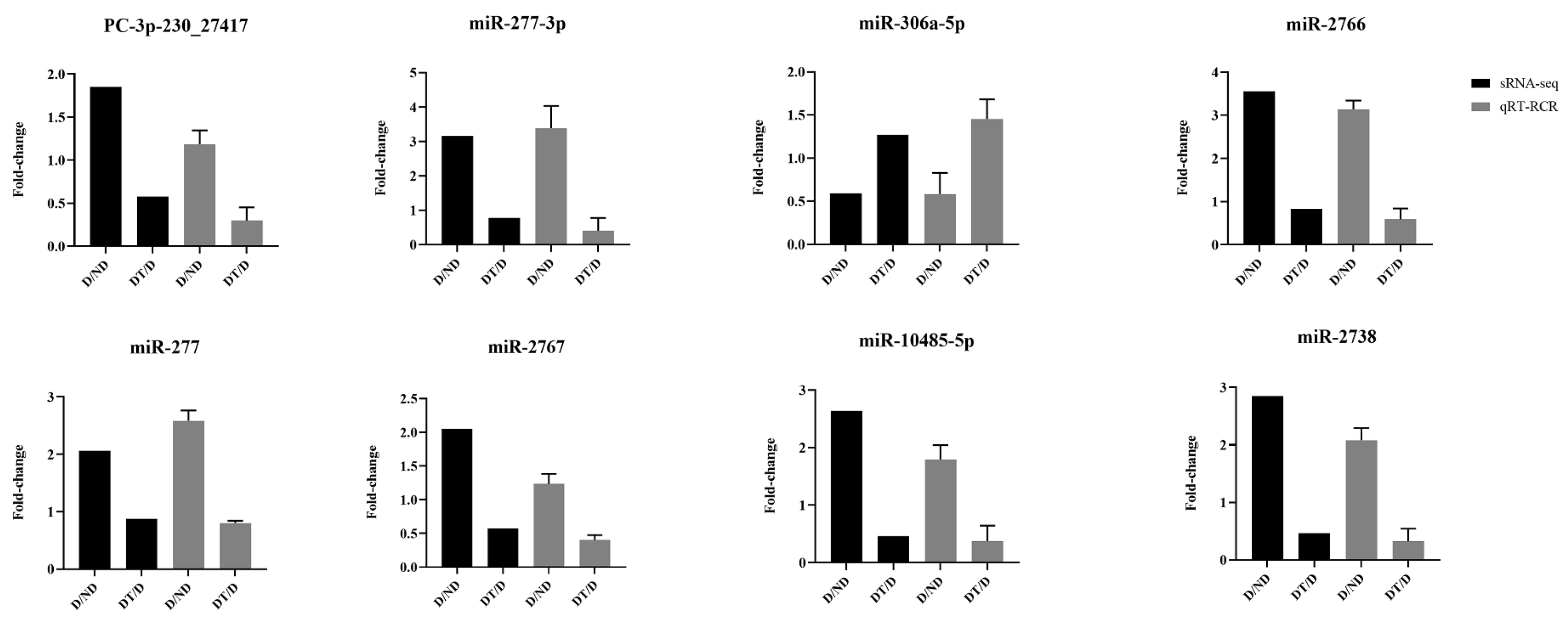
3.5. qPCR Validation of sRNA-Seq Data
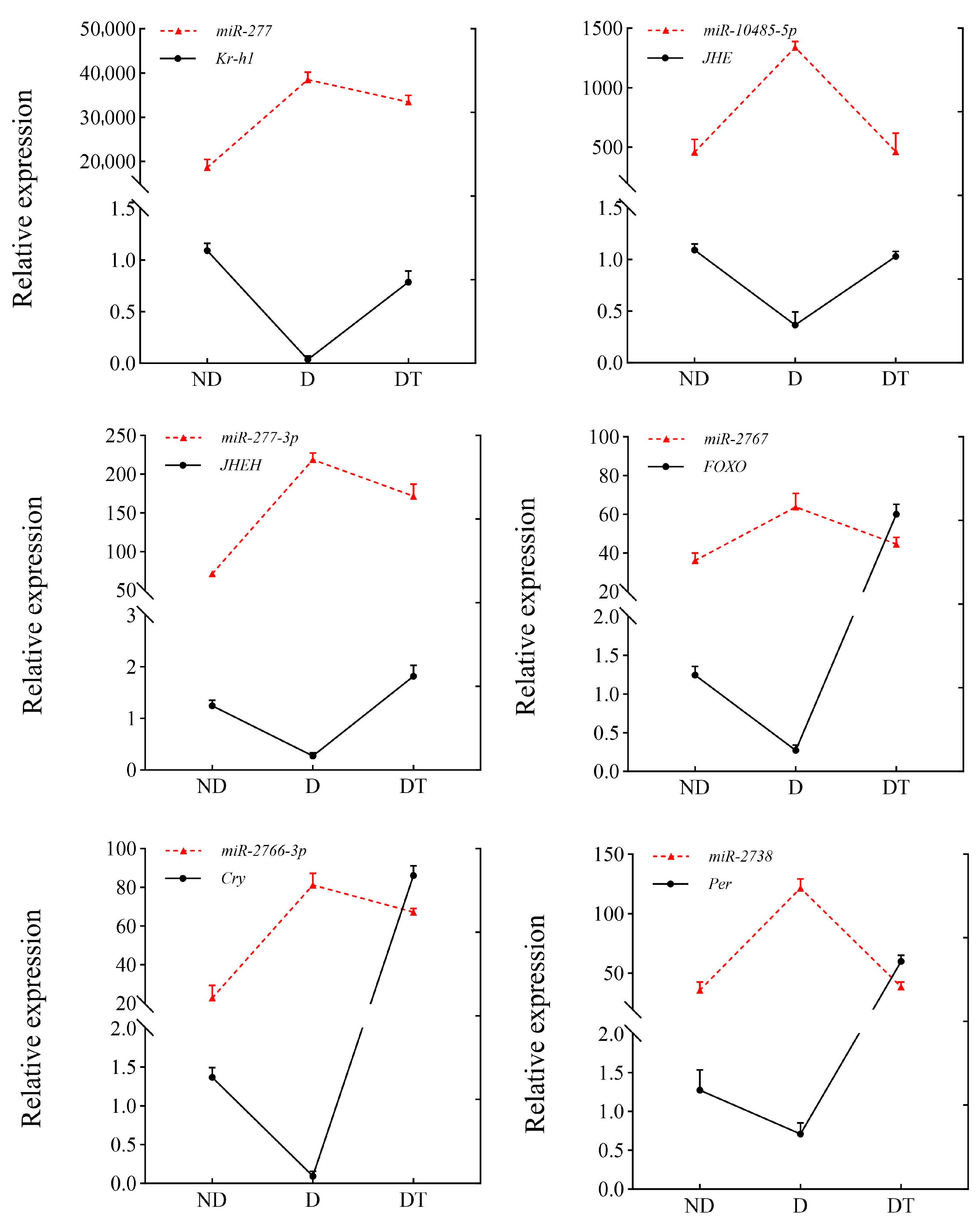
3.6. Expression Patterns of DEMs and Their Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meijer, H.A.; Kong, Y.W.; Lu, W.T.; Wilczynska, A.; Spriggs, R.V.; Robinson, S.W.; Godfrey, J.D.; Willis, A.E.; Bushell, M. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 2013, 340, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Berezikov, E.; Guryev, V.; van de Belt, J.; Wienholds, E.; Plasterk, R.H.A.; Cuppen, E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 2005, 120, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Zhou, Q.; Li, S.C.; Luo, Q.B.; Cai, Y.M.; Lin, W.C.; Chen, H.; Yang, Y.; Hu, S.N.; Yu, J. The silkworm (Bombyx mori) microRNAs and their expressions in multiple developmental stages. PLoS ONE 2008, 3, e2997. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, Y.; Jiang, J.H.; Zhong, Y.; Yang, X.N.; Li, Z.Q.; Huang, Y.P.; Tan, A.J. Identification of microRNAs in Helicoverpa armigera and Spodoptera litura based on deep sequencing and homology analysis. Int. J. Biol. Sci. 2013, 9, 1–15. [Google Scholar] [CrossRef][Green Version]
- Etebari, K.; Asgari, S. Revised annotation of Plutella xylostella microRNAs and their genome-wide target identification. Insect Mol. Biol. 2016, 25, 788–799. [Google Scholar] [CrossRef]
- Li, C.J.; Wu, W.; Tang, J.; Feng, F.; Chen, P.; Li, B. Identification and characterization of development-related microRNAs in the red flour beetle, Tribolium castaneum. Int. J. Mol. Sci. 2023, 24, 6685. [Google Scholar] [CrossRef]
- Waloff, N. Evolution of insect migration and diapause. Bull. Entomol. Soc. Am. 1979, 25, 169–170. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Clark, J.; Diakoff, S.J.; Denlinger, D.L. Transcriptional evidence for small RNA regulation of pupal diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2013, 43, 982–989. [Google Scholar] [CrossRef]
- Batz, Z.A.; Goff, A.C.; Armbruster, P.A. MicroRNAs are differentially abundant during Aedes albopictus diapause maintenance but not diapause induction. Insect Mol. Biol. 2017, 26, 721–733. [Google Scholar] [CrossRef]
