Effects of Microbes on Insect Host Physiology and Behavior Mediated by the Host Immune System
Simple Summary
Abstract
1. Introduction
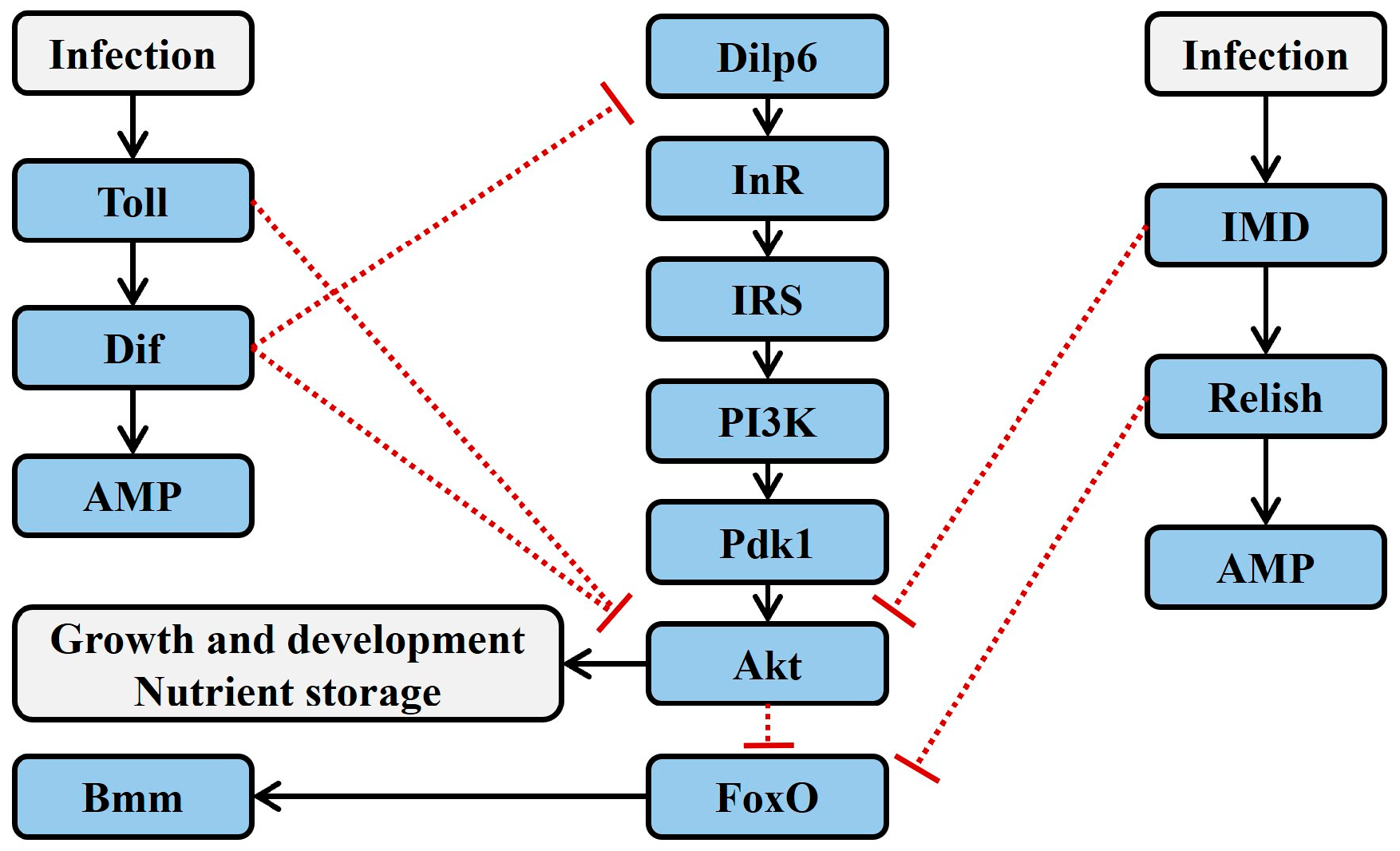
2. Major Immune and Insulin Pathway Signaling in Insects
2.1. Toll Signaling
2.2. IMD Signaling
2.3. JAK/STAT Signaling
2.4. Insulin Signaling
3. Effects of Microbes on Insects
3.1. Effects of Microbes on Insect Growth and Development
3.2. Effects of Microbes on Insect Reproduction
3.3. Effects of Microbes on Insect Resistance to Insecticides
3.4. Effects of Microbes on Insect Chemical Communication
3.5. Effects of Microbes on Insect Cell Turnover
3.6. Effects of Microbes on Insect Lifespan
3.7. Effects of Microbes on Insect Sleep
3.8. Metabolic Reprogramming Induced by Microbes
4. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Tettamanti, G.; Bassal, T.; Heryanto, C.; Eleftherianos, I.; Mohamed, A. Regulators and signalling in insect antimicrobial innate immunity: Functional molecules and cellular pathways. Cell. Signal. 2021, 83, 110003. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yong, H.Z.; Zhang, S.; Liu, Z.Y.; Zhao, Y.R. Colonization resistance of symbionts in their insect hosts. Insects 2023, 14, 594. [Google Scholar] [CrossRef]
- Zhai, Z.Z.; Huang, X.S.; Yin, Y.L. Beyond immunity: The IMD pathway as a coordinator of host defense, organismal physiology and behavior. Dev. Comp. Immunol. 2018, 83, 51–59. [Google Scholar] [CrossRef]
- Vincent, C.M.; Beckwith, E.J.; Simoes da Silva, C.J.; Pearson, W.H.; Kierdorf, K.; Gilestro, G.F.; Dionne, M.S. Infection increases activity via Toll dependent and independent mechanisms in Drosophila melanogaster. PLoS Pathog. 2022, 18, e1010826. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.M.; Lazzaro, B.P. Interactions between innate immunity and insulin signaling affect resistance to infection in insects. Front. Immunol. 2023, 14, 1276357. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Yao, X.; Bai, S.F.; Wei, J.Z.; An, S.H. Involvement of an enhanced immunity mechanism in the resistance to Bacillus thuringiensis in lepidopteran pests. Insects 2023, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.H.; Chosa, N.; Kim, S.H.; Nam, H.J.; Lemaitre, B.; Ochiai, M.; Kambris, Z.; Brun, S.; Hashimoto, C.; Ashida, M.; et al. A Spätzle-processing enzyme required for Toll signaling activation in Drosophila innate immunity. Dev. Cell 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Silverman, N.; Zhou, R.; Erlich, R.L.; Hunter, M.; Bernstein, E.; Schneider, D.; Maniatis, T. Immune activation of NF-κB and JNK requires Drosophila TAK1. J. Biol. Chem. 2003, 278, 48928–48934. [Google Scholar] [CrossRef]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd signaling pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- Iatsenko, I.; Kondo, S.; Mengin-Lecreulx, D.; Lemaitre, B. PGRP-SD, an extracellular pattern-recognition receptor, enhances peptidoglycan-mediated activation of the Drosophila Imd pathway. Immunity 2016, 45, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Valanne, S.; Wang, J.H.; Rämet, M. The Drosophila toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Ragab, A.; Buechling, T.; Gesellchen, V.; Spirohn, K.; Boettcher, A.L.; Boutros, M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011, 30, 1123–1136. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Li, H.J.; Jensen, M.B.; Maksoud, E.; Borneo, J.; Liang, Y.X.; Quake, S.R.; Luo, L.Q.; Haghighi, P.; Jasper, H. Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 2021, 596, 97–102. [Google Scholar] [CrossRef]
