Morphological Comparisons of Adult Worker Bees Developed in Chinese and Italian Honey Bee Combs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishing Experimental Bee Colonies to Rear Worker Bees
2.2. Counting Cell Contents
2.3. Measurement of Birth Weight and Morphological Indicators of Emerging Worker Bees
2.4. Statistical Analysis
3. Results
3.1. Comparison Between Cell Diameters of Chinese Worker Bees and Italian Worker Bees
3.2. Percentages of Cells with Different Contents
3.3. Comparisons of Body Weights of 6-Day-Old Larvae, White-Eyed Pupae, and Honey Stomach Capacity in Worker Bees Between the Two Groups
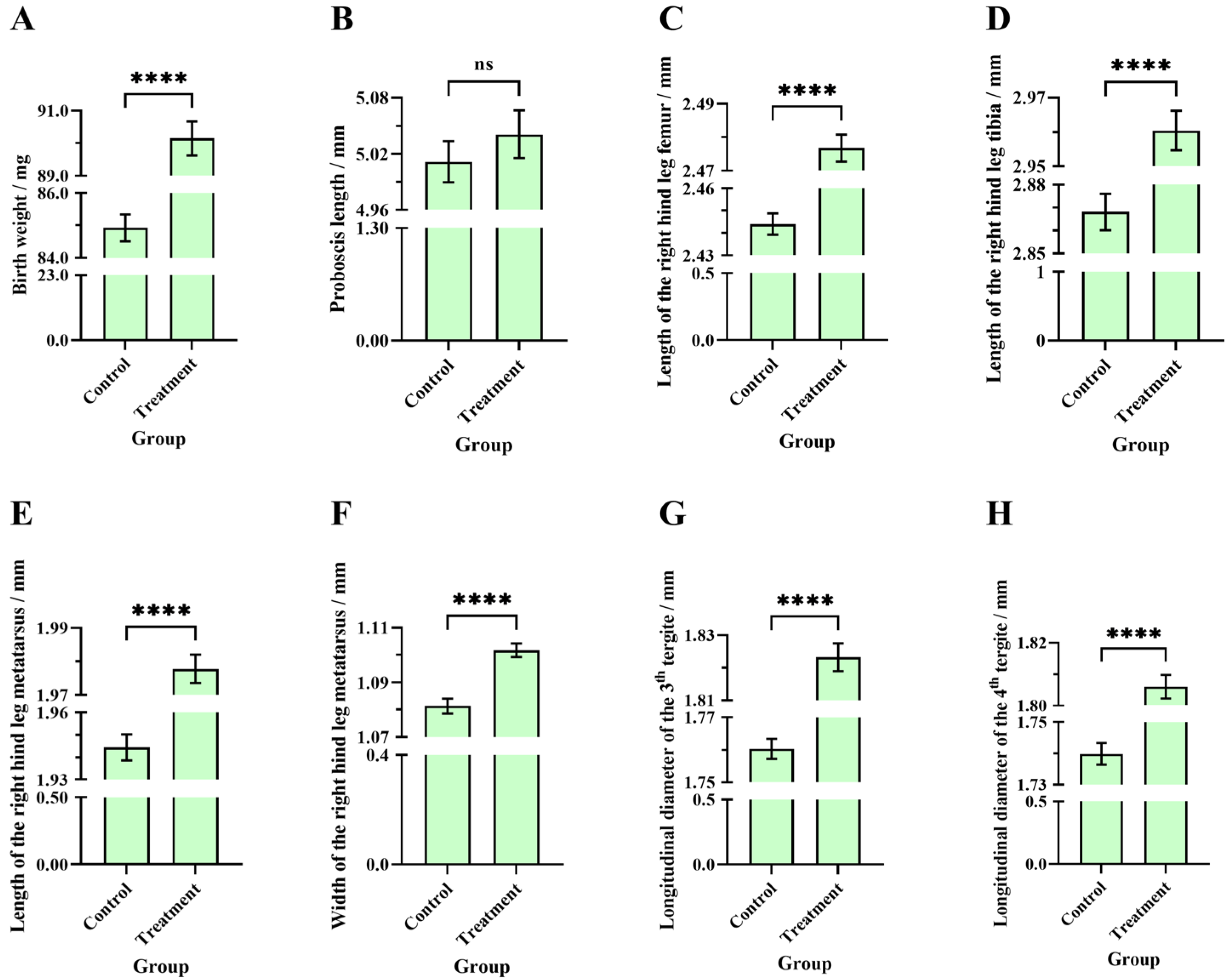
3.4. Comparisons of Birth Weight, Proboscis Length, and Longitudinal Diameter of the Third and Fourth Tergite Between the Two Groups of Worker Bees
3.5. Comparisons of the Length of the Right Hind Leg Femur, Tibia, Metatarsus, and the Metatarsus Width Between the Two Groups of Worker Bees
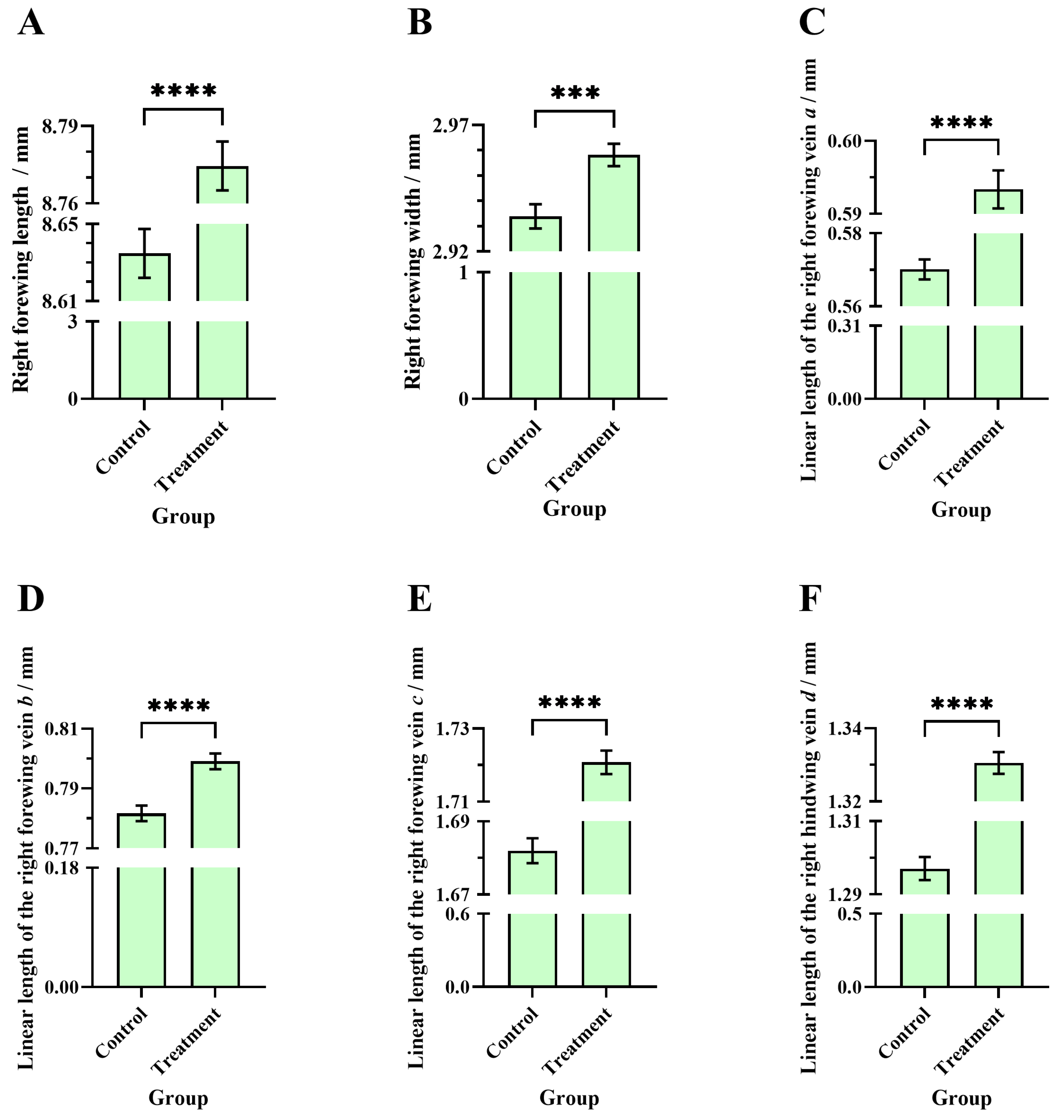
3.6. Comparisons of the Right Forewing Length, Right Forewing Width, and Linear Length of Veins a, b, c, and d Between the Two Groups of Worker Bees
3.7. Body Side Symmetry in Worker Bees
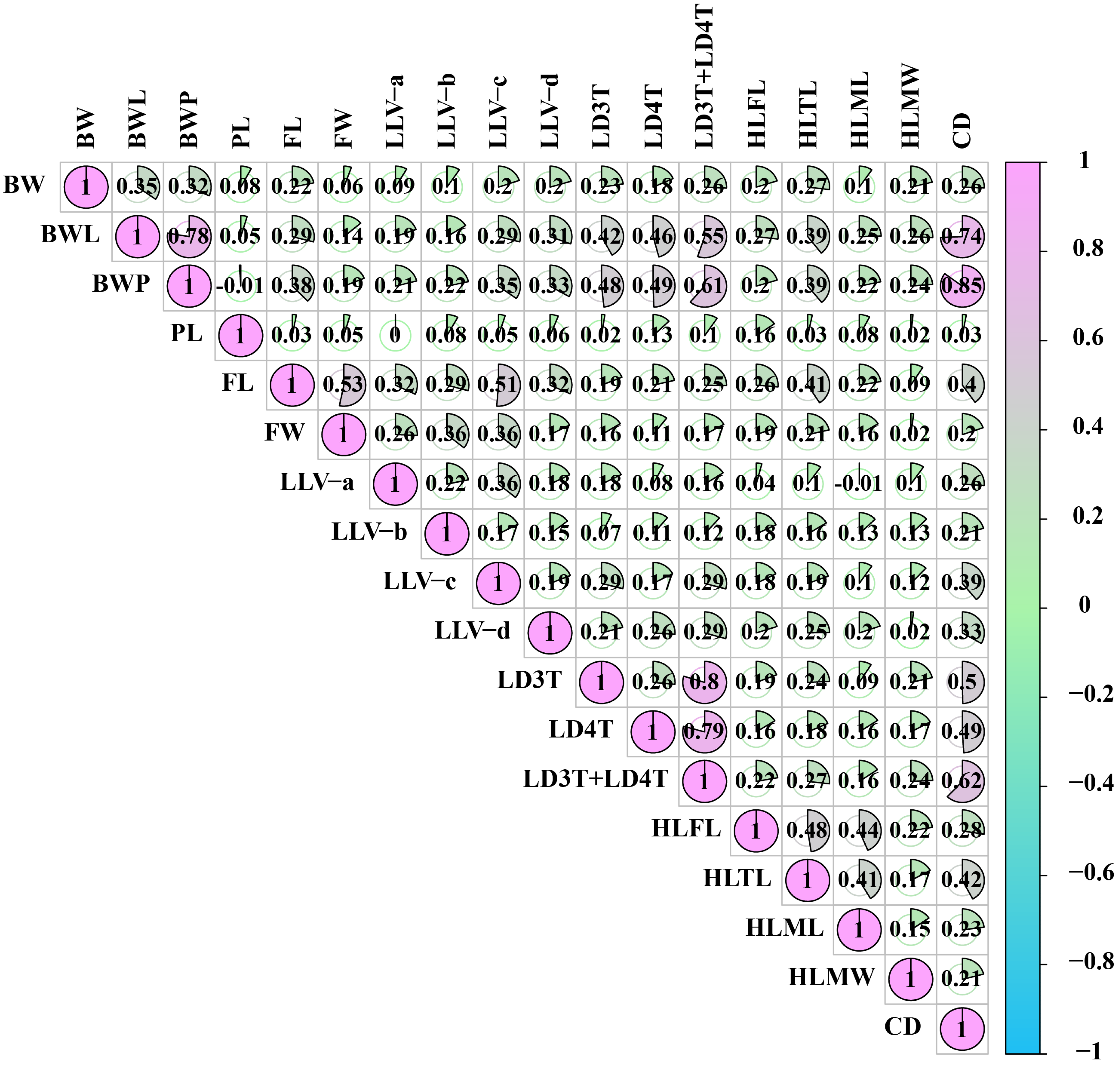
3.8. Correlation Analysis Between Worker Body Weight, Morphological Traits, and Cell Diameter
4. Discussion
4.1. Methods of Rearing Large-Bodied Chinese Worker Bees
4.2. The Advantages and Significance of Enlarged Chinese Worker Bees
4.3. How Larger Cells Aid in Rearing Larger Bees
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waddington, B.D. Body Size, Individual Behavior, and Social Behavior in Honey Bees. In Interindividual Behavioral Variability in Social Insects; Jeanne, R.L., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 385–417. [Google Scholar]
- Berg, S.; Koeniger, N.; Koeniger, G.; Fuchs, S. Body Size and Reproductive Success of Drones (Apis mellifera L.). Apidologie 1997, 28, 449–460. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Borsuk, G.; Olszewski, K. Possibility to Change the Body Size in Worker Bees by a Combination of Small-Cell and Standard-Cell Combs in the Same Nest. Apidologie 2021, 52, 1017–1032. [Google Scholar] [CrossRef]
