A Field Trial to Demonstrate the Potential of a Vitamin B Diet Supplement in Reducing Oxidative Stress and Improving Hygienic and Grooming Behaviors in Honey Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Gene Expression Analyses
2.3. Determination of Oxidative Stress Parameters
2.4. Assessment of Hygienic and Grooming Behavior
2.5. Statistical Analysis
3. Results
3.1. Comparison Between Groups
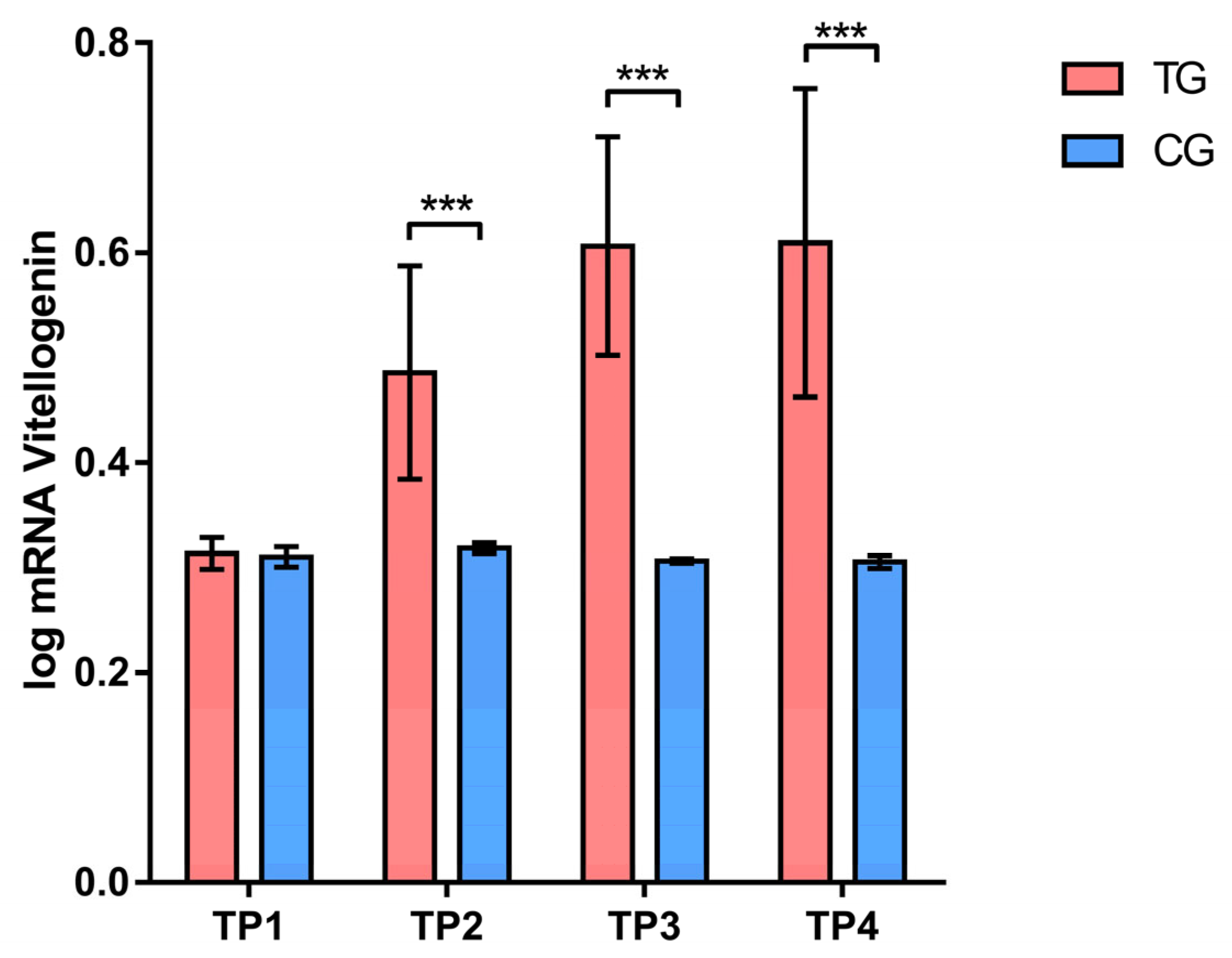
3.1.1. Gene Expression
3.1.2. Oxidative Stress Parameters
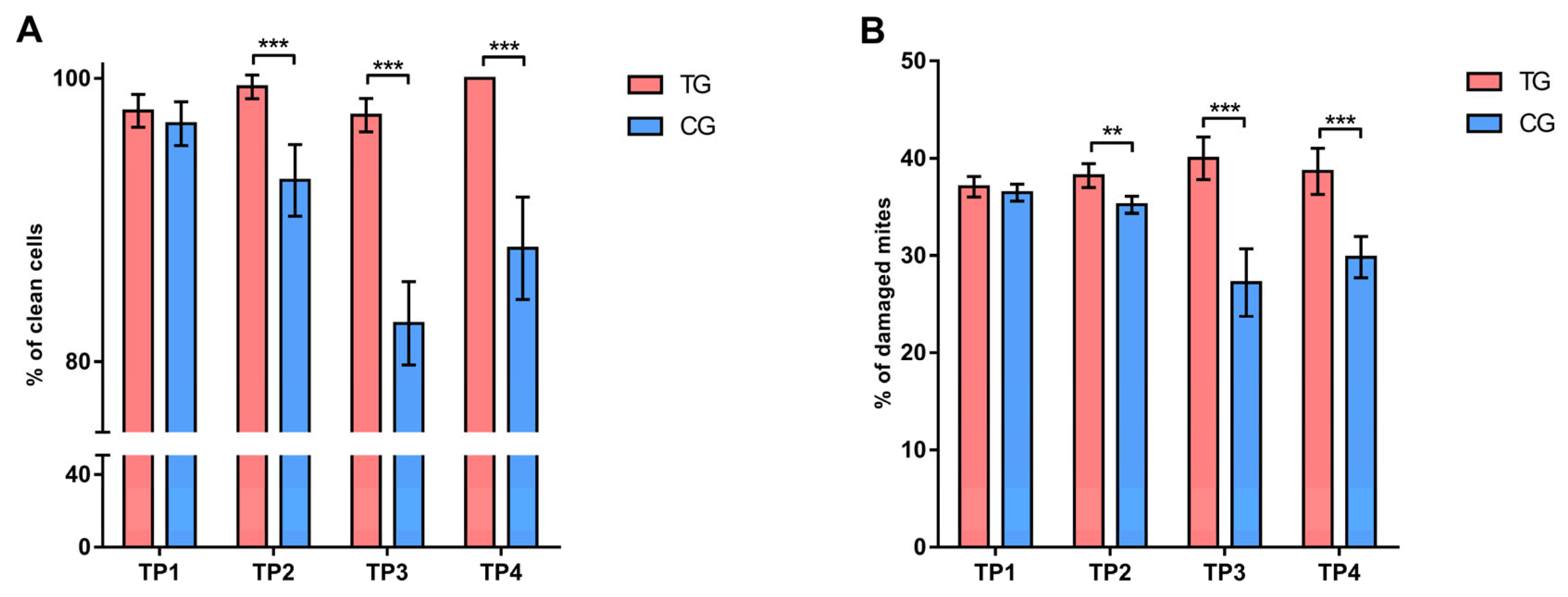
3.1.3. Hygienic and Grooming Behavior
3.2. Comparison Within Groups
3.2.1. Gene Expression
3.2.2. Oxidative Stress Parameters
3.2.3. Hygienic and Grooming Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014, 4, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Pollen nutrition affects honey bee stress resistance. Terr. Arthropod. Rev. 2012, 5, 175–189. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the availability of pollen resources affect honey bee health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef]
- Traynor, K.S.; Rennich, K.; Forsgren, E.; Rose, R.; Pettis, J.; Kunkel, G.; Madella, S.; Evans, J.; Lopez, D.; Vanengelsdorp, D. Multiyear survey targeting disease incidence in US honey bees. Apidologie 2016, 47, 325–347. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.; Vejnović, B.; Stevanović, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet. Beograd 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Carrillo-Tripp, J.; Miller, W.A.; Bonning, B.C.; Toth, A.L. Intensively cultivated landscape and Varroa mite infestation are associated with reduced honey bee nutritional state. PLoS ONE 2016, 11, e0153531. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef]
- Lopez-Uribe, M.M.; Ricigliano, V.A.; Simone-Finstrom, M. Defining pollinator health: A holistic approach based on ecological, genetic, and physiological factors. Annu. Rev. Anim. Biosci. 2020, 8, 269–294. [Google Scholar] [CrossRef] [PubMed]
- Sponsler, D.B.; Johnson, R.M. Honey bee success predicted by landscape composition in Ohio, USA. Peer J. 2015, 3, e838. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.; Wagner, D.; Danforth, B.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-associated phenological advances in bee pollinators and beepollinated plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20645–20649. [Google Scholar] [CrossRef]
- Settele, J.; Bishop, J.; Potts, S. Climate change impacts on pollination. Nat. Plants 2016, 2, 16092. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity, and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, M.; Vollmann, J.J.; Yanez, O.; Chejanovsky, N.; Crailsheim, K.; Neumann, P. Protein nutrition governs within-host race of honey bee pathogens. Sci. Rep. 2017, 7, 14988. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Brown, M.J.; Stevenson, P.C. The role of disease in bee foraging ecology. Curr. Opin. Insect Sci. 2017, 21, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Vodovnik, C.; Borshagovski, A.M.; Hakala, S.M.; Leponiemi, M.; Freitak, D. Coeffects of diet and neonicotinoid exposure on honeybee mobility and food choice. Apidologie 2021, 52, 658–667. [Google Scholar] [CrossRef]