- Erion, R.; Sehgal, A. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.M.; Xue, Z.; Dong, H.A.; Pang, J.X.; Liu, H.W.; Gong, J.; Xia, Q.Y.; Hou, Y. Nutrition regulates the expression of storage proteins in Bombyx mori via insulin-like/FoxO signaling pathway. Insect Biochem. Mol. Biol. 2022, 149, 103847. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.W.; Bitterman, M.D.; Birnbaum, M.J.; Bland, M.L. Innate immune signaling in Drosophila blocks insulin signaling by uncoupling PI(3,4,5)P3 production and Akt activation. Cell Rep. 2018, 22, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Salasc, F.; Mutuel, D.; Debaisieux, S.; Perrin, A.; Dupressoir, T.; Grenet, A.S.G.; Ogliastro, M. Role of the phosphatidylinositol-3-kinase/Akt/target of rapamycin pathway during ambidensovirus infection of insect cells. J. Gen. Virol. 2016, 97, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, M.; Muhammad, N.M.; Joseph, B.S.; Bland, M.L. The toll signaling pathway targets the insulin-like peptide Dilp6 to inhibit growth in Drosophila. Cell Rep. 2019, 28, 1439–1446. [Google Scholar] [CrossRef]
- DiAngelo, J.R.; Bland, M.L.; Bambina, S.; Cherry, S.; Birnbaum, M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20853–20858. [Google Scholar] [CrossRef]
- Davoodi, S.; Galenza, A.; Panteluk, A.; Deshpande, R.; Ferguson, M.; Grewal, S.; Foley, E. The immune deficiency pathway regulates metabolic homeostasis in Drosophila. J. Immunol. 2019, 202, 2747–2759. [Google Scholar] [CrossRef]
- Caragata, E.P.; Pais, F.S.; Baton, L.A.; Silva, J.B.L.; Sorgine, M.H.F.; Moreira, L.A. The transcriptome of the mosquito Aedes fluviatilis (Diptera: Culicidae), and transcriptional changes associated with its native Wolbachia infection. BMC Genom. 2017, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Dionne, M.S.; Pham, L.N.; Shirasu-Hiza, M.; Schneider, D.S. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 2006, 16, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Yamanaka, N.; Yagi, Y.; Nishida, Y.; Kataoka, H.; O’Connor, M.B.; Mizoguchi, A. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell 2009, 17, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Shearin, A.L.; Monks, B.R.; Seale, P.; Birnbaum, M.J. Lack of AKT in adipocytes causes severe lipodystrophy. Mol. Metab. 2016, 5, 472–479. [Google Scholar] [CrossRef]
- Softic, S.; Boucher, J.; Solheim, M.H.; Fujisaka, S.; Haering, M.F.; Homan, E.P.; Winnay, J.; Perez-Atayde, A.R.; Kahn, C.R. Lipodystrophy due to adipose tissue–specific insulin receptor knockout results in progressive NAFLD. Diabetes 2016, 65, 2187–2200. [Google Scholar] [CrossRef]
- Kamareddine, L.; Robins, W.P.; Berkey, C.D.; Mekalanos, J.J.; Watnick, P.I. The Drosophila immune deficiency pathway modulates enteroendocrine function and host metabolism. Cell Metab. 2018, 28, 449–462. [Google Scholar] [CrossRef]
