Repellent Effects of Coconut Fatty Acid Methyl Esters and Their Blends with Bioactive Volatiles on Winged Myzus persicae (Sulzer) Aphids (Hemiptera: Aphididae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Bioactive Volatile Products
2.3. Preparation of Oil-in-Water (O/W) Nanoemulsions
2.4. Repellent and Reproductive Effects of Bioactive Volatile Nanoemulsions on Winged Individuals of M. persicae on Pepper Plants
2.5. Statistical Analysis
3. Results
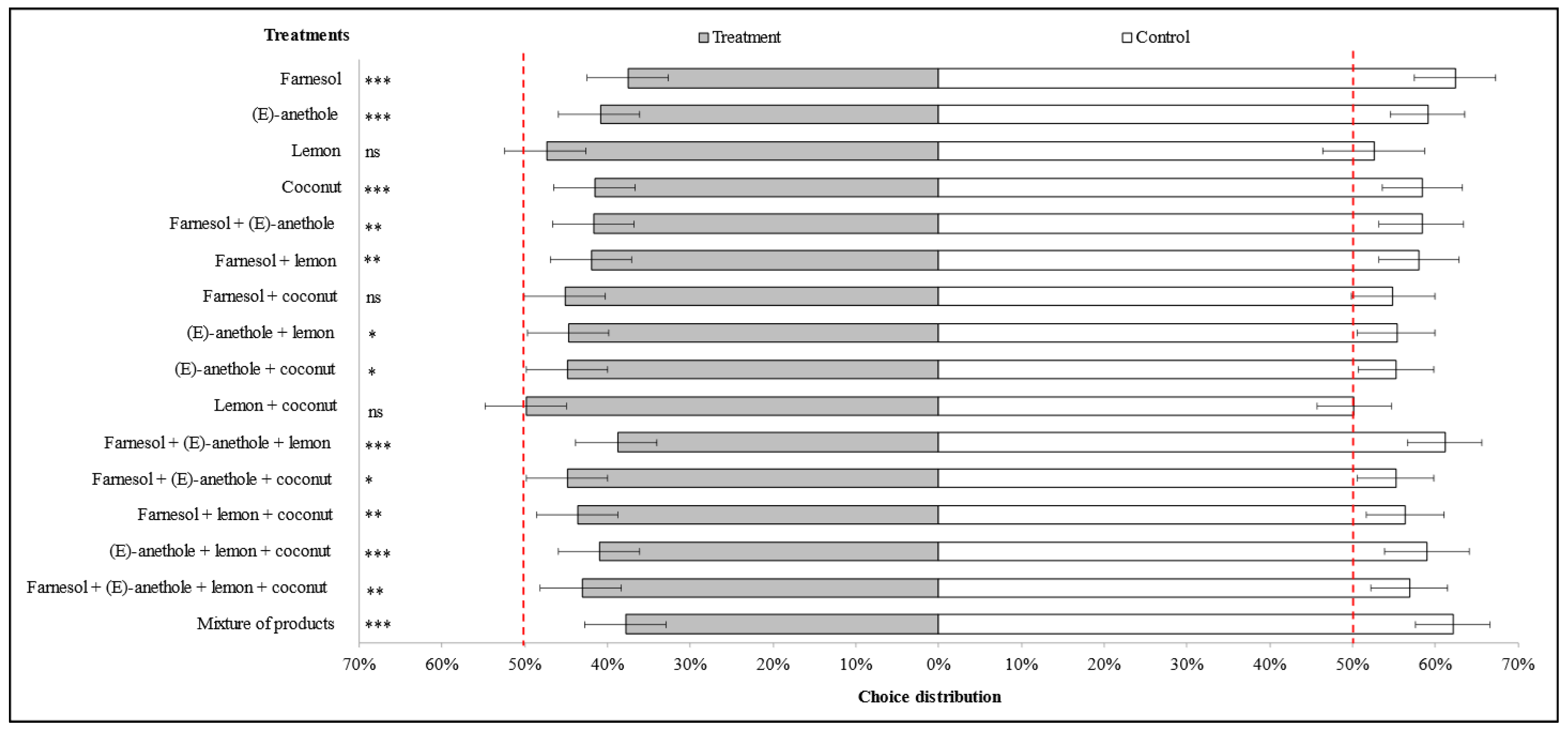
3.1. Choice Distribution of Winged Individuals of M. persicae on Pepper Plants
3.2. Reproduction of Winged Individuals of M. persicae on Pepper Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPPC Secretariat. International Year of Plant Health—Final report. In Protecting Plants, Protecting Life; FAO on behalf of the Secretariat of the International Plant Protection Convention; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Remaudiere, G.; Remaudiere, M. Catalogue des Aphididae du Monde/Catalogue of the World’s Aphididae (Homoptera, Aphidoidea); INRA Editions (Collection Techniques et Pratiques); Editions Quae: Paris, France, 1997; p. 475. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; Wiley: Chichester, UK, 2000; p. 466. [Google Scholar]
- Matthews, R.E.F.; Hull, R. Matthews Plant Virology, 4th ed.; Elsevier Academic Press: San Diego, CA, USA, 2002; p. 1056. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Taxonomic issues. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 1–36. [Google Scholar] [CrossRef]
- Nault, L.R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 1997, 90, 521–541. [Google Scholar] [CrossRef]
- Garcıa-Marı, F.; Costa-Comelles, J.; Ferragut, F. Las Plagas Agricolas, 2nd ed.; M.V. Phytoma-España, S.L.: Valencia, Spain, 1994; p. 376. [Google Scholar]
- Perez, P.; Duque, M.; Collar, J.L.; Avilla, C.; Fereres, A. Activity of alatae aphids landing on open-field pepper crops in Spain. J. Plant Dis. Prot. 2003, 110, 195–202. [Google Scholar]
- Sanchez, J.A.; La-Spina, M.; Michelena, J.M.; Lacasa, A.; Hermoso de Mendoza, A. Ecology of the aphid pests of protected pepper crops and their Parasitoids. Biocontrol Sci. Technol. 2011, 21, 171–188. [Google Scholar] [CrossRef]
- De Blas, C.; Carazo, G.; Castro, C.; Romero, J. Estudios epidemiológicos sobre el virus del mosaico del pepino en diferentes cultivos y provincias españolas; identificación serológica de los subgrupos DTL y ToRS. Bol. San. Veg. Plagas 1993, 19, 345–353. [Google Scholar]