- Mortensen, A.N.; Jack, C.J.; Bustamante, T.A.; Schmehl, D.R.; Ellis, J.D. Effects of supplemental pollen feeding on honey bee (hymenoptera: Apidae) colony strength and Nosema spp. infection. J. Econ. Entomol. 2019, 112, 60–66. [Google Scholar] [CrossRef]
- Lamontagne-Drolet, M.; Samson-Robert, O.; Giovenazzo, P.; Fournier, V. The impacts of two protein supplements on commercial honey bee (Apis mellifera L.) colonies. J. Apic. Res. 2019, 58, 800–813. [Google Scholar] [CrossRef]
- Goodrich, B.K. Do more bees imply higher fees? Honey bee colony strength as a determinant of almond pollination fees. Food Policy 2019, 83, 150–160. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Ellis, J.D. Reviewing the efficacy of pollen substitutes as a management tool for improving the health and productivity of western honey bee (Apis mellifera) colonies. Front. Sustain. Food. Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus mushroom extract on honey bees infected with Nosema ceranae. Insects 2021, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of fumagillin and Agaricus blazei mushroom extract to reduce Nosema ceranae in honey bees. Insects 2021, 12, 282. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Ilic, T.; Stevanovic, J.; Stanimirovic, Z. Effects of plant-based supplement on oxidative stress of honey bees (Apis mellifera) infected with Nosema ceranae. Animals 2023, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Jovanovic, N.M.; Dominikovic, N.; Lakic, N.; Ćosić, M.; Stevanovic, J.; Stanimirovic, Z. Potential of wormwood and oak bark-based supplement in health improvement of Nosema ceranae-infected honey bees. Animals 2024, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Jelisić, S.; Vejnović, B.; Niketić, M.; Stevanović, J. Diet supplementation helps honey bee colonies in combat infections by enhancing their hygienic behaviour. Acta Vet. Beograd 2022, 72, 145–166. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social Immunity. Curr. Biol. 2007, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Pannebakker, B.; Gengler, N.; Nguyen, B.K.; Francis, F. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): A review. J. Apic. Res. 2017, 56, 366–375. [Google Scholar] [CrossRef]
- Spivak, M.; Danka, R.G. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 2020, 52, 1–16. [Google Scholar] [CrossRef]
- Stroeymeyt, N.; Casillas-Perez, B.; Cremer, S. Organisational immunity in social insects. Curr. Opin. Insect Sci. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M. Social immunity and the superorganism: Behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World 2017, 94, 21–29. [Google Scholar] [CrossRef]