- Kang, P.; Chang, K.; Liu, Y.; Bouska, M.; Birnbaum, A.; Karashchuk, G.; Thakore, R.; Zheng, W.J.; Post, S.; Brent, C.S.; et al. Drosophila Kruppel homolog 1 represses lipolysis through interaction with dFOXO. Sci. Rep. 2017, 7, 16369. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Song, K.H.; Schneider, D.S. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster. PLoS ONE 2012, 7, e50679. [Google Scholar] [CrossRef]
- Bashir-Tanoli, S.; Tinsley, M.C. Immune response costs are associated with changes in resource acquisition and not resource reallocation. Funct. Ecol. 2014, 28, 1011–1019. [Google Scholar] [CrossRef]
- Howick, V.M.; Lazzaro, B.P. Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol. Biol. 2014, 14, 56. [Google Scholar] [CrossRef]
- Nystrand, M.; Dowling, D.K. Dose-dependent effects of an immune challenge at both ultimate and proximate levels in Drosophila melanogaster. J. Evol. Biol. 2014, 27, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Palli, S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef]
- Masuzzo, A.; Maniere, G.; Viallat-Lieutaud, A.; Avazeri, E.; Zugasti, O.; Grosjean, Y.; Kurz, C.L.; Royet, J. Peptidoglycan-dependent NF-κB activation in a small subset of brain octopaminergic neurons controls female oviposition. eLife 2019, 8, e50559. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, T.; Qian, Y.; Gkotzis, S.; Femenia, T.; Wang, T.; Udekwu, K.; Forssberg, H.; Heijtz, R.D. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry 2017, 22, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.J.; Kang, S.; Chen, D.F.; Wu, Q.J.; Wang, S.L.; Xie, W.; Zhu, X.; Baxter, S.W.; Zhou, X.G.; Jurat-Fuentes, J.L.; et al. MAPK signaling pathway alters expression of midgut ALP and ABCC genes and causes resistance to Bacillus thuringiensis Cry1Ac toxin in diamondback moth. PLoS Genet. 2015, 11, e1005124. [Google Scholar] [CrossRef]
- Davis, R.J. MAPKs: New JNK expands the group. Trends Biochem. Sci. 1994, 19, 470–473. [Google Scholar] [CrossRef]
- Tanaka, S.; Miyamoto, K.; Noda, H.; Jurat-Fuentes, J.L.; Yoshizawa, Y.; Endo, H.; Sato, R. The ATP-binding cassette transporter subfamily C member 2 in Bombyx mori larvae is a functional receptor for Cry toxins from Bacillusthuringiensis. FEBS J. 2013, 280, 1782–1794. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, X.X.; De Mandal, S.; Shakeel, M.; Hua, Y.Y.; Shoukat, R.F.; Fu, D.R.; Jin, F.L. Gut microbiota mediate Plutella xylostella susceptibility to Bt Cry1Ac protoxin is associated with host immune response. Environ. Pollut. 2021, 271, 116271. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.J.; Kang, S.; Wu, Q.J.; Wang, S.L.; Crickmore, N.; Zhou, X.G.; Bravo, A.; Soberon, M.; Zhang, Y.J. The regulation landscape of MAPK signaling cascade for thwarting Bacillus thuringiensis infection in an insect host. PLoS Pathog. 2021, 17, e1009917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhang, S.; Liu, Z.Y.; Chang, Z.Z.; Hu, H.S. Gut bacteria promote phosphine susceptibility of Tribolium castaneum by aggravating oxidative stress and fitness costs. Insects 2023, 14, 815. [Google Scholar] [CrossRef]
