Volatiles of the Predator Xylocoris flavipes Recognized by Its Prey Tribolium castaneum (Herbst) and Oryzaephilus surinamensis (Linne) as Escape Signals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Odor Choice Selection Bioassay
2.3. Volatile Analyses of X. flavipes
2.4. Olfactory Preference of Pests for Chemicals
2.5. Electroantennogram Recordings of T. castaneum and O. surinamensis to Chemicals
2.6. Repellency Evaluation of Linalool, Geraniol, and Their Mixtures

2.7. Data Analyses
3. Results
3.1. Odor Choice Selection Bioassay
3.2. Volatile Analyses of X. flavipes
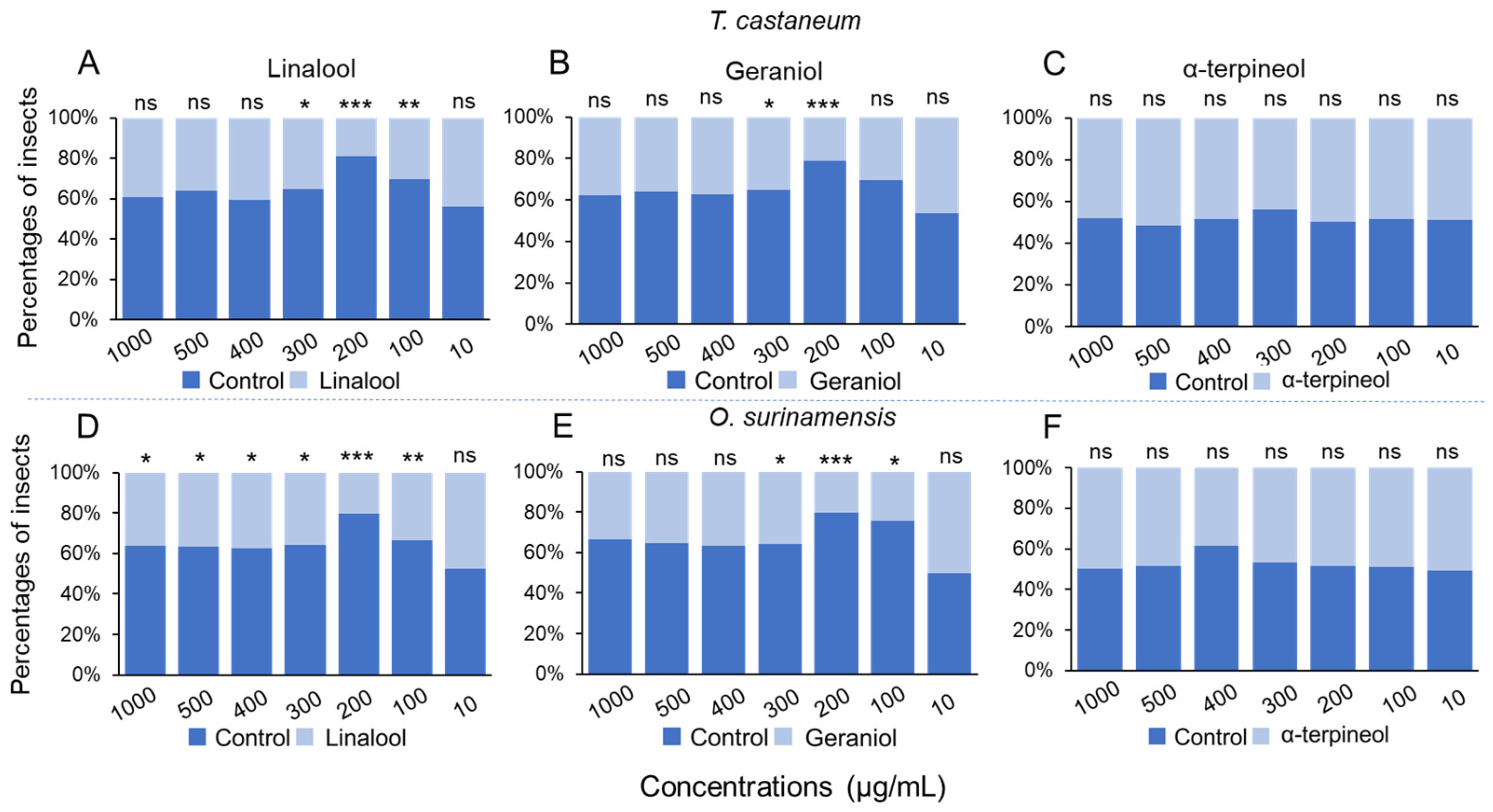
3.3. Olfactory Preference of Pests for Chemicals
3.4. Electroantennographic Responses of T. castaneum and O. surinamensis to Xylocoris flavipes Volatiles
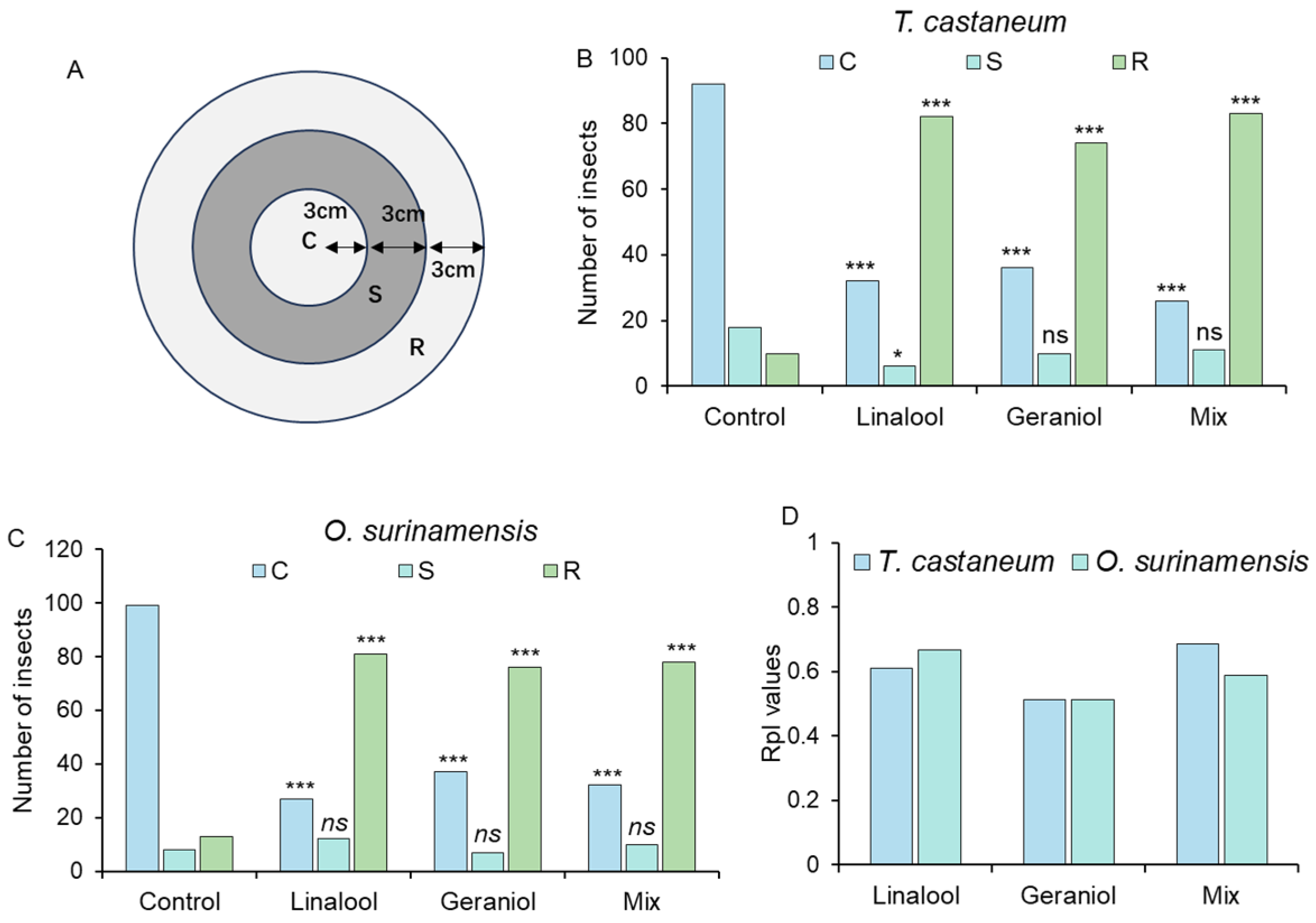
3.5. Repellency Evaluation of Linalool, Geraniol, and Their Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, J.A.; Vendl, T.; Aulicky, R.; Stejskal, V. Frass produced by the primary pest Rhyzopertha dominica supports the population growth of the secondary stored product pests Oryzaephilus surinamensis, Tribolium castaneum, and T. confusum. Bull. Entomol. Res. 2021, 111, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.F.; Athanassiou, C.G.; Hagstrum, D.W.; Zhu, K.Y. A model insect for fundamental and applied research. Annu. Rev. Entomol. 2022, 67, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Collins, P.J.; Pavic, H.; Kopittke, R.A. Inhibition of egg development by phosphine in the cosmopolitan pest of stored products Liposcelis bostrychophila (Psocoptera: Liposcelididae). Pest Manag. Sci. 2003, 59, 1191–1196. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V.; Frydova, B.; Athanassiou, C. Evaluation of phosphine resistance in populations of Sitophilus oryzae, Oryzaephilus surinamensis and Rhyzopertha dominica in the Czech Republic. Insects 2022, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Jeong, G.; Kim, H.K.; Kim, B.S.; Yang, J.O.; Koo, H.N.; Kim, G.H. Fumigation activity against phosphine-resistant Tribolium castaneum (Coleoptera: Tenebrionidae) using carbonyl sulfide. Insects 2020, 11, 750. [Google Scholar] [CrossRef]
- Shin, H.Y.; An, J.S.; Lee, J.M.; You, S.G.; Shin, I.S. Phosphine residues and physicochemical stability of Hwangtae after fumigation. Food Sci. Biotechnol. 2021, 30, 1025–1031. [Google Scholar] [CrossRef]