- Pérez, P.; Gemeno, C.; Verdugo, M.; Soto, M.J.; Ponz, F.; Fereres, A. Dinámica de poblaciones de vectores y transmisión del virus Y de la patata en cultivos de pimiento. Bol. San. Veg. Plagas 1992, 18, 225–235. [Google Scholar]
- Villanueva, V.; Castillo, P.; Font, M.I.; Alfaro-Fernández, A.; Moriones, E.; Navas-Castillo, J. First report of pepper vein yellows virus infecting sweet pepper in Spain. Plant Dis. 2013, 97, 1261. [Google Scholar] [CrossRef]
- Katis, N.I.; Tsitsipis, J.A.; Stevens, M.; Powell, G. Transmission of plant viruses. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 353–390. [Google Scholar]
- Harrington, R.; Clark, S.J.; Welham, S.J.; Verrier, P.J.; Denholm, C.H.; Hullé, M.; Maurice, D.; Rounsevell, M.D.; Cocu, N. Environmental change and the phenology of European aphids. Glob. Chang. Biol. 2007, 13, 1550–1564. [Google Scholar] [CrossRef]
- Petterson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 87–114. [Google Scholar]
- Pickett, J.A.; Bruce, T.B.A.; Glinwood, R.T. Chemical Ecology. In Aphids as Crop Pests, 2nd ed.; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 235–260. [Google Scholar]
- Rathore, H.S. Section I: Essential Oils as Green Pesticides. Chapter 1: Green pesticides for organic farming: Occurrence and properties of essential oils for use in pest control. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–25. [Google Scholar]
- Nollet, L.M.L.; Rathore, H.S. Green Pesticides Handbook: Essential Oils for Pest Control; CRC Press: Boca Raton, FL, USA, 2017; p. 571. [Google Scholar]
- Butnariu, M.; Sarac, I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018, 1, 35–43. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Regnault-Roger, C. Essential oils in insect control. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 4087–4107. [Google Scholar] [CrossRef]
- Park, Y.L.; Tak, J.H. Essential oils for arthropod pest management in agricultural production systems. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 61–70. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Martín, F.; Garzo, E.; Guirao, P.; Pascual-Villalobos, M.J.; Fereres, A.; Moreno, A. Persistence of nanoemulsions of bioactive volatiles and their impact on aphid feeding behaviour. J. Pest. Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bokonon-Ganta, A.H.; Verheggen, F.J.; Lognay, G.C.; Francis, F. Aphid behavior on Amaranthus hybridus L. (Amaranthaceae) associated with Ocimum spp. (Lamiaceae) as repellent plants. Agronomy 2020, 10, 736. [Google Scholar] [CrossRef]
