Effect of Co-Diet Supplementation on Biodegradation of Polyethylene by Galleria mellonella L. (Lepidoptera: Pyralidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyethylene (PE) Bags
2.2. Insect Collection and Rearing
2.3. Weight Loss in G. mellonella Larvae and PE, Reduction in PE Surface Area, and Diet Consumption
2.4. Statistical Analysis
3. Results
3.1. Weight Loss in G. mellonella Larvae and PE and Reduction in PE Surface Area
3.2. Diet Consumption (Wheat Germ and Honey) by G. mellonella Larvae
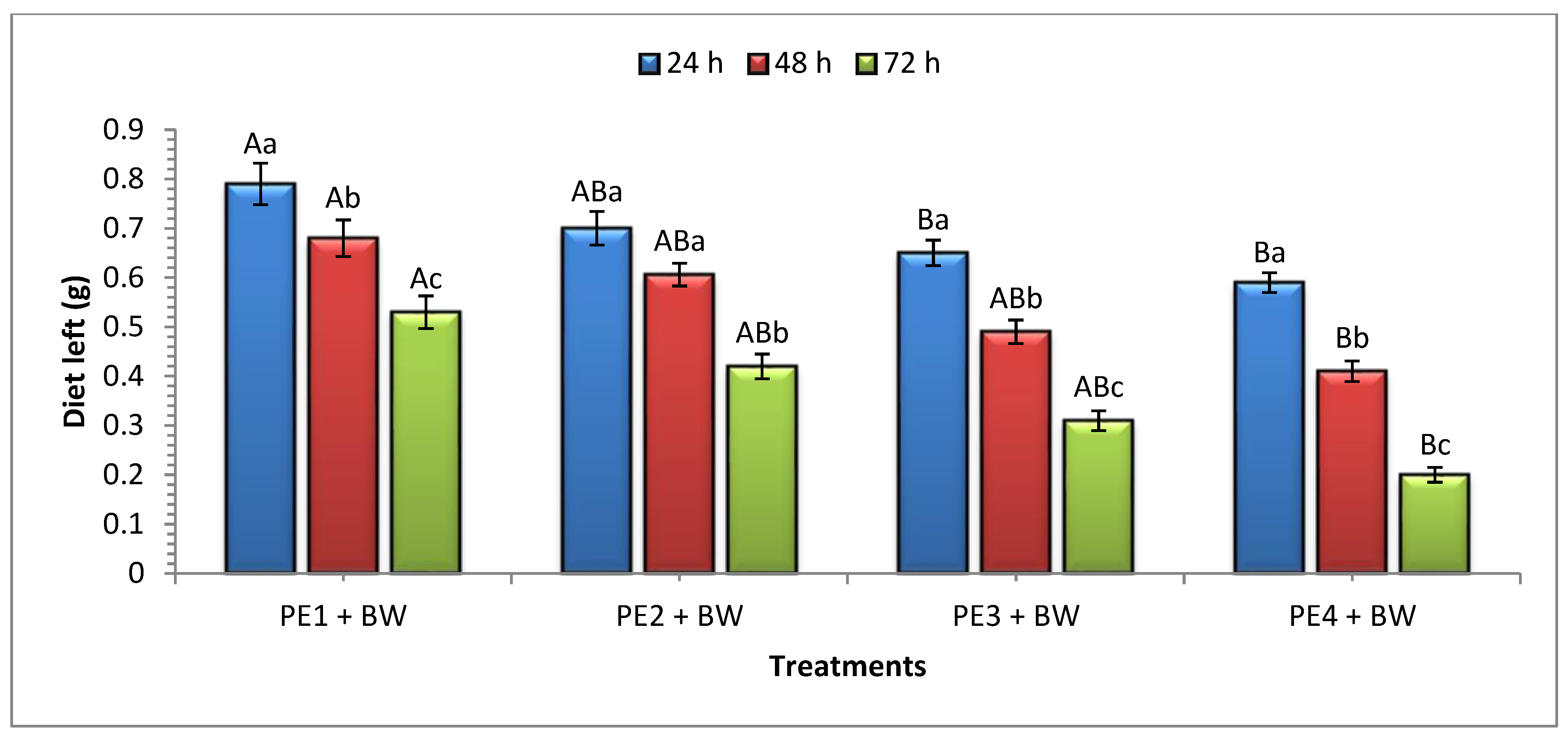
3.3. Diet Consumption (Beeswax) by G. mellonella Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, D.; Su, F.; Liu, C.; Guo, Z. Research progress for plastic waste management and manufacture of value-added products. Adv. Compos. Hybrid Mater. 2020, 3, 443–461. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotechnol. 2023, 15, 100254. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Tamoor, M.; Samak, N.A.; Jia, Y.; Mushtaq, M.U.; Sher, H.; Bibi, M.; Xing, J. Potential use of microbial enzymes for the conversion of plastic waste into value-added products: A viable solution. Front. Microbiol. 2021, 12, 777727. [Google Scholar] [CrossRef]
- Kale, S.K.; Deshmukh, A.G.; Dudhare, M.S.; Patil, V.B. Microbial degradation of plastic: A review. J. Biochem. Technol. 2015, 6, 952–961. [Google Scholar]
- Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Walker, T.R.; Fequet, L. Current trends of unsustainable plastic production and micro (nano) plastic pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar]
- Alhazmi, H.; Almansour, F.H.; Aldhafeeri, Z. Plastic waste management: A review of existing life cycle assessment studies. Sustainability 2021, 13, 5340. [Google Scholar] [CrossRef]
- Pilapitiya, P.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Bilal, H.; Raza, H.; Bibi, H.; Bibi, T. Plastic biodegradation through insects and their symbionts microbes: A review. J. Bioresour. Manag. 2021, 8, 7. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Brandon, A.M.; Xing, D.; Yang, J.; Pang, J.; Criddle, C.; Ren, N.; Wu, W. Progresses in polystyrene biodegradation and prospects for solutions to plastic waste pollution. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; p. 012005. [Google Scholar]
- Crompton, T.R. Additive Migration from Plastics into Foods: A Guide for Analytical Chemists; iSmithers Rapra Publishing: Akron, OH, USA, 2007. [Google Scholar]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.-S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Mourshed, M.; Masud, M.H.; Rashid, F.; Joardder, M.U.H. Towards the effective plastic waste management in Bangladesh: A review. Environ. Sci. Pollut. Res. 2017, 24, 27021–27046. [Google Scholar] [CrossRef]