- Zhao, Y.; Abbar, S.; Phillips, T.W.; Schilling, M.W. Phosphine fumigation and residues in dry-cured ham in commercial applications. Meat Sci. 2015, 107, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.G.; Opit, G.P.; Hosoda, E. Phosphine resistance in adult and immature life stages of Tribolium castaneum (Coleoptera: Tenebrionidae) and Plodia interpunctella (Lepidoptera: Pyralidae) populations in California. J. Econ. Entomol. 2016, 109, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.G.; Opit, G.P.; Konemann, C.; Shakya, K.; Hosoda, E. Phosphine resistance in saw-toothed grain beetle, Oryzaephilus surinamensis in the United States. J. Stored Prod. Res. 2020, 89, 101690. [Google Scholar] [CrossRef]
- van Veen, F.J.F. Plant-modified trophic interactions. Current Opin. Insect Sci. 2015, 8, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; Daniel Hare, J. Learned and naïve natural enemy responses and the interpretation of volatile organic compounds as cues or signals. New Phytol. 2009, 184, 768–782. [Google Scholar] [CrossRef]
- Meiners, T. Chemical ecology and evolution of plant–insect interactions: A multitrophic perspective. Curr. Opin. Insect Sci. 2015, 8, 22–28. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant–insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Rusman, Q.; Morrison, W.R., III; Magalhães, D.M.; Dowell, J.A.; Ngumbi, E.; Osei-Owusu, J.; Kansman, J.; Gaffke, A.; Pagadala Damodaram, K.J.; et al. Deciphering plant-insect-microorganism signals for sustainable crop production. Biomolecules 2023, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. A review on natural phenylbutanoid attractants: Occurrence, distribution, and role in nature, especially in relation to Dacini fruit fly behavior and pollination. J. Chem. Ecol. 2024, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Chen, M.S.; Lu, Y.J. Volatiles potentially useful to be used as biomarkers for monitoring the khapra beetle Trogoderma granarium for quarantine. J. Stored Prod. Res. 2024, 109, 102430. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhao, C.H.; Wang, C.Z. Comparative study of sex pheromone composition and biosynthesis in Helicoverpa armigera, H. assulta and their hybrid. Insect Biochem. Mol. Biol. 2005, 35, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Vanderplanck, M.; Verheggen, F.J. Comparison of the sex pheromone composition of Harmonia axyridis originating from native and invaded areas. Insects 2019, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Zou, Y.F.; Millar, J.G.; Hanks, L.M. Complex blends of synthetic pheromones are effective multi-species attractants for longhorned beetles (Coleoptera: Cerambycidae). J. Econ. Entomol. 2020, 113, 2269–2275. [Google Scholar] [CrossRef]