- Duan, T.F.; Li, L.; Wang, H.C.; Pang, B.P. MicroRNA miR-2765-3p regulates reproductive diapause by targeting FoxO in Galeruca daurica. Insect Sci. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, C.; Shi, Z.; Pang, L.; Chen, C.; Chen, Y.; Pan, R.; Zhou, W.; Chen, X.X.; et al. HGT is widespread in insects and contributes to male courtship in lepidopterans. Cell 2022, 185, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jin, M.; Li, H.; Zhang, L.; Zhang, L.; Yu, S. Population Genomics Provide Insights into the Evolution and Adaptation of the Asia Corn Borer. Mol. Biol. Evol. 2023, 40, masd112. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11.7.1–11.7.14. [Google Scholar] [CrossRef]
- Hofacker, L.L.; Bernhart, H.F.; Stadler, P.F. Alignment of RNA base pairing probability matrices. Bioinformatics 2004, 20, 2222–2227. [Google Scholar] [CrossRef]
- Ambady, S.; Wu, Z.Y.; Dominko, T. Identification of novel microRNAs in Xenopus laevis metaphase II arrested eggs. Genesis 2012, 50, 286–299. [Google Scholar] [CrossRef]
- Li, X.; Shahid, M.Q.; Wu, J.W.; Wang, L.; Liu, X.D.; Lu, Y.G. Comparative small RNA analysis of pollen development in autotetraploid and diploid rice. Int. J. Mol. Sci. 2016, 17, 499. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nuckeic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.X.; Gong, Y.J.; Zheng, S.C.; Liu, L.; Huang, L.H.; Feng, Q.L. De novo characterization of transcriptome and gene expression dynamics in epidermis during the larval-pupal metamorphosis of common cutworm. Insect Biochem. Mol. Biol. 2013, 43, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Zheng, Y.; Sumathipala, N.; Jiang, H.B.; Arrese, E.L.; Soulages, J.L.; Zhang, W.X.; Sunkar, R. Deep sequencing of small RNA libraries reveals dynamic regulation of conserved and novel microRNAs and microRNA-stars during silkworm development. BMC Genom. 2010, 11, 52. [Google Scholar] [CrossRef]
- Chen, Q.H.; Lu, L.; Hua, H.X.; Zhou, F.; Lu, L.X.; Lin, Y.J. Characterization and comparative analysis of small RNAs in three small RNA libraries of the brown planthopper (Nilaparvata lugens). PLoS ONE 2012, 7, e32860. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dou, W.; Liu, B.; Wei, D.; Liao, C.Y.; Smagghe, G.; Wang, J.J. Deep sequencing of small RNA libraries reveals dynamic expression patterns of microRNAs in multiple developmental stages of Bactrocera dorsalis. Insect Mol. Biol. 2014, 23, 656–667. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Chen, S.; Yang, P.C.; Ma, Z.Y.; Kang, L. Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol. 2009, 10, R6. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, R.L.; Vanlandingham, D.L.; Scholle, F.; Higgs, S.; Cullen, B.R. Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC Genom. 2010, 11, 119. [Google Scholar] [CrossRef]
- Chen, E.H.; Tao, Y.X.; Song, W.; Shen, F.; Yuan, M.L.; Tang, P.A. Transcriptome-wide identification of microRNAs and analysis of their potential roles in development of Indian meal moth (Lepidoptera:Pyralidae). J. Econ. Entomol. 2020, 113, 1535–1546. [Google Scholar] [CrossRef]
- Guo, W.; Song, J.; Yang, P.; Chen, X.; Chen, D.; Ren, L.; Kang, L.; Wang, X. Juvenile hormone suppresses aggregation behavior through influencing antennal gene expression in locusts. PLoS Genet. 2020, 16, e1008762. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Dong, W. MicroRNA miR-927 targets the juvenile hormone primary response gene Krüppel homolog1 to control Drosophila developmental growth. Insect. Mol. Biol. 2020, 29, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ge, X.; Li, Z.; Wang, Y.; Song, Q.; Stanley, W.D.; Tan, A.; Huang, Y. MicroRNA-281 regulates the expression of ecdysone receptor (EcR) isoform B in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2013, 48, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P.; Chen, C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004, 5, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef]
- Ling, L.; Ge, X.; Li, Z.Q.; Zeng, B.S.; Xu, J.; Aslam, A.F.M.; Song, Q.S.; Shang, P.; Huang, Y.P.; Tan, A.J. MicroRNA Let-7 regulates molting and metamorphosis in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2014, 53, 13–21. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhao, S.; Wu, X. BmNPV-induced hormone metabolic disorder in silkworm leads to enhanced locomotory behavior. Dev. Comp. Immunol. 2021, 121, 104036. [Google Scholar] [CrossRef]
- Lim, D.H.; Lee, S.; Choi, M.S.; Han, J.Y.; Seong, Y.; Na, D.; Kwon, Y.S.; Lee, Y.S. The conserved microRNA miR-8-3p coordinates the expression of V-ATPase subunits to regulate ecdysone biosynthesis for Drosophila metamorphosis. FASEB J. 2020, 34, 6449–6465. [Google Scholar] [CrossRef]