- Masud, M.H.; Akram, W.; Ahmed, A.; Ananno, A.A.; Mourshed, M.; Hasan, M.; Joardder, M.U.H. Towards the effective E-waste management in Bangladesh: A review. Environ. Sci. Pollut. Res. 2019, 26, 1250–1276. [Google Scholar] [CrossRef] [PubMed]
- Guptaa, B.K.; Singh, S. To Study the feasibility of Coarse and Fine plastic Aggregates in Concrete. Int. J. Appl. Eng. Res. 2018, 13, 5815–5822. [Google Scholar]
- Mousavimehr, M.; Nematzadeh, M. Post-heating flexural behavior and durability of hybrid PET–Rubber aggregate concrete. Constr. Build. Mater. 2020, 265, 120359. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Abd Manap, A.S.; Kolobe, S.D.; Monnye, M.; Yudhistira, B.; Fernando, I. Insects for plastic biodegradation—A review. Process Saf. Environ. Prot. 2024, 186, 833–849. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Gilani, I.E.; Sayadi, S.; Zouari, N.; Al-Ghouti, M.A. Plastic waste impact and biotechnology: Exploring polymer degradation, microbial role, and sustainable development implications. Bioresour. Technol. Rep. 2023, 24, 101606. [Google Scholar] [CrossRef]
- Kong, H.G.; Kim, H.H.; Chung, J.-h.; Jun, J.; Lee, S.; Kim, H.-M.; Jeon, S.; Park, S.G.; Bhak, J.; Ryu, C.-M. The Galleria mellonella hologenome supports microbiota-independent metabolism of long-chain hydrocarbon beeswax. Cell Rep. 2019, 26, 2451–2464. [Google Scholar] [CrossRef]
- Lou, Y.; Ekaterina, P.; Yang, S.-S.; Lu, B.; Liu, B.; Ren, N.; Corvini, P.F.-X.; Xing, D. Biodegradation of polyethylene and polystyrene by greater wax moth larvae (Galleria mellonella L.) and the effect of co-diet supplementation on the core gut microbiome. Environ. Sci. Technol. 2020, 54, 2821–2831. [Google Scholar] [CrossRef]
- Singh, J.K.; Vishwakarma, R.; Ray, S. Biology of greater wax moth Galleria mellonella L. during dearth period. Indian J. Entomol. 2019, 81, 656–660. [Google Scholar] [CrossRef]
- de Jong, A.W.; van Veldhuizen, D.; Groot, A.T.; Hagen, F. Standardized methods to rear high-quality Galleria mellonella larvae for the study of fungal pathogens. Entomol. Exp. Appl. 2022, 170, 1073–1080. [Google Scholar] [CrossRef]
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, 27, R292–R293. [Google Scholar] [CrossRef] [PubMed]
- Khlaif, A.T.; Abdulhay, H.S. Polyethylene Degradation by Plastivores Greater Wax Worms Larvae (Galleria mellonella). Iraqi J. Sci. 2023, 64, 2144–2152. [Google Scholar] [CrossRef]