- Yi, C.Q.; Teng, D.; Xie, J.X.; Tang, H.Y.; Zhao, D.Y.; Liu, X.X.; Liu, T.H.; Ding, W.; Khashaveh, A.; Zhang, Y.J. Volatiles from cotton aphid (Aphis gossypii) infested plants attract the natural enemy. Front Plant. Sci. 2023, 14, 1326630. [Google Scholar] [CrossRef] [PubMed]
- Hermann, S.L.; Thaler, J.S. Prey perception of predation risk: Volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 2014, 176, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Preisser, E.L.; Bolnick, D.I. The many faces of fear: Comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS ONE 2008, 3, e2465. [Google Scholar] [CrossRef] [PubMed]
- Hermann, S.L.; Thaler, J.S. The effect of predator presence on the behavioral sequence from host selection to reproduction in an invulnerable stage of insect prey. Oecologia 2018, 188, 945–952. [Google Scholar] [CrossRef]
- Wickwar, D.M.; Ramirez, R.A. Predatory arthropods and cues associated with predator presence elicit strong non-consumptive effects on bluegrass billbugs. Entomol. Exp. Appl. 2023, 171, 691–703. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhang, S.; Cao, S.; Jacquin-Joly, E.; Zhou, Q.; Liu, Y.; Wang, G.R. An odorant receptor mediates the avoidance of Plutella xylostella against parasitoid. BMC Biol. 2024, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, B.E.; Phillips, T.W. Functional response of Xylocoris flavipes (Hemiptera: Anthocoridae)-effects of prey species and habitat. Environ. Entomol. 2001, 30, 617–624. [Google Scholar] [CrossRef]
- Lu, S.H.; Zhang, L.F.; Lu, Y.J.; Chen, M.S.; Wang, Z.Y. Host volatiles potentially drive two evolutionarily related weevils to select different grains. Insects 2024, 15, 300. [Google Scholar] [CrossRef]
- Pistillo, O.M.; D’Isita, I.; Germinara, G.S. Olfactory response of the spotted asparagus beetle, Crioceris duodecimpunctata (L.) to host plant volatiles. J. Chem. Ecol. 2022, 48, 41–50. [Google Scholar] [CrossRef]
- Guo, H.; Mo, B.T.; Li, G.C.; Li, Z.L.; Huang, L.Q.; Sun, Y.L.; Dong, J.F.; Smith, D.P.; Wang, C.Z. Sex pheromone communication in an insect parasitoid, Campoletis chlorideae Uchida. Proc. Natl. Acad. Sci. USA 2022, 119, e2215442119. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- van Tol, R.W.; Diaz Rodriguez, C.M.; de Bruin, A.; Yang, D.; Taparia, T.; Griepink, F.C. Visual attraction of the European tarnished plant bug Lygus rugulipennis (Hemiptera: Miridae) to a water trap with LED light in chrysanthemum greenhouses and olfactory attraction to novel compounds in Y-tube tests. Pest Manag. Sci. 2022, 78, 2523–2533. [Google Scholar] [CrossRef]
