Eco-Morphological Responses of Camponotus japonicus (Hymenoptera: Formicidae) to Varied Climates and Habitats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Ant Sampling and Identification
2.3. Measurements of Ant Morphological Traits
2.4. Data Analysis
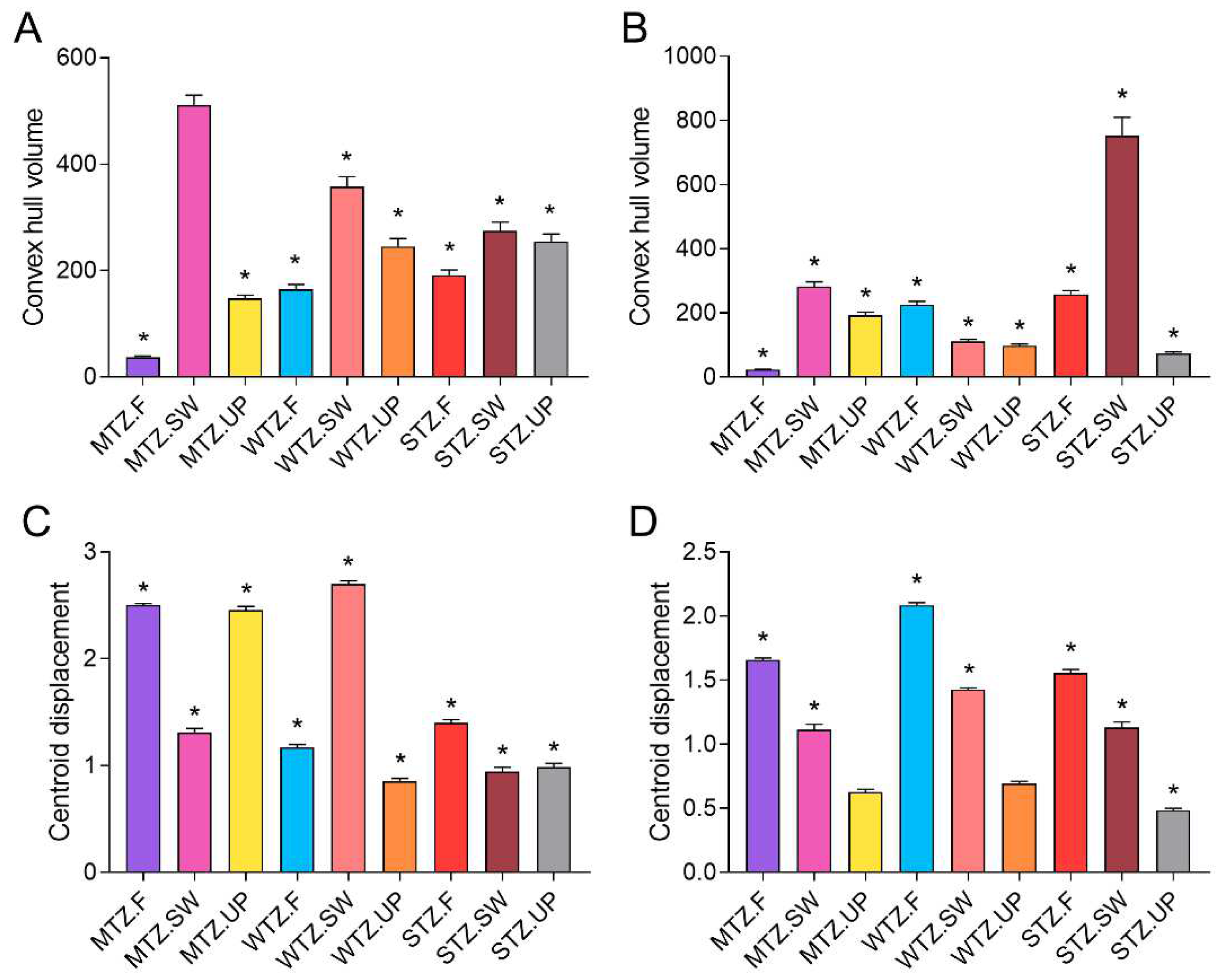
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bergmann, C. Über die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Gottinger Stud. 1848, 3, 595–708. [Google Scholar]
- Allen, J. The influence of physical conditions in the genesis of species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Rensch, B. Some problems of geographical variation and species-formation. Proc. Linn. Soc. Lond. 1936, 150, 275–285. [Google Scholar] [CrossRef]
- Ashton, K.G. Patterns of within-species body size variation of birds: Strong evidence for Bergmann’s rule. Glob. Ecol. Biogeogr. 2002, 11, 505–523. [Google Scholar] [CrossRef]
- Heinze, J.; Foitzik, S.; Fischer, B. The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography 2003, 26, 349–355. [Google Scholar] [CrossRef]
- Classen, A.; Steffan-Dewenter, I.; Kindeketa, W.J.; Peters, M.K.; Rezende, E. Integrating intraspecific variation in community ecology unifies theories on body size shifts along climatic gradients. Funct. Ecol. 2017, 31, 768–777. [Google Scholar] [CrossRef]
- Tian, L.; Benton, M.J. Predicting biotic responses to future climate warming with classic ecogeographic rules. Curr. Biol. 2020, 30, 744–749. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer: Sunderland, MA, USA, 1998. [Google Scholar]
- Moczek, A.P.; Sultan, S.; Foster, S.; Ledón-Rettig, C.; Dworkin, I.F.; Nijhout, H.F.; Abouheif, E.; Pfennig, D.W. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B 2011, 278, 2705–2713. [Google Scholar] [CrossRef]
- Sommer, R.J. Phenotypic plasticity: From theory and genetics to current and future challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Algar, A.C.; López-Darias, M. Sex-specific responses of phenotypic diversity to environmental variation. Ecography 2016, 39, 715–725. [Google Scholar] [CrossRef]
- Gomez, J.P.; Bravo, G.A.; Brumfield, R.T.; Tello, J.G.; Cadena, C.D. A phylogenetic approach to disentangling the role of competition and habitat filtering in community assembly of Neotropical forest birds. J. Anim. Ecol. 2010, 79, 1181–1192. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, Q.M.; Newman, C.; Huang, X.Q.; Luo, X.Y.; Zhou, Z.M. Variation in Pheidole nodus (Hymenoptera: Formicidae) functional morphology across urban parks. PeerJ 2023, 11, e15679. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Chang. Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.; Larigauderie, A.; Cesario, M.; Elmquist, T.; Hoegh-Guldberg, O.; Lavorel, S.; Mace, M.G.; Palmer, M.; Scholes, R.; Yahara, T. Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ. Sustain. 2009, 1, 46–54. [Google Scholar] [CrossRef]
- Perkins, D.M.; Reiss, J.; Yvon-Durocher, G.; Woodward, G. Global change and food webs in running waters. Hydrobiologia 2010, 657, 181–198. [Google Scholar] [CrossRef]