- Smith, M.L.; Napp, N.; Petersen, K.H. Imperfect Comb Construction Reveals the Architectural Abilities of Honeybees. Proc. Natl. Acad. Sci. USA 2021, 118, e2103605118. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Damiani, N.; Ruffinengo, S.; Jong, D.D.; Principal, J.; Eguaras, M. Brood Cell Size of Apis mellifera Modifies the Reproductive Behavior of Varroa destructor. Exp. Appl. Acarol. 2010, 50, 269–279. [Google Scholar] [CrossRef]
- Bishop, G.H. Growth Rates of Honey Bee Larva. J. Exp. Zool. 1961, 146, 11–20. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.T.; Benton, A.W. Rates of Growth of Honeybee Larvae. J. Apic. Res. 1982, 21, 189–192. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; AL-Kahtani, S.N. The Relationship between Comb Age and Performance of Honey Bee (Apis mellifera) Colonies. Saudi J. Biol. Sci. 2020, 27, 30–34. [Google Scholar] [CrossRef]
- Shawer, M.B.; Elnabawy, E.M.; Mousa, K.M.; Gaber, S.; Ueno, T. Impact of Different Comb Age on Morphological and Biological Characteristics of Honeybee Workers (Apis mellifera L.). J.-Fac. Agric. Kyushu Univ. 2020, 65, 277–282. [Google Scholar] [CrossRef]
- Glushkov, N.M. Results of Testing Combs with Enlarged Cell Diameters. Pchelovodstvo 1956, 33, 32–40. [Google Scholar]
- Kotelkov, N.Z.; Blashkin, V.I. Experiment with Rearing Worker Bees and Queens in Enlarged Cells. Pchelovodstvo 1957, 34, 33–36. [Google Scholar]
- Mikhaylov, K.I. The Testing of the Comb Foundation with Enlarged Cells. Pchelovodstvo 1957, 9, 29–34. [Google Scholar]
- Michener, C.D. The Social Behavior of the Bees: A Comparative Study; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Brian, A.D. Division of Labour and Foraging in Bombus Agrorum Fabricius. J. Anim. Ecol. 1952, 21, 223–240. [Google Scholar] [CrossRef]
- Kerr, W.E.; Hebling, N.J. Influence of the Weight of Worker Bees on Division of Labor. Evolution 1964, 18, 267–270. [Google Scholar] [CrossRef]
- Wells, P.H.; Giacchino, J. Relationship between the Volume and the Sugar Concentration of Loads Carried by Honeybees. J. Apic. Res. 1968, 7, 77–82. [Google Scholar] [CrossRef]
- Milne, C.P.; Pries, K.J. Honeybee Corbicular Size and Honey Production. J. Apic. Res. 1984, 23, 11–14. [Google Scholar] [CrossRef]