- Wu, H.H.; Liu, J.Y.; Liu, Y.M.; Abbas, M.; Zhang, Y.C.; Kong, W.A.; Zhao, F.; Zhang, X.Y.; Zhang, J.Z. CYP6FD5, an antenna-specific P450 gene, is potentially involved in the host plant recognition in Locusta migratoria. Pestic. Biochem. Phys. 2022, 188, 105255. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Y.; Liu, Y.P.; Wang, G.R.; Zhou, Q. Identification and functional analysis of a chemosensory protein from Bactrocera minax (Diptera: Tephritidae). Pest Manag. Sci. 2022, 78, 3479–3488. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Z.; Zhou, A.M.; Chen, J. Olfactory and behavioral responses to acetate esters in red imported fire ant, Solenopsis invicta. Pest Manag. Sci. 2021, 77, 1371–1382. [Google Scholar] [CrossRef]
- Snyder, W.E.; Wise, D.H. Antipredator behavior of spotted cucumber beetles (Coleoptera: Chrysomelidae) in response to predators that pose varying risks. Environ. Entomol. 2000, 29, 35–42. [Google Scholar] [CrossRef]
- Silberbush, A.; Markman, S.; Lewinsohn, E.; Bar, E.; Cohen, J.E.; Blaustein, L. Predator-released hydrocarbons repel oviposition by a mosquito. Ecol. Lett. 2010, 13, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jacquin-Joly, E.; Wang, G.R. The Role of (E)-β-Farnesene in tritrophic interactions: Biosynthesis, Chemoreception, and Evolution. Annu. Rev. Entomol. 2025, 70, 313–335. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Fu, Z.; Lee, B.W.; Crowder, D.W. Insect alarm pheromones in response to predators: Ecological trade-offs and molecular mechanisms. Insect Biochem. Mol. Biol. 2021, 128, 103514. [Google Scholar] [CrossRef] [PubMed]
- Hawlena, D.; Schmitz, O.J. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl. Acad. Sci. USA 2010, 107, 15503–15507. [Google Scholar] [CrossRef]
- Wirsing, A.J.; Heithaus, M.R.; Brown, J.S.; Kotler, B.P.; Schmitz, O.J. The context dependence of non-consumptive predator effects. Ecol. Lett. 2021, 24, 113–129. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.R.M.; Ricci-Júnior, E. An approach to natural insect repellent formulations: From basic research to technological development. Acta Trop. 2020, 212, 105419. [Google Scholar] [CrossRef] [PubMed]
- Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Neurotoxic effects of linalool and β-pinene on Tribolium castaneum Herbst. Molecules 2017, 22, 2052. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiao, X.M.; Haji, D.; Zhou, T.H.; Liu, Z.H.; Whiteman, N.K.; Huang, J. Convergent resistance to GABA receptor neurotoxins through plant-insect coevolution. Nat. Ecol. Evol. 2023, 7, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Christie, N.; Acosta, J.J.; Mphahlele, M.M.; Payn, K.G.; Myburg, A.A.; Külheim, C. Terpenes associated with resistance against the gall wasp, Leptocybe invasa, in Eucalyptus grandis. Plant Cell Environ. 2018, 41, 1840–1851. [Google Scholar] [CrossRef]
- Sharma, E.; Anand, G.; Kapoor, R. Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann. Bot. 2017, 119, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Liu, G.; Xuan, N.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Comprehensive history of CSP genes: Evolution, phylogenetic distribution and functions. Genes 2020, 11, 413. [Google Scholar] [CrossRef]



| No. | Retention Time/Min | Retention Index | Compound | Relative Content/% |
|---|---|---|---|---|
| 1 | 6.45 | 1082 | linalool | 67.55 ± 7.33 |
| 2 | 8.28 | 1079 | α-terpineol | 14.86 ± 4.37 |
| 3 | 8.72 | 1125 | geraniol | 10.43 ± 3.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Yang, L.; Wu, Z.; Chen, M.; Lu, Y. Volatiles of the Predator Xylocoris flavipes Recognized by Its Prey Tribolium castaneum (Herbst) and Oryzaephilus surinamensis (Linne) as Escape Signals. Insects 2025, 16, 31. https://doi.org/10.3390/insects16010031
Lu S, Yang L, Wu Z, Chen M, Lu Y. Volatiles of the Predator Xylocoris flavipes Recognized by Its Prey Tribolium castaneum (Herbst) and Oryzaephilus surinamensis (Linne) as Escape Signals. Insects. 2025; 16(1):31. https://doi.org/10.3390/insects16010031
Chicago/Turabian StyleLu, Shaohua, Li Yang, Zonglin Wu, Mingshun Chen, and Yujie Lu. 2025. "Volatiles of the Predator Xylocoris flavipes Recognized by Its Prey Tribolium castaneum (Herbst) and Oryzaephilus surinamensis (Linne) as Escape Signals" Insects 16, no. 1: 31. https://doi.org/10.3390/insects16010031
APA StyleLu, S., Yang, L., Wu, Z., Chen, M., & Lu, Y. (2025). Volatiles of the Predator Xylocoris flavipes Recognized by Its Prey Tribolium castaneum (Herbst) and Oryzaephilus surinamensis (Linne) as Escape Signals. Insects, 16(1), 31. https://doi.org/10.3390/insects16010031