- Scanes, C.G. Human activity and habitat loss: Destruction, fragmentation, and degradation. In Animals and Human Society; Academic Press: Cambridge, MA, USA, 2018; pp. 451–482. [Google Scholar]
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.N.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.W.; Ananthakrishnan, T.N. Phenotypic Plasticity of Insects: Mechanisms and Consequences; Science Publishers: Hackensack, NJ, USA, 2009. [Google Scholar]
- Dalrymple, R.L.; Flores-Moreno, H.; Kemp, D.J.; White, T.E.; Laffan, S.W.; Hemmings, F.A.; Hitchcock, T.D.; Moles, A.T. Abiotic and biotic predictors of macroecological patterns in bird and butterfly coloration. Ecol. Monogr. 2018, 88, 204–224. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Passera, L.; Roncin, E.; Kaufmann, B.; Keller, L. Increased soldier production in ant colonies exposed to intraspecific competition. Nature 1996, 379, 630–631. [Google Scholar] [CrossRef]
- Tuma, J.; Eggleton, P.; Fayle, T.M. Ant-termite interactions: An important but under-explored ecological linkage. Biol. Rev. 2020, 95, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, P.; Nooten, S.S.; Wang, R.; Wong, M.K.; Brassard, F.; Guénard, B. The abundance, biomass, and distribution of ants on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2201550119. [Google Scholar] [CrossRef]
- Wills, B.; Landis, D. The role of ants in north temperate grasslands: A review. Oecologia 2018, 186, 323–338. [Google Scholar] [CrossRef]
- Elizalde, L.; Arbetman, M.; Arnan, X.; Eggleton, P.; Leal, I.R.; Lescano, M.N.; Saez, A.; Werenkraut, V.; Pirk, G.I. The ecosystem services provided by social insects: Traits, management tools and knowledge gaps. Biol. Rev. 2020, 95, 1418–1441. [Google Scholar] [CrossRef]
- Oster, G.F.; Wilson, E.O. Caste and Ecology in the Social Insects; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Ferguson-Gow, H.; Sumner, S.; Bourke, A.F.; Jones, K.E. Colony size predicts the division of labour in attine ants. Proc. R. Soc. B 2014, 281, 20141411. [Google Scholar] [CrossRef]
- McCreery, H.F.; Breed, M. Cooperative transport in ants: A review of proximate mechanisms. Insectes Soc. 2014, 61, 99–110. [Google Scholar] [CrossRef]
- Giraldo, Y.M.; Traniello, J.F. Worker senescence and the sociobiology of aging in ants. Behav. Ecol. Sociobiol. 2014, 68, 1901–1919. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E. The developmental basis of worker caste polymorphism in ants. Am. Nat. 1991, 138, 1218–1238. [Google Scholar] [CrossRef]
- Smith, C.; Anderson, K.; Tillberg, C.; Gadau, J.; Suarez, A. Caste determination in a polymorphic social insect: Nutritional, social, and genetic factors. Am. Nat. 2008, 172, 497–507. [Google Scholar] [CrossRef]
- Segev, U.; Burkert, L.; Feldmeyer, B.; Foitzik, S. Pace-of-life in a social insect: Behavioral syndromes in ants shift along a climatic gradient. Behav. Ecol. 2017, 28, 1149–1159. [Google Scholar] [CrossRef]