- Milne, C.P.; Pries, K.J. Honeybees with Larger Corbiculae Carry Larger Pollen Pellets. J. Apic. Res. 1986, 25, 53–54. [Google Scholar] [CrossRef]
- Abd Al-Fattah, M.A.A.-W.; Yehia Ibrahim, Y.; Ibrahim Haggag, M. Some Biological Aspects of Honey Bee Colonies in Relation to the Age of Beeswax Combs. J. Apic. Res. 2021, 60, 405–413. [Google Scholar] [CrossRef]
- Cideciyan, M. The Relationships between Size and Behavior in Worker Honey Bees (Apis mellifera). Master’s Thesis, University of Miami, Coral Gables, FL, USA, 1984. [Google Scholar]
- Taha, E.-K.A.; Rakha, O.M.; Elnabawy, E.-S.M.; Hassan, M.M.; Shawer, D.M.B. Comb Age Significantly Influences the Productivity of the Honeybee (Apis mellifera) Colony. J. King Saud Univ.-Sci. 2021, 33, 101436. [Google Scholar] [CrossRef]
- Waddington, K.D. Patterns of Size Variation in Bees and Evolution of Communication Systems. Evolution 1981, 35, 813–814. [Google Scholar] [CrossRef]
- Scholze, E.; Pichler, H.; Heran, H. Zur Entfernungsschtzung Der Bienen Nach Dem Kraftaufwand. Die Naturwissenschaften 1964, 51, 69–70. [Google Scholar] [CrossRef]
- Lindauer, M. The Dance Language of Honeybees: The History of a Discovery. In Experimental Behavioral Ecology and Sociobiology; Memoriam Karl Von Frisch 1886–1982; Hölldobler, B., Lindauer, M., Eds.; Gustav Fischer Verlag Stuttgart: New York, NY, USA, 1985; pp. 129–140. [Google Scholar]
- Frisch, K.v. The Dance Language and Orientation of Bees; Harvard University Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Waddington, K.D. Cost-Intake Information Used in Foraging. J. Insect Physiol. 1985, 31, 891–897. [Google Scholar] [CrossRef]
- Waddington, K.D. Implications of Variation in Worker Body Size for the Honey Bee Recruitment System. J. Insect Behav. 1989, 2, 91–103. [Google Scholar] [CrossRef]
- Roulston, T.a.H.; Cane, J.H. The Effect of Diet Breadth and Nesting Ecology on Body Size Variation in Bees (Apiformes). J. Kans. Entomol. Soc. 2000, 73, 129–142. [Google Scholar]
- Milne, C.P. The Need for Using Laboratory Tests in Breeding Honeybees for Improved Honey Production. J. Apic. Res. 1985, 24, 237–242. [Google Scholar] [CrossRef]