- Cremer, S.; Pull, C.D.; Furst, M.A. Social immunity: Emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 2018, 63, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.; Pincheira-Donoso, D.; Thorogood, R. Defences against brood parasites from a social immunity perspective. Philos. Trans. R. Soc. B 2019, 374, 20180207. [Google Scholar] [CrossRef] [PubMed]
- Naug, D.; Camazine, S. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 2002, 215, 427–439. [Google Scholar] [CrossRef]
- Baracchi, D.; Cini, A. A socio-spatial combined approach confirms a highly compartmentalised structure in honeybees. Ethology 2014, 120, 1167–1176. [Google Scholar] [CrossRef]
- Lecocq, A.; Jensen, A.B.; Kryger, P.; Nieh, J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016, 6, 22042. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Fadda, A.; Turillazzi, S. Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J. Insect Physiol. 2012, 58, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Biganski, S.; Kurze, C.; Muller, M.Y.; Moritz, R.F.A. Social response of healthy honeybees towards Nosema ceranae-infected workers: Care or kill? Apidologie 2018, 49, 325–334. [Google Scholar] [CrossRef]
- Rueppell, O.; Hayworth, M.K.; Ross, N.P. Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 2010, 23, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.S.; Fang, Y.; Xu, S.; Ge, L.; Nasr, M.E. The resistance mechanism of the Asian honey bee, Apis cerana Fabr, to an ectoparasitic mite Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Arathi, H.S.; Burns, I.; Spivak, M. Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of hygienic bees. Ethology 2000, 106, 365–379. [Google Scholar] [CrossRef]
- Bigio, G.; Schurch, R.; Ratnieks, F.L.W. Hygienic behavior in honey bees (Hymenoptera: Apidae): Effects of brood, food, and time of the year. J. Econ. Entomol. 2013, 106, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.J. Grooming by honey bees as a component of varroa resistant behavior. J. Apic. Res. 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Dadoun, N.; Nait-Mouloud, M.; Mohammedi, A.; Sadeddine Zennouche, O. Differences in grooming behavior between susceptible and resistant honey bee colonies after 13 years of natural selection. Apidologie 2020, 51, 793–801. [Google Scholar] [CrossRef]
- Russo, R.M.; Liendo, M.C.; Landi, L.; Pietronave, H.; Merke, J.; Fain, H.; Muntaabski, I.; Palacio, M.A.; Rodríguez, G.A.; Lanzavecchia, S.B.; et al. Grooming behavior in naturally Varroa-resistant Apis mellifera colonies from north-central Argentina. Front. Ecol. Evol. 2020, 8, 590281. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Emsen, B.; Unger, P.; Espinosa-Montaño, L.G.; Petukhova, T. Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J. Invertebr. Pathol. 2012, 110, 314–320. [Google Scholar] [CrossRef]