- Roy, D. On the nutrition of larvae of bee-wax moth, Galleria mellonella. Z. Vgl. Physiol. 1937, 24, 638–643. [Google Scholar] [CrossRef]
- Sanluis-Verdes, A.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Bello-Villarino, M.; Spínola-Amilibia, M.; Ruiz-Lopez, E.; Illanes-Vicioso, R.; Castroviejo, P.; Aiese Cigliano, R.; Montoya, M. Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat. Commun. 2022, 13, 5568. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Ren, L.; Men, L.; Zhang, Z.; Guan, F.; Tian, J.; Wang, B.; Wang, J.; Zhang, Y.; Zhang, W. Biodegradation of polyethylene by Enterobacter sp. D1 from the guts of wax moth Galleria mellonella. Int. J. Environ. Res. Public Health 2019, 16, 1941. [Google Scholar] [CrossRef]
- Cassone, B.J.; Grove, H.C.; Elebute, O.; Villanueva, S.M.; LeMoine, C.M. Role of the intestinal microbiome in low-density polyethylene degradation by caterpillar larvae of the greater wax moth, Galleria mellonella. Proc. R. Soc. B 2020, 287, 20200112. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Hickin, M.; Nadel, H.; Schal, C.; Cohen, A.C. Optimization of a diet for the greater wax moth (Lepidoptera: Pyralidae) using full factorial and mixture design. J. Econ. Entomol. 2021, 114, 1091–1103. [Google Scholar] [CrossRef]
- Dhakal, S.; Panthi, S.; Khanal, B.K.; Pandey, L.; Bhattarai, S.S. The Feeding Potential of Greater Wax Moth (Galleria melonella) in Different Gauzes of Plastics. Int. J. Appl. Sci. Biotechnol. 2020, 8, 235–240. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1995; pp. 451–554. [Google Scholar]
- Minitab, L. Getting Started with Minitab 17; Minitab Inc.: State College, PA, USA, 2017; Volume 73. [Google Scholar]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Burelo, M.; Hernández-Varela, J.D.; Medina, D.I.; Treviño-Quintanilla, C.D. Recent developments in bio-based polyethylene: Degradation studies, waste management and recycling. Heliyon 2023, 9, e21374. [Google Scholar] [CrossRef] [PubMed]
- Cassone, B.J.; Grove, H.C.; Kurchaba, N.; Geronimo, P.; LeMoine, C.M. Fat on plastic: Metabolic consequences of an LDPE diet in the fat body of the greater wax moth larvae (Galleria mellonella). J. Hazard. Mater. 2022, 425, 127862. [Google Scholar] [CrossRef]
- Barrionuevo, J.M.R.; Reines, M.G.; Martin, E.; Malizia, A.; Galindo-Cardona, A.; Escobar, L.; Garzia, A.C.M. Polyethylene fragments affected the development of Galleria mellonella L. (Lepidoptera, Pyralidae). Acta Zool. Lilloana 2023, 67, 361–368. [Google Scholar] [CrossRef]