- Segev, U.; Foitzik, S. Ant personalities and behavioral plasticity along a climatic gradient. Behav. Ecol. Sociobiol. 2019, 73, 84. [Google Scholar] [CrossRef]
- Lange, D.; Calixto, E.S.; Rosa, B.B.; Sales, T.A.; Del-Claro, K. Natural history and ecology of foraging of the Camponotus crassus Mayr, 1862 (Hymenoptera: Formicidae). J. Nat. Hist. 2019, 53, 1737–1749. [Google Scholar] [CrossRef]
- Belchior, C.; Del-Claro, K.; Oliveira, P.S. Seasonal patterns in the foraging ecology of the harvester ant Pogonomyrmex naegelii (Formicidae, Myrmicinae) in a Neotropical savanna: Daily rhythms, shifts in granivory and carnivory, and home range. Arthropod-Plant Interact. 2012, 6, 571–582. [Google Scholar] [CrossRef]
- Santos, J.C.; Yamamoto, M.; Oliveira, F.R.; Del-Claro, K. Behavioral repertory of the weaver ant Camponotus (Myrmobrachys) senex (Hymenoptera: Formicidae). Sociobiology 2005, 46, 27–38. [Google Scholar]
- Lawes, M.J.; Moore, A.M.; Andersen, A.N.; Preece, N.D.; Franklin, D.C. Ants as ecological indicators of rainforest restoration: Community convergence and the development of an Ant Forest Indicator Index in the Australian wet tropics. Ecol. Evol. 2017, 7, 8442–8455. [Google Scholar] [CrossRef]
- Ma, R.Q.; Zhang, L.L.; Xu, Y.; Wei, C.; He, H. The influence of climate oscillations and geological events on population differentiation of Camponotus japonicus in the Chinese mainland. Ecol. Evol. 2024, 14, e11077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Study on Worker Polymorphism of Camponotus japonicus. Master’s Thesis, Shanxi Normal University, Xi’an, China, 2013. [Google Scholar]
- Zhang, L.; Ma, R.; Xu, W.; Billen, J.; He, H. Comparative morphology and ultrastructure of the labial gland among castes of Camponotus japonicus (Hymenoptera: Formicidae). Arthropod Struct. Dev. 2023, 72, 101236. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Xiao, G. Study on the biological characteristics of Camponotus japonicus and their predation on the masson pine caterpillar. J. For. Res. 1991, 4, 405–408. [Google Scholar]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche complementarity due to plasticity in resource use: Plant partitioning of chemical N forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Courbaud, B.; Vieilledent, G.; Kunstler, G. Intra-specific variability and the competition-colonisation trade-off: Coexistence, abundance and stability patterns. Theor. Ecol. 2012, 5, 61–71. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C. The Ants of China; China Forestry Publishing House: Beijing, China, 1995. [Google Scholar]
- Wang, C.L.; Xiao, G.R.; Wu, J. Taxonomic Studies on the Genus Camponotus Mayr in China (Hymenoptera, Formicidae). For. Res. 1989, 2, 221. [Google Scholar]
- Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kato, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kalyanamoorthy, S.; Minh, B.Q.; Wong, T.K.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bertelsmeier, C.; Blight, O.; Courchamp, F. Invasions of ants (Hymenoptera: Formicidae) in light of global climate change. Myrmecol. News 2016, 22, 25–42. [Google Scholar]