- Invernizzi, C.; Zefferino, I.; Santos, E.; Sánchez, L.; Mendoza, Y. Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J. Apic. Res. 2015, 54, 321–327. [Google Scholar] [CrossRef]
- Nganso, B.T.; Fombong, A.T.; Yusuf, A.A.; Pirk, C.W.; Stuhl, C.; Torto, B. Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 2017, 12, e0179329. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.; Al-Abbadi, A.A.; Khan, K.A.; Ghramh, H.A.; Ahmed, A.M.; Ansari, M.J. In vitro antagonistic potential of gut bacteria isolated from indigenous honey bee race of Saudi Arabia against Paenibacillus larvae. J. Apic. Res. 2020, 59, 825–833. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Ghamdi, M.S.; Ahmed, A.M.; Mohamed, A.S.A.; Shaker, G.H.; Ansari, M.J.; Dorrah, M.A.; Khan, K.A.; Ayaad, T.H. 2020. Immune investigation of the honeybee Apis mellifera jemenitica broods: A step toward production of a bee-derived antibiotic against the American foulbrood. Saudi J. Biol. Sci. 2020, 28, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.A.; Rodriguez, E.; Goncalves, L.; Bedascarrasbure, E.; Spivak, M. Hygienic behaviors of honey bees in response to brood experimentally pinkilled or infected with Ascosphaera apis. Apidologie 2010, 41, 602–612. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; de Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Muñoz, I.; Stevanovic, J.; Stanimirovic, Z.; De la Rúa, P. Genetic variation of Apis mellifera from Serbia inferred from mitochondrial analysis. J. Apic. Sci. 2012, 56, 59–69. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Stevanovic, J.; Cosic, M.; Stanimirovic, Z. Contact varroacidal efficacy of lithium citrate and its influence on viral loads, immune parameters and oxidative stress of honey bees in a field experiment. Front. Physiol. 2022, 13, 1000944. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, B.; Wang, Y.; Yang, Z.; Yang, W. Protein content in larval diet affects adult longevity and antioxidant gene expression in honey bee workers. Entomol. Exp. Appl. 2014, 151, 19–26. [Google Scholar] [CrossRef]
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1984; pp. 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J. Trauma Acute Care Surg. 1991, 31, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Stanimirović, Z.; Stevanović, J.; Mirilović, M.; Stojić, V. Heritability of hygienic behavior in grey honey bees (Apis mellifera carnica). Acta Vet. Beograd 2008, 58, 593–601. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Lakic, N.; Djelic, N.; Radovic, I. Stimulating effect of sugar dusting on honey bee grooming behaviour. Entomol. Exp. Appl. 2012, 143, 23–30. [Google Scholar] [CrossRef]
- Bienefeld, K.; Zautke, F.; Pronin, D.; Mazedd, A. Recording the proportion of damaged Varroa jacobsoni Oud. in the debris of honey bee colonies (Apis mellifera). Apidologie 1999, 30, 249–256. [Google Scholar] [CrossRef]