- Réjasse, A.; Waeytens, J.; Deniset-Besseau, A.; Crapart, N.; Nielsen-Leroux, C.; Sandt, C. Plastic biodegradation: Do Galleria mellonella larvae bioassimilate polyethylene? A spectral histology approach using isotopic labeling and infrared microspectroscopy. Environ. Sci. Technol. 2021, 56, 525–534. [Google Scholar] [CrossRef]
- Noel, G.; Serteyn, L.; Sare, A.R.; Massart, S.; Delvigne, F.; Francis, F. Co-diet supplementation of low density polyethylene and honeybee wax did not influence the core gut bacteria and associated enzymes of Galleria mellonella larvae (Lepidoptera: Pyralidae). Int. Microbiol. 2023, 26, 397–409. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Alkassab, A.T.; Thorbahn, D.; Frommberger, M.; Bischoff, G.; Pistorius, J. Effect of contamination and adulteration of wax foundations on the brood development of honeybees. Apidologie 2020, 51, 642–651. [Google Scholar] [CrossRef]
- Mandal, S.; Vishwakarma, R. Population dynamics of greater wax moth (Galleria mellonella L.) infesting Apis mellifera L. combs during dearth period. Ecoscan (Spec. Issue) 2016, 9, 93–98. [Google Scholar]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The biology and control of the greater wax moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Rodríguez Vega, L.C. Ciclo Biológico de Galleria mellonella Linnaeus (Lepidoptera: Pyralidae). 2015. Available online: https://hdl.handle.net/20.500.12996/2107 (accessed on 1 September 2024).
- Poma, O.; Ricce, B.; Beraún, J.; Perez Carpio, J.E.; Fernandez, H.; Soria, J. Biodegradation of Low-Density Polyethylene—LDPE by the Lepidopteran Galleria Mellonella Reusing Beekeeping Waste. Front. Bioeng. Biotechnol. 2022, 10, 915331. [Google Scholar] [CrossRef]

| Treatments | Composition |
|---|---|
| PE1 | Polyethylene 1.0 mm |
| PE2 | Polyethylene 2.0 mm |
| PE3 | Polyethylene 3.0 mm |
| PE4 | Polyethylene 4.0 mm |
| PE1 + WgH | Polyethylene 1.0 mm + Wheat germ and Honey |
| PE2 + WgH | Polyethylene 2.0 mm + Wheat germ and Honey |
| PE3 + WgH | Polyethylene 3.0 mm + Wheat germ and Honey |
| PE4 + WgH | Polyethylene 4.0 mm + Wheat germ and Honey |
| PE1 + BW | Polyethylene 1.0 mm + Beeswax |
| PE2 + BW | Polyethylene 2.0 mm + Beeswax |
| PE3 + BW | Polyethylene 3.0 mm + Beeswax |
| PE4 + BW | Polyethylene 4.0 mm + Beeswax |
| Effect | Df | Larval Weight Loss | Surface Area Reduction | PE Weight Loss | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Treatment | 11 | 75.20 | ≤0.05 | 76.29 | ≤0.05 | 412.33 | ≤0.05 |
| Time | 2 | 118.89 | ≤0.05 | 215.12 | ≤0.05 | 108.51 | ≤0.05 |
| Treatment × Time | 22 | 0.56 | 0.93 | 2.91 | ≤0.05 | 0.62 | 0.8947 |
| Treatments | Final Larval Weight (mg) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | F2,8 | p | |