- Norris, K. Agriculture and biodiversity conservation: Opportunity knocks. Conserv. Lett. 2008, 1, 2–11. [Google Scholar] [CrossRef]
- Marini, L.; Fontana, P.; Klimek, S.; Battisti, A.; Gaston, K.J. Impact of farm size and topography on plant and insect diversity of managed grasslands in the Alps. Biol. Conserv. 2009, 142, 394–403. [Google Scholar] [CrossRef]
- Wright, T.E.; Kasel, S.; Tausz, M.; Bennett, L.T. Edge microclimate of temperate woodlands as affected by adjoining land use. Agric. For. Meteorol. 2010, 150, 1138–1146. [Google Scholar] [CrossRef]
- Gallé, R.; Urák, I.; Nikolett, G.S.; Hartel, T. Sparse trees and shrubs confers a high biodiversity to pastures: Case study on spiders from Transylvania. PLoS ONE 2017, 12, e0183465. [Google Scholar] [CrossRef]
- Hall, M.A.; Nimmo, D.G.; Cunningham, S.A.; Walker, K.; Bennett, A.F. The response of wild bees to tree cover and rural land use is mediated by species’ traits. Biol. Conserv. 2019, 231, 1–12. [Google Scholar] [CrossRef]
- New, T.R. Providing habitats for urban insects. In Insect Conservation and Urban Environments; Springer: Berlin/Heidelberg, Germany, 2015; pp. 163–202. [Google Scholar]
- Bengston, S.E.; Domhaus, A. Be meek or be bold? A colony-level behavioural syndrome in ants. Proc. R. Soc. B 2014, 281, 20140518. [Google Scholar] [CrossRef]
- Bishop, T.R.; Robertson, M.P.; Gibb, H.; van Rensburg, B.J.; Braschler, B.; Chown, S.L.; Foord, S.H.; Munyai, T.C.; Okey, I.; Tshivhandekano, P.G.; et al. Ant assemblages have darker and larger members in cold environments. Glob. Ecol. Biogeogr. 2016, 25, 1489–1499. [Google Scholar] [CrossRef]
- Pinkert, S.; Brandl, R.; Zeuss, D. Colour lightness of dragonfly assemblages across North America and Europe. Ecography 2017, 40, 1110–1117. [Google Scholar] [CrossRef]
- Wetterer, J.K. Ontogenetic changes in forager polymorphism and foraging ecology in the leaf-cutting ant, Atta cephalotes. Oecologia 1994, 98, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.; Pirogan, D.; Avril, A.; Bouyarden, F.; Chapuisat, M. Environmental influence on the phenotype of ant workers revealed by common garden experiment. Behav. Ecol. Sociobiol. 2016, 70, 357–367. [Google Scholar] [CrossRef]
- Shik, J.Z.; Arnan, X.; Oms, C.S.; Cerdá, X.; Boulay, R. Evidence for locally adaptive metabolic rates among ant populations along an elevational gradient. J. Anim. Ecol. 2019, 88, 1240–1249. [Google Scholar] [CrossRef]
- Reymond, A.; Purcell, J.; Cherix, D.; Guisan, A.; Pellissier, L. Functional diversity decreases with temperature in high elevation ant fauna. Ecol. Entomol. 2013, 38, 364–373. [Google Scholar] [CrossRef]
- Gibb, H.; Cunningham, S.A. Restoration of trophic structure in an assemblage of omnivores, considering a revegetation chronosequence. J. Appl. Ecol. 2013, 50, 449–458. [Google Scholar] [CrossRef]
- Kaspari, M. Body size and microclimate use in Neotropical granivorous ants. Oecologia 1993, 96, 500–507. [Google Scholar] [CrossRef]