- Oldroyd, B.; Rinderer, T.; Buco, S. Heritability of Morphological Characters Used to Distinguish European and Africanized Honeybees. Theor. Appl. Genet. 1991, 82, 499–504. [Google Scholar] [CrossRef]
- Mostajeran, M.; Edriss, M.A.; Basiri, M.R. Analysis of Colony and Morphological Characters in Honey Bees (Apis mellifera meda). Pak. J. Biol. Sci. 2006, 9, 2685–2688. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Collins, A.M.; Tucker, K.W. Honey Production and Underlying Nectar Harvesting Activities of Africanized and European Honeybees. J. Apic. Res. 1985, 24, 161–167. [Google Scholar] [CrossRef]
- Inouye, D.W. The Effect of Proboscis and Corolla Tube Lengths on Patterns and Rates of Flower Visitation by Bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.; Cruz, C.D.; Campos, L.A.d.O.; Regazzi, A.J. Correlation between Honey Production and Some Morphological Traits in Africanized Honey Bees (Apis melifera). Ciência Rural 2002, 32, 869–872. [Google Scholar] [CrossRef]
- Rahimi, A.; Hasheminasab, H.; Ezati, N. Predicting Honey Production Based on Morphological Characteristics of Honey Bee (Apis mellifera L.) Using Multiple Regression Model. Ecol. Environ. Conserv. 2015, 21, 29–33. [Google Scholar]
- Zeng, Z. Analysis of the Correlation between Morphological Indicators and Bee Products Yield of Worker Honey Bees. Apic. China 1992, 34, 23. (In Chinese) [Google Scholar]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA; London, UK, 1987. [Google Scholar]
- Liu, Y. The Relationship between the Morphological Characteristics of Chinese Honey Bees and the Productivity of Bee Colonies. Apic. China 1964, 13, 133–137. (In Chinese) [Google Scholar]
- Huang, W.; Yang, G.; Chen, S. Preliminary Study on the Biological Characteristics of Chinese Honey Bees. Sci. Agric. Sin. 1963, 13, 43–44. (In Chinese) [Google Scholar]
- Seeley, T.D. Honeybee Ecology: A Study of Adaptation in Social Life; Edited by Monographs in Behavior and Ecology; Princeton University Press: Princeton, NJ, USA, 1985. [Google Scholar]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- He, Z. Experiment on Adding Italian Honey Bee Wax Comb Foundation to Chinese Honey Bee Colonies. Apic. Mon. 1931, 2, 16–21. (In Chinese) [Google Scholar]