- Davis, A.R. Regular dorsal dimples on Varroa destructor—Damage symptoms or developmental origin? Apidologie 2009, 40, 151–162. [Google Scholar] [CrossRef]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking Measures of Colony and Individual Honey Bee Health to Survival among Apiaries Exposed to Varying Agricultural Land Use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Allier, F.; Decourtye, A.; Odoux, J.F.; Tamic, T.; Chabirand, M.; Delestra, E.; Decugis, F.; Le Conte, Y.; Henry, M. A “Landscape physiology” approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 2017, 7, 40568. [Google Scholar] [CrossRef] [PubMed]
- Corby-Harris, V.; Maes, P.; Anderson, K.E. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 2014, 9, e95056. [Google Scholar] [CrossRef]
- Azzouz-Olden, F.; Hunt, A.; DeGrandi-Hoffman, G. Transcriptional response of honey bee (Apis mellifera) to differential nutritional status and Nosema infection. BMC Genom. 2018, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US conservation reserve program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T.V.; Purać, J.; Orčić, S.; Kojić, D.; Vujanović, D.; Stanimirović, Z.; Gržetić, I.; Ilijević, K.; Šikoparija, B.; Blagojević, D.P. Environmental effects on superoxide dismutase and catalase activity and expression in honey bee. Arch. Insect Biochem. Physiol. 2015, 90, 181–194. [Google Scholar] [CrossRef]
- Spremo, J.; Purać, J.; Čelić, T.; Đorđievski, S.; Pihler, I.; Kojić, D.; Vukašinović, E. Assessment of oxidative status, detoxification capacity and immune responsiveness in honey bees with ageing. J. Comp. Physiol. A 2024, 298, 111735. [Google Scholar] [CrossRef]
- De Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Badiou-Beneteau, A.; Carvalho, S.M.; Brunet, J.L.; Carvalho, G.A.; Bulete, A.; Giroud, B.; Belzunces, L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 2012, 82, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Tchamitchian, S.; Bonnet, M.; Cousin, M.; Kretzschmar, A.; Brunet, J.L.; Le Conte, Y. Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Sci. Rep. 2016, 6, 31430. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Papežíková, I.; Palíková, M.; Syrová, E.; Zachová, A.; Somerlíková, K.; Kovácová, V.; Pecková, L. Effect of feeding honey bee (Apis mellifera Hymenoptera: Apidae) colonies with honey, sugar solution, inverted sugar, and wheat starch syrup on nosematosis prevalence and intensity. J. Econ. Entomol. 2020, 113, 26–33. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Stevanović, J.; Aleksić, N.; Stojić, V. Heritability of grooming behaviour in grey honey bees (Apis mellifera carnica). Acta Vet. Beograd 2010, 60, 313–323. [Google Scholar] [CrossRef]