- Dardouri, T.; Gautier, H.; Ben Issa, R.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of two modes of action of volatiles from selected living aromatic plants. Pest. Manag. Sci. 2019, 75, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Hori, M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 1998, 24, 1425–1432. [Google Scholar] [CrossRef]
- Hori, M. The effects of rosemary and ginger oils on the alighting behavior of Myzus persicae (Sulzer) (Homoptera: Aphididae) and on the incidence of yellow spotted streakspotted streak. Appl. Entomol. Zool. 1999, 34, 351–358. [Google Scholar] [CrossRef]
- Martín-López, B.; López-López, V.; Cabaleiro Sobrino, C. Short communication: Repellency and toxicity of oils from different origins on Myzus persicae (Sulzer) (Hemiptera: Aphididae) in pepper. Span. J. Agric. Res. 2003, 1, 73–77. [Google Scholar] [CrossRef]
- Halbert, S.E.; Corsini, D.; Wiebe, M.; Vaughn, S.F. Plant-derived compounds and extracts with potential as aphid repellents. Ann. Appl. Biol. 2009, 154, 303–307. [Google Scholar] [CrossRef]
- Cantó-Tejero, M.; Casas, J.L.; Marcos-Garcia, M.A.; Pascual-Villalobos, M.J.; Florencio-Ortiz, V.; Guirao, P. Essential oils-based repellents for the management of Myzus persicae and Macrosiphum euphorbiae. J. Pest. Sci. 2022, 95, 365–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Wang, X.; Bian, Z.; Zhang, X.; Yang, G.; Lu, Y. Volatiles from essential oils of three Lamiaceae plants repel the winged cotton aphid, disturb its feeding behavior and reduce its fecundity. Pest. Manag. Sci. 2024, 80, 9. [Google Scholar] [CrossRef]
- Zhu, J.J.; Cermak, S.C.; Kenar, J.A.; Brewer, G.; Haynes, K.F.; Boxler, D.; Bajer, P.D.; Wang, D.; Wang, C.; Li, A.Y.; et al. Better than DEET repellent compounds derived from coconut oil. Sci. Rep. 2018, 8, 14053. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Benelli, G.; Lukehart, C.M. Special issue: Applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J. Clust. Sci. 2017, 28, 1–2. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid. Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Philògene, B.J.R.; Regnault-Roger, C.; Vincent, C. Productos fitosanitarios insecticidas de origen vegetal: Promesas de ayer y hoy. In Biopesticidas de Origen Vegetal; Regnault-Roger, C., Philògene, B.J.R., Vincent, C., Eds.; Ediciones Mundi Prensa: Madrid, Spain, 2004; pp. 1–18. [Google Scholar]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodriguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Gutierrez, C.; Fereres, A.; Reina, M.; Cabrera, R.; Gonzalez-Coloma, A. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae. J. Chem. Ecol. 1997, 23, 1641–1650. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 March 2024).