| PE1 | 62.21 ± 2.02 D a | 55.42 ± 1.84 F b | 47.89 ± 1.44 F c | 48.4 | ≤0.05 |
| PE2 | 65.24 ± 2.19 CD a | 58.31 ± 2.02 EF b | 51.67 ± 1.73 EF c | 34.9 | ≤0.05 |
| PE3 | 67.32 ± 2.48 B–D a | 61.43 ± 2.13 DEF b | 53.93 ± 2.02 D–F c | 27.4 | ≤0.05 |
| PE4 | 68.65 ± 2.59 A–D a | 63.12 ± 2.25 C–F a | 55.12 ± 2.30 D–F b | 24.4 | ≤0.05 |
| PE1 + WgH | 70.31 ± 2.88 A–D a | 66.31 ± 2.30 B–F ab | 60.12 ± 2.59 C–F b | 11.7 | ≤0.05 |
| PE2 + WgH | 73.67 ± 3.17 A–D a | 69.24 ± 2.42 A–E ab | 62.32 ± 2.88 B–F b | 12.2 | ≤0.05 |
| PE3 + WgH | 76.35 ± 3.46 A–D a | 71.52 ± 2.59 A–E ab | 66.52 ± 3.06 A–E b | 7.75 | ≤0.05 |
| PE4 + WgH | 77.58 ± 3.75 A–D a | 72.15 ± 2.77 A–D ab | 68.04 ± 3.23 A–D b | 6.41 | ≤0.05 |
| PE1 + BW | 80.41 ± 3.81 A–C a | 75.42 ± 2.88 A–C ab | 70.67 ± 3.46 A–C b | 6.14 | ≤0.05 |
| PE2 + BW | 81.63 ± 3.92 A–C a | 77.12 ± 3.06 AB ab | 71.32 ± 3.63 A–C b | 6.34 | ≤0.05 |
| PE3 + BW | 83.25 ± 4.04 AB a | 79.63 ± 3.17 AB a | 76.15 ± 3.81 AB a | 2.77 | 0.1402 |
| PE4 + BW | 85.09 ± 4.44 A a | 81.19 ± 3.46 A a | 78.11 ± 4.04 A a | 2.29 | 0.1820 |
| F11,35 | 5.17 | 10.3 | 11.1 | ||
| p | ≤0.05 | ≤0.05 | ≤0.05 | ||
| Treatments | Final Surface Area (cm2) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | F2,8 | p | |
| PE1 | 20.00 ± 0.59 F a | 18.50 ± 0.57 C b | 15.00 ± 0.60 E c | 57.4 | ≤0.05 |
| PE2 | 20.50 ± 0.28 F a | 19.00 ± 0.69 BC b | 16.50 ± 0.71 DE c | 34.7 | ≤0.05 |
| PE3 | 21.00 ± 0.34 EF a | 20.00 ± 0.80 A–C a | 17.50 ± 0.75 DE b | 22.2 | ≤0.05 |
| PE4 | 21.50 ± 0.23 DEF a | 20.50 ± 0.75 A–C a | 18.00 ± 1.03 C–E b | 17.4 | ≤0.05 |
| PE1 + WgH | 23.75 ± 0.57 AB a | 22.75 ± 0.92 AB ab | 21.75 ± 0.75 A–C b | 5.19 | ≤0.05 |
| PE2 + WgH | 24.00 ± 0.28 AB a | 23.00 ± 0.55 AB ab | 22.00 ± 0.57 AB b | 12.8 | ≤0.05 |
| PE3 + WgH | 24.25 ± 0.02 AB a | 23.15 ± 1.06 AB a | 22.75 ± 0.46 A a | 4.07 | 0.0765 |
| PE4 + WgH | 24.50 ± 0.04 A a | 23.50 ± 0.75 A ab | 23.00 ± 0.28 A b | 8.17 | ≤0.05 |
| PE1 + BW | 21.75 ± 0.57 C–F a | 20.25 ± 1.15 A–C a | 17.00 ± 0.98 DE b | 20.3 | ≤0.05 |
| PE2 + BW | 22.50 ± 0.28 B–E a | 21.00 ± 0.92 A–C a | 18.50 ± 0.86 B–E b | 22.1 | ≤0.05 |
| PE3 + BW | 23.00 ± 0.23 A–D a | 22.00 ± 0.86 A–C a | 19.50 ± 0.92 A–D b | 17.8 | ≤0.05 |
| PE4 + BW | 23.50 ± 0.17 A–C a | 22.50 ± 0.57 A–C a | 20.00 ± 0.57 A–D b | 43.1 | ≤0.05 |
| F11,35 | 18.9 | 4.24 | 12.8 | ||
| p | ≤0.05 | ≤0.05 | ≤0.05 | ||
| Treatments | Final Weight of PE (mg) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | F2,8 | p | |
| PE1 | 21.12 ± 1.25 G a | 17.11 ± 1.15 I b | 10.61 ± 0.57 I c | 78.9 | ≤0.05 |
| PE2 | 31.65 ± 1.44 EF a | 27.85 ± 1.73 GH b | 22.35 ± 1.27 GH c | 29.5 | ≤0.05 |