- Wu, W.; Zhai, M.; Li, C.; Yu, X.; Song, X.; Gao, S.; Li, B. Multiple functions of miR-8-3p in the development and metamorphosis of the red flour beetle, Tribolium castaneum. Insect Mol. Biol. 2019, 28, 208–221. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xu, J.; Ling, L.; Luo, X.Y.; Yang, D.H.; Yang, X.; Zhang, X.Q.; Huang, Y.P. miR-34 regulates larval growth and wing morphogenesis by directly modulating ecdysone signalling and cuticle protein in Bombyx mori. RNA Biol. 2020, 17, 1342–1351. [Google Scholar] [CrossRef]
- Hardin, P.E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 2011, 74, 141–143. [Google Scholar]
- Ikeno, T.; Numata, H.; Goto, S.G. Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem. Biophys. Res. Commun. 2011, 410, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Kotwica-Rolinska, J.; Damulewicz, M.; Chodakova, L.; Kristofova, L.; Dolezel, D. Pigment dispersing factor is a circadian clock output and regulates photoperiodic response in the linden bug, Pyrrhocoris apterus. Front. Physiol. 2022, 13, 884909. [Google Scholar] [CrossRef] [PubMed]
- Meuti, M.E.; Stone, M.; Ikeno, T.; Denlinger, D.L. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J. Exp. Biol. 2015, 218 Pt 3, 412–422. [Google Scholar] [CrossRef] [PubMed]



| Type | ND | D | DT | Combined | ||||
|---|---|---|---|---|---|---|---|---|
| Reads | % | Reads | % | Reads | % | Reads | % | |
| Known miRNA | 4,635,549 | 20.24 | 5,009,956 | 23.91 | 5,522,041 | 24.28 | 15,167,546 | 22.77 |
| Novel miRNA | 5,282,122 | 23.07 | 5,960,892 | 28.45 | 5,459,801 | 24.00 | 16,702,815 | 25.08 |
| rRNA | 638,409 | 2.79 | 507,609 | 2.42 | 439,204 | 1.93 | 1,585,222 | 2.38 |
| tRNA | 303,522 | 1.33 | 231,957 | 1.11 | 212,673 | 0.93 | 748,152 | 1.12 |
| snoRNA | 2560 | 0.01 | 3142 | 0.01 | 3076 | 0.01 | 8778 | 0.01 |
| snRNA | 11,402 | 0.05 | 7285 | 0.03 | 16,048 | 0.0 | 34,735 | 0.05 |
| other Rfam RNA | 122,015 | 0.53 | 95,717 | 0.46 | 83,110 | 0.37 | 300,842 | 0.45 |
| Transcriptome (mRNA) | 3,137,096 | 13.70 | 2,377,816 | 11.35 | 2,713,959 | 11.93 | 8,228,871 | 12.36 |
| Unannotated | 8,767,533 | 38.29 | 6,758,616 | 32.26 | 8,295,952 | 36.47 | 23,822,101 | 35.77 |
| Total (clean reads) | 22,900,208 | 100 | 20,952,990 | 100 | 22,745,864 | 100 | 66,599,060 | 100 |