- Dai, G. Providing Italian Worker Bee Combs to Chinese Honey Bee Colonies Results in Better Egg Laying Effects for Chinese Honey Bee Queens. Apic. China 1978, 20, 13. (In Chinese) [Google Scholar]
- Xu, C. Improving the Technology of Chinese Honey Bees: Chinese Honey Bee Colonies Use Italian Worker Bee Combs to Lay Eggs. J. Bee 2010, 30, 18. (In Chinese) [Google Scholar]
- Yang, W. The Use of Italian Worker Bee Combs for Reproduction in Chinese Honey Bee Colony Yields Good Results. J. Bee 1987, 9, 35. (In Chinese) [Google Scholar]
- Zhang, Q. Study on the Use of Italian Worker Bee Wax Comb Foundation in Chinese Bee Colonies. Apic. Sci. Technol. 2000, 15, 12. (In Chinese) [Google Scholar]
- Liao, D. Rearing Chinese Worker Bees in Italian Worker Cells Can Increase Their Body Size. J. Bee 2005, 27, 30. (In Chinese) [Google Scholar]
- Li, S. It Is Feasible to Provide a New Queen of Chinese Honey Bees with a Italian Worker Bee Comb for Egg Laying. J. Bee 2011, 31, 4. (In Chinese) [Google Scholar]
- Wang, Z.; Li, W. A Way to Improve the Economic Efficiency of Chinese Honey Bee Production: Preliminary Report on the Experiment of Using Italian Worker Bee Combs to Produce Chinese Drone Bee Pupae. J. Bee 1988, 10, 6–7. (In Chinese) [Google Scholar]
- Yang, W. The Use of Italian Worker Bee Combs in the Reproduction of Chinese Bee Colonies Can Be Resistant to the Wax Worms. J. Bee 1984, 6, 33. (In Chinese) [Google Scholar]
- Harbo, J.R. Effect of Population Size on Brood Production, Worker Survival and Honey Gain in Colonies of Honeybees. J. Apic. Res. 1986, 25, 22–29. [Google Scholar] [CrossRef]
- Chole, H.; Woodard, S.H.; Bloch, G. Body Size Variation in Bees: Regulation, Mechanisms, and Relationship to Social Organization. Curr. Opin. Insect Sci. 2019, 35, 77–87. [Google Scholar] [CrossRef]
- Vlasov, V.H. Testing Foundation with Enlarged Cells. Pchelovodstvo 1960, 37, 15–17. [Google Scholar]
- Constantin, A. Erfolg Der Anwendung Von Waben Mit Vergrosserten Zellen in Der Den Bedingungen Der Sozialistischen Republik Rumanien. In Proceedings of the XX Congreso Internacional Jubileo de los Apicultores (XX Jubilee Apimondia Congress), Bucharest, Romania, 26–31 August 1965; Georgescu, D.I.D., Ed.; pp. 742–744. [Google Scholar]