| PE3 | 44.01 ± 2.05 CD a | 40.99 ± 2.02 D–F ab | 35.97 ± 2.54 D–F b | 10.1 | ≤0.05 |
| PE4 | 57.38 ± 2.79 AB a | 54.41 ± 2.51 A–C ab | 49.91 ± 2.88 A–C b | 5.69 | ≤0.05 |
| PE1 + WgH | 29.21 ± 1.27 E–G a | 27.13 ± 1.15 GH a | 23.05 ± 1.73 GH b | 14.9 | ≤0.05 |
| PE2 + WgH | 37.97 ± 1.38 DE a | 35.91 ± 1.78 E–G ab | 31.92 ± 2.25 E–G b | 8.40 | ≤0.05 |
| PE3 + WgH | 50.63 ± 2.50 BC a | 49.54 ± 2.21 B–D a | 46.14 ± 2.65 B–D a | 2.72 | 0.1444 |
| PE4 + WgH | 63.11 ± 3.23 A a | 62.01 ± 3.04 A a | 58.91 ± 2.82 A a | 1.55 | 0.2875 |
| PE1 + BW | 25.23 ± 1.44 FG a | 21.19 ± 1.38 HI b | 15.69 ± 0.86 HI c | 43.7 | ≤0.05 |
| PE2 + BW | 35.88 ± 1.73 DE a | 32.08 ± 1.90 FG a | 27.00 ± 2.16 F–H b | 15.9 | ≤0.05 |
| PE3 + BW | 48.03 ± 2.52 C a | 44.93 ± 2.36 C–E ab | 39.96 ± 2.77 C–E b | 7.61 | ≤0.05 |
| PE4 + BW | 60.79 ± 3.01 A a | 57.92 ± 2.96 AB a | 53.52 ± 3.00 AB a | 4.50 | 0.0640 |
| F11,35 | 65.9 | 59.1 | 46.3 | ||
| p | ≤0.05 | ≤0.05 | ≤0.05 | ||
| Effect | Df | Consumption of WgH | Consumption of Beeswax | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Treatment | 3 | 122.39 | ≤0.05 | 60.98 | ≤0.05 |
| Time | 2 | 290.96 | ≤0.05 | 155.48 | ≤0.05 |
| Treatment × Time | 6 | 1.95 | 0.1137 | 1.47 | 0.2310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfooz, A.; Yasin, M.; Qayyum, M.A.; Abbasi, A.; Hashem, A.; Almutairi, K.F.; Abd_Allah, E.F.; Farhan, M.; Aqueel, M.A.; Subhan, M. Effect of Co-Diet Supplementation on Biodegradation of Polyethylene by Galleria mellonella L. (Lepidoptera: Pyralidae). Insects 2024, 15, 704. https://doi.org/10.3390/insects15090704
Mahfooz A, Yasin M, Qayyum MA, Abbasi A, Hashem A, Almutairi KF, Abd_Allah EF, Farhan M, Aqueel MA, Subhan M. Effect of Co-Diet Supplementation on Biodegradation of Polyethylene by Galleria mellonella L. (Lepidoptera: Pyralidae). Insects. 2024; 15(9):704. https://doi.org/10.3390/insects15090704
Chicago/Turabian StyleMahfooz, Areej, Muhammad Yasin, Mirza Abdul Qayyum, Asim Abbasi, Abeer Hashem, Khalid F. Almutairi, Elsayed Fathi Abd_Allah, Muhammad Farhan, Muhammad Anjum Aqueel, and Mishal Subhan. 2024. "Effect of Co-Diet Supplementation on Biodegradation of Polyethylene by Galleria mellonella L. (Lepidoptera: Pyralidae)" Insects 15, no. 9: 704. https://doi.org/10.3390/insects15090704
APA StyleMahfooz, A., Yasin, M., Qayyum, M. A., Abbasi, A., Hashem, A., Almutairi, K. F., Abd_Allah, E. F., Farhan, M., Aqueel, M. A., & Subhan, M. (2024). Effect of Co-Diet Supplementation on Biodegradation of Polyethylene by Galleria mellonella L. (Lepidoptera: Pyralidae). Insects, 15(9), 704. https://doi.org/10.3390/insects15090704