- Burand, J.P.; Tan, W.J.; Kim, W.J.; Nojima, S.; Roelofs, W. Infection with the insect virus Hz-2v alters mating behavior and pheromone production in female Helicoverpa zea moths. J. Insect Sci. 2005, 5, 6. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Alaux, C.; Tchamitchan, S.; Brunet, J.L.; Plettner, E.; Belzunces, L.P.; Le Conte, Y. Nosema spp. infection alters pheromone production in honey bees (Apis mellifera). J. Chem. Ecol. 2010, 36, 522–525. [Google Scholar] [CrossRef]
- Keesey, I.W.; Koerte, S.; Khallaf, M.A.; Retzke, T.; Guillou, A.; Grosse-Wilde, E.; Buchon, N.; Knaden, M.; Hansson, B.S. Pathogenic bacteria enhance dispersal through alteration of Drosophila social communication. Nat. Commun. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Kobler, J.M.; Jimenez, F.J.R.; Petcu, I.; Kadow, I.C.G. Immune receptor signaling and the mushroom body mediate post-ingestion pathogen avoidance. Curr. Biol. 2020, 30, 4693–4709. [Google Scholar] [CrossRef] [PubMed]
- Owald, D.; Waddell, S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr. Opin. Neurobiol. 2015, 35, 178–184. [Google Scholar] [CrossRef]
- Harris, N.; Braiser, D.J.; Dickman, D.K.; Fetter, R.D.; Tong, A.; Davis, G.W. The innate immune receptor PGRP-LC controls presynaptic homeostatic plasticity. Neuron 2015, 88, 1157–1164. [Google Scholar] [CrossRef]
- Basset, A.; Khush, R.S.; Braun, A.; Gardan, L.; Boccard, F.; Hoffmann, J.A.; Lemaitre, B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. USA 2000, 97, 3376–3381. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes. Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Karpac, J.; Tran, S.L.; Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 2014, 156, 109–122. [Google Scholar] [CrossRef]
- Georgel, P.; Naitza, S.; Kappler, C.; Ferrandon, D.; Zachary, D.; Swimmer, C.; Kopczynski, C.; Duyk, G.; Reichhart, J.M.; Hoffmann, J.A. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 2001, 1, 503–514. [Google Scholar] [CrossRef]
- Zhai, Z.Z.; Boquete, J.P.; Lemaitre, B. Cell-specific Imd-NF-κB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection. Immunity 2018, 48, 897–910. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 2009, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Q.; Patel, P.H.; Kohlmaier, A.; Grenley, M.O.; McEwen, D.G.; Edgar, B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 2009, 137, 1343–1355. [Google Scholar] [CrossRef]
- Hori, A.; Kurata, S.; Kuraishi, T. Unexpected role of the IMD pathway in Drosophila gut defense against Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2017, 495, 395–400. [Google Scholar] [CrossRef]
- Tavignot, R.; Chaduli, D.; Djitte, F.; Charroux, B.; Royet, J. Inhibition of a NF-κB/Diap1 pathway by PGRP-LF is required for proper apoptosis during Drosophila development. PLoS Genet. 2017, 13, e1006569. [Google Scholar] [CrossRef] [PubMed]
- Iatsenko, I.; Boquete, J.P.; Lemaitre, B. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens Drosophila lifespan. Immunity 2018, 49, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Shirasu-Hiza, M.M.; Dionne, M.S.; Pham, L.N.; Ayres, J.S.; Schneider, D.S. Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr. Biol. 2007, 17, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Mallon, E.B.; Alghamdi, A.; Holdbrook, R.T.K.; Rosato, E. Immune stimulation reduces sleep and memory ability in Drosophila melanogaster. PeerJ 2014, 2, e434. [Google Scholar] [CrossRef]