- Kaspari, M.; Weiser, M.D. The size–grain hypothesis and interspecific scaling in ants. Funct. Ecol. 1999, 13, 530–538. [Google Scholar] [CrossRef]
- Parr, C.L.; Dunn, R.R.; Sanders, N.J.; Weiser, M.D.; Photakis, M.; Bishop, T.R.; Gibb, H. GlobalAnts: A new database on the geography of ant traits (Hymenoptera: Formicidae). Insect Conserv. Divers. 2017, 10, 5–20. [Google Scholar] [CrossRef]
- Sarty, M.; Abbott, K.L.; Lester, P.J. Habitat complexity facilitates coexistence in a tropical ant community. Oecologia 2006, 14, 465–473. [Google Scholar] [CrossRef]
- Weber, N.A. The biology of the fungus-growing ants. Part VII. The Barro Colorado Island, Canal Zone, species. Rev. Entomol. 1941, 12, 93–130. [Google Scholar]
- Weiser, M.D.; Kaspari, M. Ecological morphospace of New World ants. Ecol. Entomol. 2006, 31, 131–142. [Google Scholar]
- Wiernasz, D.C.; Cole, B.J. Queen size mediates queen survival and colony fitness in harvester ants. Evolution 2003, 57, 2179–2183. [Google Scholar] [PubMed]
- Yates, M.L.; Andrew, N.R.; Binns, M.; Gibb, H. Morphological traits: Predictable responses to macrohabitats across a 300 km scale. PeerJ 2014, 2, e271. [Google Scholar] [CrossRef] [PubMed]
- Grevé, M.E.; Bláha, S.; Teuber, J.; Rothmaier, M.; Feldhaar, H. The effect of ground surface rugosity on ant running speed is species-specific rather than size dependent. Insectes Soc. 2019, 66, 355–364. [Google Scholar] [CrossRef]



| Abbreviation | Location | Longitude | Latitude |
|---|---|---|---|
| BJHD | Haidian, Beijing | 116°21′47.73″ | 39°58′31.44″ |
| CQWL | Wulong, Chongqing | 107°46′3.00″ | 29°19′55.56″ |
| FJNA | Nanan, Fujian | 118°27′35.64″ | 25°05′36.96″ |
| GDRY | Ruyuan, Guangdong | 113°14′13.92″ | 24°47′18.60″ |
| GZGY | Guiyang, Guizhou | 106°41′56.76″ | 26°36′16.92″ |
| HBHD | Handan, Hebei | 114°28′45.12″ | 36°37′48.72″ |
| HBWH | Wuhan, Hubei | 114°26′51.36″ | 30°33′52.56″ |
| HLHE | Harbin, Heilongjiang | 126°43′24.96″ | 45°43′30.36″ |
| HNLY | Luoyang, Henan | 112°27′18.86″ | 34°35′53.69″ |
| HNSY | Shaoyang, Hunan | 111°28′5.95″ | 27°12′26.84″ |
| JSNJ | Nanjing, Jiangsu | 118°51′35.28″ | 32°04′33.96″ |
| JXNC | Nanchang, Jiangxi | 115°45′56.52″ | 28°46′37.92″ |
| LNSY | Shenyang, Liaoning | 123°27′50.52″ | 41°50′41.64″ |
| NMCF | Chifeng, Neimenggu | 119°0′18.36″ | 42°17′56.04″ |
| NMHH | Hohhot, Neimenggu | 111°37′30.31″ | 40°52′17.73″ |
| SCMY | Mianyang, Sichuan | 104°43′35.40″ | 31°28′35.40″ |
| SDTA | Taian, Shandong | 117°09′57.26″ | 36°13′35.86″ |
| SXJC | Jiaocheng, Shanxi | 112°08′45.96″ | 37°34′40.08″ |
| SXXX | Xixiang, Shannxi | 107°45′58.32″ | 32°59′4.92″ |
| SXYL | Yulin, Shannxi | 109°43′15.75″ | 38°19′48.43″ |
| XJAL | Altay, Xinjiang | 88°07′21.62″ | 47°51′51.37″ |
| ZJYY | Yuyao, Zhejiang | 121°05′35.88″ | 29°43′50.52″ |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Body length | −0.443 | −0.023 | 0.053 | −0.272 |
| Head length | −0.425 | 0.357 | 0.126 | 0.138 |
| Head width | −0.426 | 0.359 | 0.071 | 0.192 |