- Jagannadham, B.; Goyal, N.P. Morphological and Behavioural Characteristics of Honeybees. Workers Reared in Combs with Larger Cells. In Proceedings of the Second International Conference on Apiculture in Tropical Climates, New Delhi, India, 29 February–4 March 1980; pp. 238–253. [Google Scholar]
- Couvillon, M.J.; Schürch, R.; Ratnieks, F.L.W. Waggle Dance Distances as Integrative Indicators of Seasonal Foraging Challenges. PLoS ONE 2014, 9, e93495. [Google Scholar] [CrossRef]
- Araújo, E.D.; Costa, M.; Chaud-Netto, J.; Fowler, H.G. Body Size and Flight Distance in Stingless Bees (Hymenoptera: Meliponini): Inference of Flight Range and Possible Ecological Implications. Braz. J. Biol. 2004, 64, 563–568. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee Foraging Ranges and Their Relationship to Body Size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Neto, B.; Contrera, F.A.L.; Castro, M.S.; Nieh, J.C. Long Distance Foraging and Recruitment by a Stingless Bee, Melipona Mandacaia. Apidologie 2009, 40, 472–480. [Google Scholar] [CrossRef]
- Waddington, K.D.; Herbst, L.H. Body Size and the Functional Length of the Proboscis of Honey Bees. Fla. Entomol. 1987, 70, 124–128. [Google Scholar] [CrossRef]
- Sauthier, R.; I’Anson Price, R.; Grüter, C. Worker Size in Honeybees and Its Relationship with Season and Foraging Distance. Apidologie 2017, 48, 234–246. [Google Scholar] [CrossRef]
- Osterlund, E. The Cell--Heart of the Hive. Am. Bee J. 2001, 141, 568–572. [Google Scholar]
- Gontarski, H. Über Phänotypische Beeinflussbarkeit Der Arbeiterinnen Von Apis mellifica Durch Die Brutzellengrösse. Z. Morphol. Und Okol. Tiere 1935, 29, 455–471. [Google Scholar] [CrossRef]
- Buchner, R. Beeinflussung Der Grösse Der Arbeitsbiene Durch Raum- Und Nahrungsmangel Während Der Larvenzeit. Wilhelm Roux’ Arch. Entwicklungsmechanik Org. 1953, 146, 544–579. [Google Scholar] [CrossRef] [PubMed]
- Gontarski, H. Zur Brutbiologie Der Honigbiene. Z. Bienenforsch. 1953, 2, 7–10. [Google Scholar]
- Kulzhinskaya, K.P. The Role of the Food Factor in the Growth of Bees. Pchelovodstvo 1955, 32, 36–37. [Google Scholar]
- Gontarski, H. Mikrochemische Futtersaftuntersuchungen Und Die Frage Der Königinnenentstehung. Hessische Biene 1949, 85, 89–92. [Google Scholar]