- Colin, T.; Lim, M.Y.; Quarrell, S.R.; Allen, G.R.; Barron, A. B Effects of thymol on European honey bee hygienic behaviour. Apidoogie 2019, 50, 141–152. [Google Scholar] [CrossRef]
- Tison, L.; Riva, C.; Maisonnasse, A.; Kretzschmar, A.; Hervé, M.R.; Le Conte, Y.; Mondet, F. Seasonal and environmental variations infl uencing the Varroa Sensitive Hygiene trait in the honey bee. Entomol. Gen. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Stevanovic, J.; Cirkovic, D. Investigations of reproductive, productive, hygienic and grooming features of Syenichko-Peshterski honey bee ecotype. Apidologie 2003, 34, 487–488. [Google Scholar]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 2016, 6, 32108. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Stevanovic, J.; Cirkovic, D. Behavioural defenses of the honey bee ecotype from Sjenica—Pester against Varroa destructor. Acta Vet. Beograd 2005, 55, 69–82. [Google Scholar] [CrossRef]
- Currie, R.W.; Tahmasbi, G.H. The ability of high- and low grooming lines of honey bees to remove the parasitic mite Varroa destructor is affected by environmental conditions. Can. J. Zool. 2008, 86, 1059–1067. [Google Scholar] [CrossRef]
- Tahmasbi, G.H. The effect of temperature and humidity on grooming behaviour of honeybee, Apis mellifera (Hym.: Apidae) colonies against varroa mite, Varroa destructor (Acari: Varroidae). J. Entomol. Soc. Iran 2009, 28, 7–23. [Google Scholar]
- Zaitoun, S.T.; Al-Ghzawi, A. Monthly changes in the natural grooming response in workers of three honey bee subspecies against the bee parasitic mite Varroa destructor. Jordan J. Agric. Sci. 2009, 5, 207–217. [Google Scholar]
- Russo, R.M.; Pietronave, H.; Conte, C.A.; Liendo, M.C.; Basilio, A.; Lanzavecchia, S.B.; Scannapieco, A.C. Stimulus-specific gene expression profiles associated with grooming behavior and Varroa destructor resistance in honey bees. Front. Bee Sci. 2024, 2, 1441317. [Google Scholar] [CrossRef]
- Lapidge, K.L.; Oldroyd, B.P.; Spivak, M. Seven suggestive quantitative trait loci influence hygienic behavior of honey bees. Naturwissenschaften 2002, 89, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Oxley, P.R.; Spivak, M.; Oldroyd, B.P. Six quantitative trait loci infl uence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol. Ecol. 2010, 19, 1452–1461. [Google Scholar] [CrossRef]
- Le Conte, Y.; Alaux, C.; Martin, J.F.; Harbo, J.R.; Harris, J.W.; Dantec, C.; Séverac, D.; Cros-Artei, S.; Navajas, M. Social immunity in honeybees (Apis mellifera): Transcriptome analysis of Varroa–hygienic behaviour. Insect Mol. Biol. 2011, 20, 399–408. [Google Scholar] [CrossRef]
- Tsuruda, J.M.; Harris, J.W.; Bourgeois, L.; Danka, R.G.; Hunt, G.J. High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. PLoS ONE 2012, 7, e48276. [Google Scholar] [CrossRef]
- Boutin, S.; Alburaki, M.; Mercier, P.L.; Giovenazzo, P.; Derome, N. Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genom. 2015, 16, 500. [Google Scholar] [CrossRef]