| Scape length | −0.188 | −0.466 | 0.776 | 0.066 |
| Pronotum width | −0.359 | 0.145 | −0.075 | 0.470 |
| Weber’s length | −0.370 | −0.212 | 0.003 | −0.571 |
| Mandible length | −0.332 | −0.208 | −0.526 | −0.235 |
| Eye width | −0.172 | −0.646 | −0.301 | 0.513 |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Body length | 0.389 | 0.032 | −0.216 | 0.398 |
| Head length | 0.415 | −0.409 | 0.122 | −0.204 |
| Head width | 0.421 | −0.335 | 0.110 | −0.379 |
| Scape length | 0.272 | 0.536 | 0.363 | 0.269 |
| Pronotum width | 0.429 | −0.112 | 0.077 | 0.005 |
| Weber’s length | 0.358 | 0.227 | 0.340 | 0.210 |
| Mandible length | 0.198 | 0.607 | −0.280 | −0.683 |
| Eye width | 0.274 | −0.001 | −0.771 | 0.270 |
| Explanatory Variables | Degrees of Freedom | Sum of Squares | H value | Original p-Value | Bonferroni- Corrected p-Value | |
|---|---|---|---|---|---|---|
| PC1 | Climate zones | 2 | 2,580,699 | 144.78 | <0.001 | <0.001 |
| Habitat types | 2 | 28,454 | 1.60 | 0.045 | 1 | |
| Climate zones × Habitat types | 4 | 891,941 | 50.04 | <0.001 | <0.001 | |
| Residuals | 453 | 4,555,362 | ||||
| PC2 | Climate zones | 2 | 4161 | 0.23 | 0.015 | 1 |
| Habitat types | 2 | 553,548 | 31.05 | <0.001 | <0.001 | |
| Climate zones × Habitat types | 4 | 1,059,262 | 59.42 | <0.001 | <0.001 | |
| Residuals | 453 | 6,576,795 | ||||
| PC3 | Climate zones | 2 | 127,764 | 7.17 | <0.001 | 0.333 |
| Habitat types | 2 | 6972 | 0.39 | 0.031 | 1 | |
| Climate zones × Habitat types | 4 | 780,054 | 43.76 | <0.001 | <0.001 | |
| Residuals | 453 | 7,275,911 | ||||
| PC4 | Climate zones | 2 | 170,646 | 9.57 | 0.0011 | 0.1 |
| Habitat types | 2 | 313,024 | 17.56 | <0.001 | 0.0018 | |
| Climate zones × Habitat types | 4 | 643,018 | 36.07 | <0.001 | <0.001 | |
| Residuals | 453 | 7,116,562 |
| Explanatory Variables | Degrees of Freedom | Sum of Squares | H Value | Original p-Value | Bonferroni- Corrected p-Value | |
|---|---|---|---|---|---|---|
| PC1 | Climate zones | 2 | 533,849 | 29.95 | <0.001 | <0.001 |
| Habitat types | 2 | 14,133 | 0.79 | 0.673 | 1 | |
| Climate zones × Habitat types | 4 | 1,194,862 | 67.03 | <0.001 | <0.001 | |
| Residuals | 453 | 6,478,590 | ||||
| PC2 | Climate zones | 2 | 436,362 | 24.48 | <0.001 | <0.001 |
| Habitat types | 2 | 921,037 | 51.67 | <0.001 | <0.001 | |
| Climate zones × Habitat types | 4 | 709,591 | 39.81 | <0.001 | <0.001 | |
| Residuals | 453 | 6,200,106 | ||||
| PC3 | Climate zones | 2 | 333,929 | 18.73 | <0.001 | 0.0011 |
| Habitat types | 2 | 228,464 | 12.82 | 0.002 | 0.020 | |
| Climate zones × Habitat types | 4 | 1,660,191 | 93.14 | <0.001 | <0.001 | |
| Residuals | 453 | 6,081,880 | ||||
| PC4 | Climate zones | 2 | 793,628 | 44.52 | <0.001 | <0.001 |
| Habitat types | 2 | 815,734 | 45.76 | <0.001 | <0.001 | |
| Climate zones × Habitat types | 4 | 557,720 | 31.29 | <0.001 | <0.001 | |
| Residuals | 453 | 6,192,454 | 29.95 |
| Independent Variable | Estimate | Std. Error | t-Value | |
|---|---|---|---|---|
| PC1 | Intercept | −3.4236 | 0.5091 | −6.7245 |