- Nold, R. Ueber Den Einfluss Des Futtersaftes Auf Die Sich Entwickelnde Königin. Hessische Biene 1949, 86, 171–172. [Google Scholar]
- Haydak, M.H. Do the Nurse Honey Bees Recognize the Sex of the Larvae? Science 1958, 127, 1113. [Google Scholar] [CrossRef] [PubMed]
- Komarov, P.M. Influence of the Age of the Larvae and of the Number of Generations Upon the Development of the Queen’s Sex Organs. Bee World 1934, 15, 81–84. [Google Scholar] [CrossRef]
- Melampy, R.M.; Willis, E.R. Respiratory Metabolism During Larval and Pupal Development of the Female Honeybee (Apis mellifica L.). Physiol. Zool. 1939, 12, 302–311. [Google Scholar] [CrossRef]
- Wolschin, F.; Shpigler, H.; Amdam, G.V.; Bloch, G. Size-Related Variation in Protein Abundance in the Brain and Abdominal Tissue of Bumble Bee Workers. Insect Mol. Biol. 2012, 21, 319–325. [Google Scholar] [CrossRef]
- Porath, H.T.; Hazan, E.; Shpigler, H.; Cohen, M.; Band, M.; Ben-Shahar, Y.; Levanon, E.Y.; Eisenberg, E.; Bloch, G. Rna Editing Is Abundant and Correlates with Task Performance in a Social Bumblebee. Nat. Commun. 2019, 10, 1605. [Google Scholar] [CrossRef]


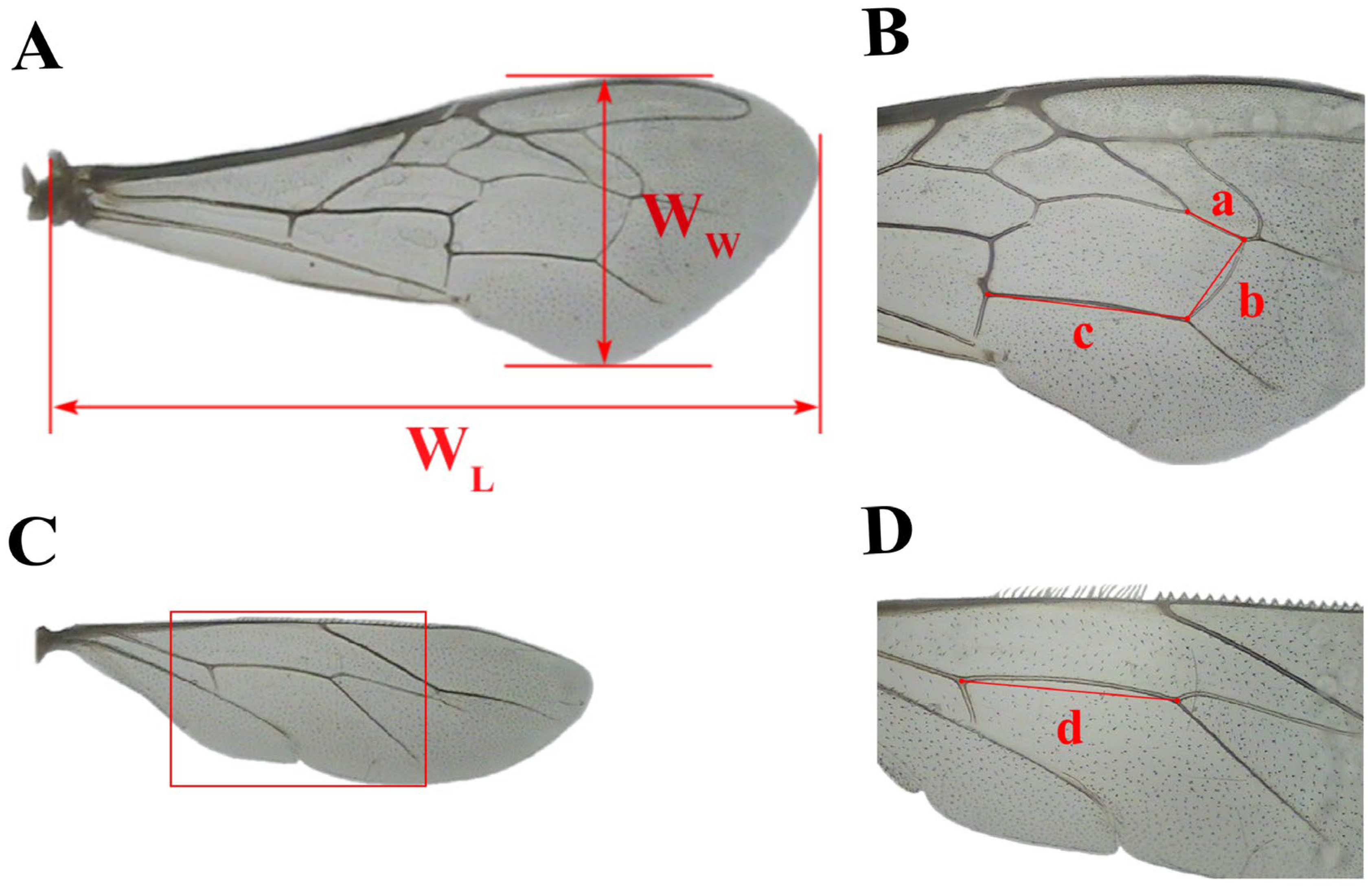
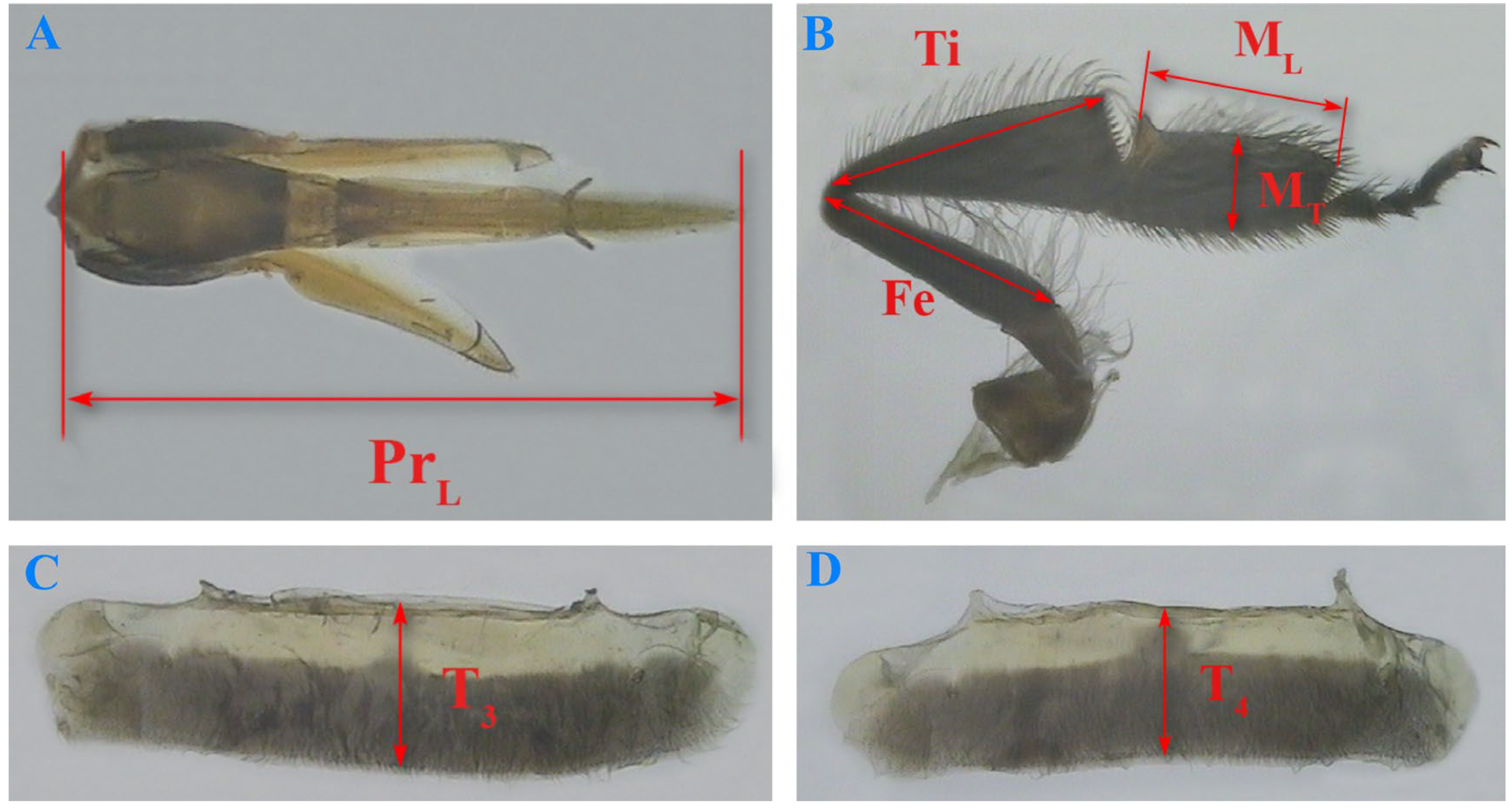



| Group | Index | Mean of Differences (Left–Right) | SD of Differences | 95% Confidence Interval | t | df | p-Value | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Control | Forewing length | −0.0095 | 0.0559 | −0.0171 to −0.0019 | 2.4620 | 209 | 0.0146 | 0.1699 |
| Forewing width | −0.0031 | 0.0566 | −0.0108 to 0.0046 | 0.7956 | 209 | 0.4272 | 0.0549 | |
| Linear length of vein a | −0.0009 | 0.0314 | −0.0052 to 0.0034 | 0.4271 | 209 | 0.6698 | 0.0295 | |
| Linear length of vein b | 0.0032 | 0.0324 | −0.0012 to 0.0076 | 1.4130 | 209 | 0.1593 | 0.0975 | |
| Linear length of vein c | −0.0022 | 0.0355 | −0.0070 to 0.0026 | 0.9099 | 209 | 0.3639 | 0.0628 | |
| Linear length of vein d | −0.0005 | 0.0135 | −0.0024 to 0.0013 | 0.5778 | 209 | 0.5640 | 0.0399 | |
| Hind leg femur length | −0.0020 | 0.0322 | −0.0063 to 0.0024 | 0.8880 | 209 | 0.3756 | 0.0613 | |
| Hind leg tibia length | −0.0076 | 0.0565 | −0.0152 to 0.0001 | 1.9370 | 209 | 0.0541 | 0.1337 | |