| miRNAs | Log2 (Fold Change) | p-Value | Up/Down |
| D/ND | |||
| miR-14 | 2.54 | 9.93 × 10−5 | Up |
| miR-277 | 1.04 | 1.70 × 10−4 | Up |
| miR-2766-3p | 1.83 | 3.39 × 10−4 | Up |
| miR-10-5p | −1.43 | 7.87 × 10−5 | Down |
| miR-305-5p | −3.43 | 6.90 × 10−4 | Down |
| mir-3344-p5 | -inf | 9.10 × 10−4 | Down |
| DT/D | |||
| miR-6307 | 0.50 | 9.02 × 10−3 | Up |
| miR-970-5p | -inf | 1.13 × 10−2 | Up |
| PC-3p-81145 | -inf | 1.22 × 10−2 | Up |
| PC-3p-431 | −0.41 | 2.27 × 10−3 | Down |
| PC-5p-66538 | -inf | 8.90 × 10−3 | Down |
| PC-3p-230 | −0.78 | 1.12 × 10−2 | Down |
| Common DEMs Overlapping between the Two Comparisons | |||
| miRNAs | D/ND | DT/D | |
| miR-970-5p | Down | Up | |
| miR-306a-5p | Down | Up | |
| mir-3344-p5 | Down | Up | |
| PC-3p-230 | Up | Down | |
| miR-277 | Up | Down | |
| miR-263b | Up | Down | |
| miR-263b-5p | Up | Down | |
| miR-2738 | Up | Down | |
| miR-2767 | Up | Down | |
| miR-277-3p | Up | Down | |
| miR-10485-5p | Up | Down | |
| miR-2766-3p | Up | Down | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Liu, Y.; Tian, X.; Chen, Y.; Gao, S. Transcriptome-Wide Evaluation Characterization of microRNAs and Assessment of Their Functional Roles as Regulators of Diapause in Ostrinia furnacalis Larvae (Lepidoptera: Crambidae). Insects 2024, 15, 702. https://doi.org/10.3390/insects15090702
Ma H, Liu Y, Tian X, Chen Y, Gao S. Transcriptome-Wide Evaluation Characterization of microRNAs and Assessment of Their Functional Roles as Regulators of Diapause in Ostrinia furnacalis Larvae (Lepidoptera: Crambidae). Insects. 2024; 15(9):702. https://doi.org/10.3390/insects15090702
Chicago/Turabian StyleMa, Hongyue, Ye Liu, Xun Tian, Yujie Chen, and Shujing Gao. 2024. "Transcriptome-Wide Evaluation Characterization of microRNAs and Assessment of Their Functional Roles as Regulators of Diapause in Ostrinia furnacalis Larvae (Lepidoptera: Crambidae)" Insects 15, no. 9: 702. https://doi.org/10.3390/insects15090702
APA StyleMa, H., Liu, Y., Tian, X., Chen, Y., & Gao, S. (2024). Transcriptome-Wide Evaluation Characterization of microRNAs and Assessment of Their Functional Roles as Regulators of Diapause in Ostrinia furnacalis Larvae (Lepidoptera: Crambidae). Insects, 15(9), 702. https://doi.org/10.3390/insects15090702