| Annual mean temperature | 0.113 | 0.0183 | 6.1702 | |
| Annual mean precipitation | 0.0175 | 0.0028 | 6.2281 | |
| Relative humidity | −0.0025 | 0.009 | −0.2737 | |
| Elevation | 0.0012 | 0.0002 | 6.6169 | |
| PC2 | Intercept | 1.0217 | 0.3749 | 2.7249 |
| Annual mean temperature | −0.0495 | 0.0135 | −3.666 | |
| Annual mean precipitation | 0.0046 | 0.0021 | 2.2362 | |
| Relative humidity | −0.0111 | 0.0066 | −1.6787 | |
| Elevation | −0.0001 | 0.0001 | −0.3823 | |
| PC3 | Intercept | 1.0373 | 0.3164 | 3.2781 |
| Annual mean temperature | −0.0026 | 0.0114 | −0.227 | |
| Annual mean precipitation | 0.0082 | 0.0017 | 4.721 | |
| Relative humidity | −0.0223 | 0.0056 | −3.9902 | |
| Elevation | −0.0007 | 0.0001 | −6.1976 | |
| PC4 | Intercept | −1.4203 | 0.296 | −4.7983 |
| Annual mean temperature | 0.0106 | 0.0107 | 0.9957 | |
| Annual mean precipitation | −0.0087 | 0.0016 | −5.3408 | |
| Relative humidity | 0.0332 | 0.0052 | 6.3475 | |
| Elevation | −0.0002 | 0.0001 | −1.5807 |
| Independent Variable | Estimate | Std. Error | t-Value | |
|---|---|---|---|---|
| PC1 | Intercept | 1.9129 | 0.3213 | 5.9534 |
| Annual mean temperature | −0.0418 | 0.0116 | −3.6132 | |
| Annual mean precipitation | 0.0074 | 0.0018 | 4.1812 | |
| Relative humidity | −0.0253 | 0.0057 | −4.4553 | |
| Elevation | −0.0008 | 0.0001 | −6.8904 | |
| PC2 | Intercept | 1.9129 | 0.3213 | 5.9534 |
| Annual mean temperature | −0.0418 | 0.0116 | −3.6132 | |
| Annual mean precipitation | 0.0074 | 0.0018 | 4.1812 | |
| Relative humidity | −0.0253 | 0.0057 | −4.4553 | |
| Elevation | −0.0008 | 0.0001 | −6.8904 | |
| PC3 | Intercept | 1.9129 | 0.3213 | 5.9534 |
| Annual mean temperature | −0.0418 | 0.0116 | −3.6132 | |
| Annual mean precipitation | 0.0074 | 0.0018 | 4.1812 | |
| Relative humidity | −0.0253 | 0.0057 | −4.4553 | |
| Elevation | −0.0008 | 0.0001 | −6.8904 | |
| PC4 | Intercept | 1.1869 | 0.3072 | 3.8636 |
| Annual mean temperature | 0.0263 | 0.0111 | 2.3809 | |
| Annual mean precipitation | −0.0014 | 0.0017 | −0.8359 | |
| Relative humidity | −0.0188 | 0.0054 | −3.461 | |
| Elevation | −0.0004 | 0.0001 | −4.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Zhang, L.; He, H. Eco-Morphological Responses of Camponotus japonicus (Hymenoptera: Formicidae) to Varied Climates and Habitats. Insects 2024, 15, 719. https://doi.org/10.3390/insects15090719
Ma R, Zhang L, He H. Eco-Morphological Responses of Camponotus japonicus (Hymenoptera: Formicidae) to Varied Climates and Habitats. Insects. 2024; 15(9):719. https://doi.org/10.3390/insects15090719
Chicago/Turabian StyleMa, Ruoqing, Liangliang Zhang, and Hong He. 2024. "Eco-Morphological Responses of Camponotus japonicus (Hymenoptera: Formicidae) to Varied Climates and Habitats" Insects 15, no. 9: 719. https://doi.org/10.3390/insects15090719
APA StyleMa, R., Zhang, L., & He, H. (2024). Eco-Morphological Responses of Camponotus japonicus (Hymenoptera: Formicidae) to Varied Climates and Habitats. Insects, 15(9), 719. https://doi.org/10.3390/insects15090719