- Taglienti, A.; Donati, L.; Dragone, I.; Ferretti, L.; Gentili, A.; Araniti, F.; Sapienza, F.; Astolfi, R.; Fiorentino, S.; Vecchiarelli, V.; et al. In vivo antiphytoviral and aphid repellency activity of essential oils and hydrosols from Mentha suaveolens and Foeniculum vulgare to control Zucchini Yellow Mosaic Virus and its vector Aphis gossypii. Plants 2023, 12, 1078. [Google Scholar] [CrossRef]
- Lacotte, V.; Rey, M.; Peigner, S.; Mercier, P.E.; Rahioui, I.; Sivignon, C.; Razy, L.; Benhamou, S.; Livi, S.; da Silva, P. Bioactivity and chemical composition of forty plant essential oils against the pea aphid Acyrthosiphon pisum revealed peppermint oil as a promising biorepellent. Ind. Crops Prod. 2023, 197, 116610. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Guirao, P.; Diaz-Baños, F.G.; Cantó-Tejero, M.; Villora, G. Oil in water nanoemulsion formulations of botanical active substances. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2019; pp. 223–247. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Liu, Y.; Chen, J.; Francis, F. Use of slow-release plant infochemicals to control aphids: A first investigation in a Belgian wheat field. Sci. Rep. 2016, 6, 31552. [Google Scholar] [CrossRef] [PubMed]
- Martín Pérez, F.; Pascual-Villalobos, M.J.; Guirao, P.; Kenar, J.A.; Cermak, S.C. Setting up preliminary tests to prove the effect of coconut fatty acid as an aphid repellent in pepper. In Proceedings of the 32nd Annual Meeting AAIC, Bologna, Italy, 5–8 September 2021. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.S.; Darwish, H.; Alzahrani, A.K.; Alharthi, S.; Alghamdi, A.S.; Al-Barty, A.M.; Helal, M.; Maghrabi, A.; Baazeem, A.; Alamari, H.A.; et al. Environment-friendly control potential of two citrus essential oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Agronomy 2022, 12, 2040. [Google Scholar] [CrossRef]
- Girardi, J.; Berķe-Ļubinska, K.; Mežaka, I.; Nakurte, I.; Skudriņš, G.; Pastare, L. In vivo bioassay of the repellent activity of caraway essential oil against green peach aphid. Insects 2023, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and antifeedant activities of citral-derived lactones against the peach potato aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef]
- Khaled-Gasmi, W.; Hamouda, A.B.; Chaieb, I.; Souissi, R.; Ascrizzi, R.; Flamini, G.; Boukhris-Bouhachem, S. Natural repellents based on three botanical species essential oils as an eco-friendly approach against aphids. S. Afr. J. Bot. 2021, 141, 133–141. [Google Scholar] [CrossRef]
- Sayed, S.; Soliman, M.M.; Al-Otaibi, S.; Hassan, M.M.; Elarrnaouty, S.-A.; Abozeid, S.M.; El-Shehawi, A.M. Toxicity, deterrent and repellent activities of four essential oils on Aphis punicae (Hemiptera: Aphididae). Plants 2022, 11, 463. [Google Scholar] [CrossRef]

| Treatments 2 | Number of Aphids | % Choice | % No Choice | RI (%) 3 | |||
|---|---|---|---|---|---|---|---|
| C | T | Choice | No Choice | ||||
| Farnesol | 245 | 147 | 392 | 208 | 65.33 | 34.67 | 40.24 |
| (E)-anethole | 282 | 195 | 477 | 123 | 79.50 | 20.50 | 30.85 |
| Lemon | 141 | 127 | 268 | 332 | 44.67 | 55.33 | 9.93 |
| Coconut | 242 | 172 | 414 | 186 | 69.00 | 31.00 | 28.93 |
| Farnesol + (E)-anethole | 219 | 156 | 375 | 225 | 62.50 | 37.50 | 28.77 |
