Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Generator Device
2.2. Field Investigation Study Sites
Field Investigations Mosquito Trap Comparisons
3. Results
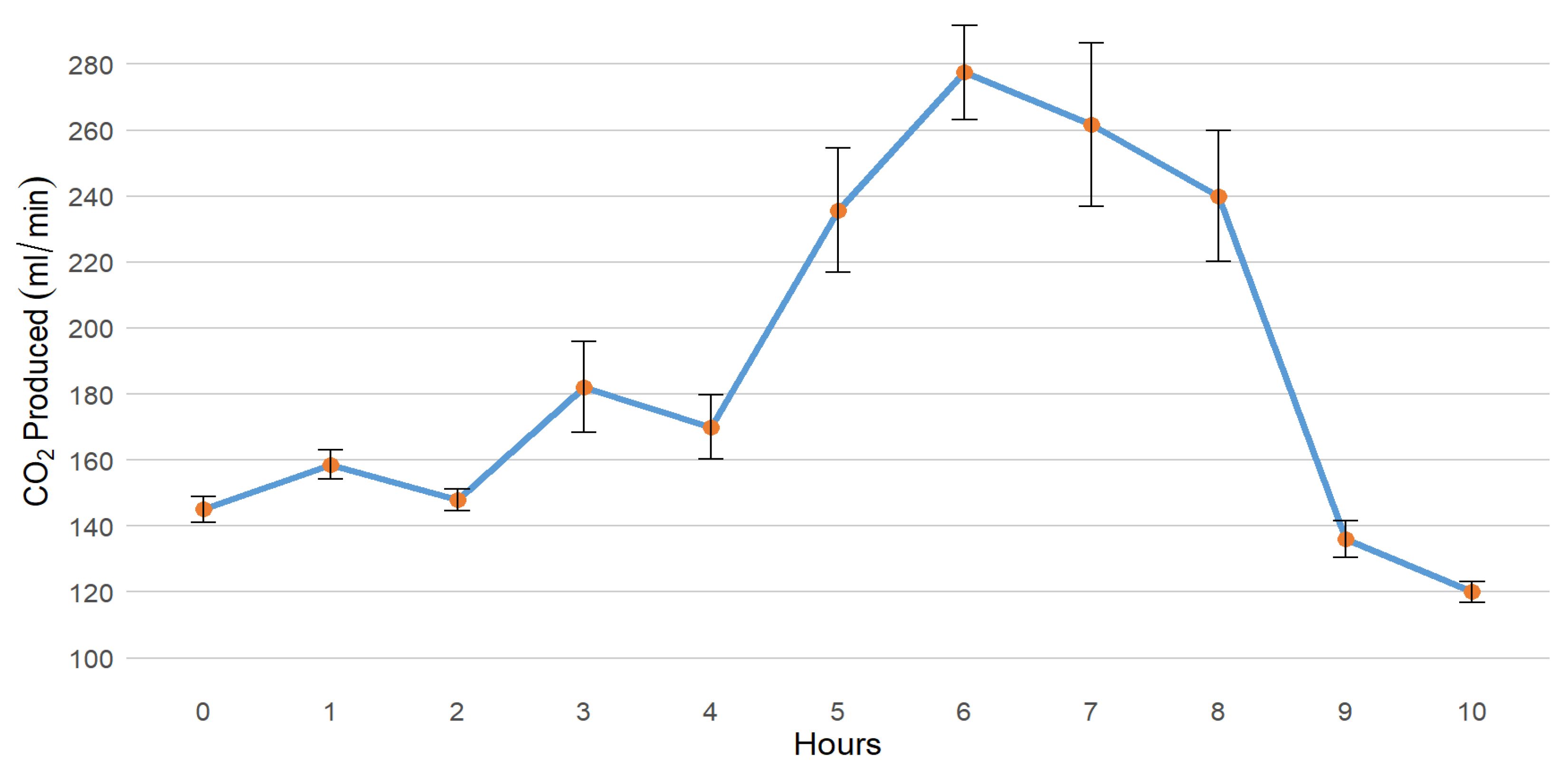
3.1. Carbon Dioxide Production
3.2. Field Investigations Mosquito Collections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. A Global Brief on Vector-Borne Diseases. Available online: https://iris.who.int/handle/10665/111008 (accessed on 15 March 2024).