- Kuo, T.H.; Pike, D.H.; Beizaeipour, Z.; Williams, J.A. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFκB Relish. BMC Neurosci. 2010, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Edery, I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr. Biol. 2008, 18, 195–199. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A.J. The Warburg effect then and now: From cancer to inflammatory diseases. BioEssays 2013, 35, 965–973. [Google Scholar] [CrossRef]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Science’s STKE 2005, 2005, re12. [Google Scholar] [CrossRef] [PubMed]
- Siegert, I.; Schödel, J.; Nairz, M.; Schatz, V.; Dettmer, K.; Dick, C.; Kalucka, J.; Franke, K.; Ehrenschwender, M.; Schley, G.; et al. Ferritin-mediated iron sequestration stabilizes hypoxia-inducible factor-1α upon LPS activation in the presence of ample oxygen. Cell Rep. 2015, 13, 2048–2055. [Google Scholar] [CrossRef]
- Bandarra, D.; Biddlestone, J.; Mudie, S.; Müller, H.A.J.; Rocha, S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis. Models Mech. 2015, 8, 169–181. [Google Scholar] [CrossRef]
- Van Uden, P.; Kenneth, N.S.; Webster, R.; Müller, H.A.; Mudie, S.; Rocha, S. Evolutionary conserved regulation of HIF-1β by NF-κB. PLoS Genet. 2011, 7, e1001285. [Google Scholar] [CrossRef]
- Shelby, K.S.; Popham, H.J.R. RNA-Seq study of microbially induced hemocyte transcripts from larval Heliothis virescens (Lepidoptera: Noctuidae). Insects 2012, 3, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, A.; Kucerova, K.; Jonatova, L.; Tomcala, A.; Schneedorferova, I.; Okrouhlik, J.; Dolezal, T. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol. 2015, 13, e1002135. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, A.; Dolezal, T. Extracellular adenosine modulates host-pathogen interactions through regulation of systemic metabolism during immune response in Drosophila. PLoS Pathog. 2018, 14, e1007022. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.B.; Zhu, F.; Xu, C.J.; Li, Y. Metabolomics meets systems immunology. EMBO Rep. 2023, 24, e55747. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.E.; Thummel, C.S.; Tennessen, J.M. Metabolomic studies in Drosophila. Genet. 2017, 206, 1169–1185. [Google Scholar] [CrossRef]
- Droujinine, I.A.; Perrimon, N. Interorgan communication pathways in physiology: Focus on Drosophila. Annu. Rev. Genet. 2016, 50, 539–570. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Z.; Luo, Q.; Zhou, L.; Du, X.; Ren, Y. Effects of Microbes on Insect Host Physiology and Behavior Mediated by the Host Immune System. Insects 2025, 16, 82. https://doi.org/10.3390/insects16010082
Zhang S, Wang Z, Luo Q, Zhou L, Du X, Ren Y. Effects of Microbes on Insect Host Physiology and Behavior Mediated by the Host Immune System. Insects. 2025; 16(1):82. https://doi.org/10.3390/insects16010082
Chicago/Turabian StyleZhang, Shan, Zhengyan Wang, Qiong Luo, Lizhen Zhou, Xin Du, and Yonglin Ren. 2025. "Effects of Microbes on Insect Host Physiology and Behavior Mediated by the Host Immune System" Insects 16, no. 1: 82. https://doi.org/10.3390/insects16010082
APA StyleZhang, S., Wang, Z., Luo, Q., Zhou, L., Du, X., & Ren, Y. (2025). Effects of Microbes on Insect Host Physiology and Behavior Mediated by the Host Immune System. Insects, 16(1), 82. https://doi.org/10.3390/insects16010082