| Farnesol + lemon | 245 | 177 | 422 | 178 | 70.33 | 29.67 | 27.76 |
| Farnesol + coconut | 213 | 175 | 388 | 212 | 64.67 | 35.33 | 17.84 |
| (E)-anethole + lemon | 249 | 201 | 450 | 150 | 75.00 | 25.00 | 19.28 |
| (E)-anethole + coconut | 264 | 214 | 478 | 122 | 79.67 | 20.33 | 18.94 |
| Lemon + coconut | 255 | 253 | 508 | 92 | 84.67 | 15.33 | 0.78 |
| Farnesol + (E)-anethole + lemon | 290 | 184 | 474 | 126 | 79.00 | 21.00 | 36.55 |
| Farnesol + (E)-anethole + coconut | 260 | 211 | 471 | 129 | 78.50 | 21.50 | 18.85 |
| Farnesol + lemon + coconut | 247 | 191 | 438 | 162 | 73.00 | 27.00 | 22.67 |
| (E)-anethole + lemon + coconut | 222 | 154 | 376 | 224 | 62.67 | 37.33 | 30.63 |
| Farnesol + (E)-anethole + lemon + coconut | 264 | 200 | 464 | 136 | 77.33 | 22.67 | 24.24 |
| Mixture of products 4 | 288 | 175 | 463 | 137 | 77.17 | 22.83 | 39.24 |
| Treatments 2 | Mean Number of Nymphs/Adult per Day 3 | t-Test | p-Value 4 | |
|---|---|---|---|---|
| T | C | |||
| Farnesol | 0.54 ± 0.10 | 0.68 ± 0.09 | 1.841 | 0.079 |
| (E)-anethole | 1.16 ± 0.15 | 0.99 ± 0.13 | −1.700 | 0.103 |
| Lemon | 0.40 ± 0.07 | 0.34 ± 0.09 | −0.898 | 0.379 |
| Coconut | 0.55 ± 0.06 | 0.54 ± 0.08 | −0.175 | 0.863 |
| Farnesol + (E)-anethole | 0.75 ± 0.12 | 0.72 ± 0.09 | −0.290 | 0.774 |
| Farnesol + lemon | 0.73 ± 0.07 | 0.79 ± 0.07 | 0.468 | 0.645 |
| Farnesol + coconut | 0.76 ± 0.15 | 0.70 ± 0.11 | −0.571 | 0.574 |
| (E)-anethole + lemon | 0.66 ± 0.07 | 0.78 ± 0.08 | 1.235 | 0.229 |
| (E)-anethole + coconut | 0.89 ± 0.09 | 0.78 ± 0.08 | −0.941 | 0.356 |
| Lemon + coconut | 0.99 ± 0.12 | 0.89 ± 0.11 | −0.817 | 0.422 |
| Farnesol + (E)-anethole + lemon | 0.60 ± 0.09 | 0.67 ± 0.07 | 0.901 | 0.377 |
| Farnesol + (E)-anethole + coconut | 0.58 ± 0.07 | 0.57 ± 0.06 | −0.266 | 0.792 |
| Farnesol + lemon + coconut | 0.65 ± 0.10 | 0.97 ± 0.13 | 2.301 | 0.031 * |
| (E)-anethole + lemon + coconut | 0.44 ± 0.10 | 0.41 ± 0.05 | −0.266 | 0.793 |
| Farnesol + (E)-anethole + lemon + coconut | 0.69 ± 0.11 | 0.66 ± 0.07 | −0.424 | 0.676 |
| Mixture of products 5 | 0.60 ± 0.07 | 0.52 ± 0.06 | −1.095 | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, F.; Guirao, P.; Pascual-Villalobos, M.J. Repellent Effects of Coconut Fatty Acid Methyl Esters and Their Blends with Bioactive Volatiles on Winged Myzus persicae (Sulzer) Aphids (Hemiptera: Aphididae). Insects 2024, 15, 731. https://doi.org/10.3390/insects15090731
Martín F, Guirao P, Pascual-Villalobos MJ. Repellent Effects of Coconut Fatty Acid Methyl Esters and Their Blends with Bioactive Volatiles on Winged Myzus persicae (Sulzer) Aphids (Hemiptera: Aphididae). Insects. 2024; 15(9):731. https://doi.org/10.3390/insects15090731
Chicago/Turabian StyleMartín, Félix, Pedro Guirao, and María Jesús Pascual-Villalobos. 2024. "Repellent Effects of Coconut Fatty Acid Methyl Esters and Their Blends with Bioactive Volatiles on Winged Myzus persicae (Sulzer) Aphids (Hemiptera: Aphididae)" Insects 15, no. 9: 731. https://doi.org/10.3390/insects15090731
APA StyleMartín, F., Guirao, P., & Pascual-Villalobos, M. J. (2024). Repellent Effects of Coconut Fatty Acid Methyl Esters and Their Blends with Bioactive Volatiles on Winged Myzus persicae (Sulzer) Aphids (Hemiptera: Aphididae). Insects, 15(9), 731. https://doi.org/10.3390/insects15090731