| Hind leg metatarsus length | 0.0067 | 0.0561 | −0.0010 to 0.0143 | 1.7230 | 209 | 0.0863 | 0.1189 | |
| Hind leg metatarsus width | −0.0019 | 0.0265 | −0.0055 to 0.0017 | 1.0400 | 209 | 0.2997 | 0.0717 | |
| Treated | Forewing length | 0.0008 | 0.0306 | −0.0034 to 0.0049 | 0.3641 | 209 | 0.7161 | 0.0251 |
| Forewing width | −0.0071 | 0.0428 | −0.0129 to −0.0013 | 2.4030 | 209 | 0.0171 | 0.1658 | |
| Linear length of vein a | 0.0017 | 0.0224 | −0.0013 to 0.0048 | 1.1300 | 209 | 0.2596 | 0.0780 | |
| Linear length of vein b | −0.0004 | 0.0332 | −0.0050 to 0.0041 | 0.1908 | 209 | 0.8489 | 0.0132 | |
| Linear length of vein c | −0.0006 | 0.0197 | −0.0033 to 0.0021 | 0.4450 | 209 | 0.6568 | 0.0307 | |
| Linear length of vein d | −0.0012 | 0.0090 | −0.0024 to 0.0000 | 1.9210 | 209 | 0.0560 | 0.1326 | |
| Hind leg femur length | −0.0049 | 0.0206 | −0.0078 to −0.0021 | 3.4740 | 209 | 0.0006 | 0.2397 | |
| Hind leg tibia length | −0.0083 | 0.0379 | −0.0135 to −0.0032 | 3.1940 | 209 | 0.0016 | 0.2204 | |
| Hind leg metatarsus length | −0.0019 | 0.0374 | −0.0070 to 0.0032 | 0.7429 | 209 | 0.4584 | 0.0513 | |
| Hind leg metatarsus width | −0.0068 | 0.0258 | −0.0104 to −0.0033 | 3.8370 | 209 | 0.0002 | 0.2648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Li, H.; Wu, P.; Yue, D.; Guo, Y.; Zhao, W.; Dong, K. Morphological Comparisons of Adult Worker Bees Developed in Chinese and Italian Honey Bee Combs. Insects 2025, 16, 104. https://doi.org/10.3390/insects16010104
Yang S, Li H, Wu P, Yue D, Guo Y, Zhao W, Dong K. Morphological Comparisons of Adult Worker Bees Developed in Chinese and Italian Honey Bee Combs. Insects. 2025; 16(1):104. https://doi.org/10.3390/insects16010104
Chicago/Turabian StyleYang, Shunhua, Hui Li, Pingqing Wu, Dan Yue, Yulong Guo, Wenzheng Zhao, and Kun Dong. 2025. "Morphological Comparisons of Adult Worker Bees Developed in Chinese and Italian Honey Bee Combs" Insects 16, no. 1: 104. https://doi.org/10.3390/insects16010104
APA StyleYang, S., Li, H., Wu, P., Yue, D., Guo, Y., Zhao, W., & Dong, K. (2025). Morphological Comparisons of Adult Worker Bees Developed in Chinese and Italian Honey Bee Combs. Insects, 16(1), 104. https://doi.org/10.3390/insects16010104