- Scannapieco, A.C.; Mannino, M.C.; Soto, G.; Palacio, M.A.; Cladera, J.L.; Lanzavecchia, S.B. Expression analysis of genes putatively associated with hygienic behavior in selected stocks of Apis mellifera L. from Argentina. Insectes Soc. 2017, 64, 485–494. [Google Scholar] [CrossRef]
- Harpur, B.A.; Guarna, M.M.; Huxter, E.; Higo, H.; Moon, K.M.; Hoover, S.E.; Ibrahim, A.; Melathopoulos, A.P.; Desai, S.; Currie, R.W.; et al. Integrative genomics reveals the genetics and evolution of the honey bee’s social immune system. Genome Biol. Evol. 2019, 11, 937–948. [Google Scholar] [CrossRef]
- Teixeira, É.W.; de Paiva Daibert, R.M.; Glatzl Júnior, L.A.; da Silva, M.V.; Alves, M.L.; Evans, J.D.; Toth, A.L. Transcriptomic analysis suggests candidate genes for hygienic behavior in African-derived Apis mellifera honeybees. Apidologie 2021, 52, 447–462. [Google Scholar] [CrossRef]



| Primer | Sequence 5′–3′ | Reference |
|---|---|---|
| Cu/ZnSOD-F | TCAACTTCAAGGACCACATAGTG | [62] |
| Cu/ZnSOD-R | ATAACACCACAAGCAAGACGAG | |
| MnSOD-F | GTCGCCAAAGGTGATGTCAATAC | [62] |
| MnSOD-R | CGTCTGGTTTACCGCCATTTG | |
| GST-F | AGGAGAGGTGTGGAGAGATAGTG | [62] |
| GST-R | CGCAAATGGTCGTGTGGATG | |
| CAT-F | TTCTACTGTGGGTGGCGAAAG | [62] |
| CAT-R | GTGTGTTGTTACCGACCAAATCC | |
| VgMC-F | AGTTCCGACCGACGACGA | [63] |
| VgMC-R | TTCCCTCCCACGGAGTCC | |
| β-actin-F | TTGTATGCCAACACTGTCCTTT | [63] |
| β-actin-R | TGGCGCGATGATCTTAATTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovic, N.M.; Glavinic, U.; Stevanovic, J.; Ristanic, M.; Vejnovic, B.; Dolasevic, S.; Stanimirovic, Z. A Field Trial to Demonstrate the Potential of a Vitamin B Diet Supplement in Reducing Oxidative Stress and Improving Hygienic and Grooming Behaviors in Honey Bees. Insects 2025, 16, 36. https://doi.org/10.3390/insects16010036
Jovanovic NM, Glavinic U, Stevanovic J, Ristanic M, Vejnovic B, Dolasevic S, Stanimirovic Z. A Field Trial to Demonstrate the Potential of a Vitamin B Diet Supplement in Reducing Oxidative Stress and Improving Hygienic and Grooming Behaviors in Honey Bees. Insects. 2025; 16(1):36. https://doi.org/10.3390/insects16010036
Chicago/Turabian StyleJovanovic, Nemanja M., Uros Glavinic, Jevrosima Stevanovic, Marko Ristanic, Branislav Vejnovic, Slobodan Dolasevic, and Zoran Stanimirovic. 2025. "A Field Trial to Demonstrate the Potential of a Vitamin B Diet Supplement in Reducing Oxidative Stress and Improving Hygienic and Grooming Behaviors in Honey Bees" Insects 16, no. 1: 36. https://doi.org/10.3390/insects16010036
APA StyleJovanovic, N. M., Glavinic, U., Stevanovic, J., Ristanic, M., Vejnovic, B., Dolasevic, S., & Stanimirovic, Z. (2025). A Field Trial to Demonstrate the Potential of a Vitamin B Diet Supplement in Reducing Oxidative Stress and Improving Hygienic and Grooming Behaviors in Honey Bees. Insects, 16(1), 36. https://doi.org/10.3390/insects16010036