- Gyawali, N.; Taylor-Robinson, A. Confronting the Emerging Threat to Public Health in Northern Australia of Neglected Indigenous Arboviruses. Trop. Med. Infect. Dis. 2017, 2, 55. [Google Scholar] [CrossRef]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Johansen, C.A.; Warrilow, D.; Ritchie, S.A. Evolution of Mosquito-Based Arbovirus Surveillance Systems in Australia. J. Biomed. Biotechnol. 2012, 2012, 325659. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, N.; Taylor-Robinson, A.W.; Bradbury, R.S.; Pederick, W.; Faddy, H.M.; Aaskov, J.G. Neglected Australian Arboviruses Associated with Undifferentiated Febrile Illnesses. Front. Microbiol. 2019, 10, 2818. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Sobur, A.; Islam, S.; Ievy, S.; Hossain, J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Koh, W.L.; Casteriano, A.; Beijerink, N.; Godfrey, C.; Brown, G.; Emery, D.; Šlapeta, J. Mosquito-Borne Heartworm Dirofilaria Immitis in Dogs from Australia. Parasit. Vectors. 2016, 9, 535. [Google Scholar] [CrossRef]
- Park, S.L.; Huang, Y.-J.S.; Vanlandingham, D.L. Re-Examining the Importance of Pigs in the Transmission of Japanese Encephalitis Virus. Pathogens 2022, 11, 575. [Google Scholar] [CrossRef]
- Osório, H.C.; Zé-Zé, L.; Amaro, F.; Alves, M.J. Mosquito Surveillance for Prevention and Control of Emerging Mosquito-Borne Diseases in Portugal—2008–2014. Int. J. Environ. Res. Public Health 2014, 11, 11583–11596. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Van den Hurk, A.F.; Meyer, D.B.; Ritchie, S.A. Searching for the Proverbial Needle in a Haystack: Advances in Mosquito-Borne Arbovirus Surveillance. Parasit. Vectors. 2018, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Brugman, V.A.; Sato, S.; Ant, T.H.; Tojo, B.; Masuda, G.; Kaneko, S.; Moji, K.; Medlock, J.M.; Logan, J.G. Field Testing of a Lightweight, Inexpensive, and Customisable 3D-Printed Mosquito Light Trap in the UK. Sci. Rep. 2019, 9, 11412. [Google Scholar] [CrossRef]
- Degener, C.M.; Staunton, K.M.; Bossin, H.; Marie, J.; Diogo, R.; Lima, D.C.; Eiras, Á.E.; Akaratovic, K.I.; Kiser, J.; Gordon, S.W. Evaluation of the New Modular Biogents BG-pro Mosquito Trap in Comparison to CDC, EVS, BG-Sentinel, and BG-Mosquitaire Traps. J. Am. Mosq. Control Assoc. 2021, 37, 224–241. [Google Scholar] [CrossRef]
- Hoel, D.F.; Dunford, J.C.; Kline, D.L.; Irish, S.R.; Weber, M.; Richardson, A.G.; Doud, C.W.; Wirtz, R.A. A Comparison of Carbon Dioxide Sources for Mosquito Capture in Centers for Disease Control and Prevention Light Traps on the Florida Gulf Coast1. J. Am. Mosq. Control Assoc. 2015, 31, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Degener, C.M.; Geier, M.; Kline, D.; Urban, J.; Willis, S.; Ramirez, K.; Cloherty, E.R.; Gordon, S.W. Field Trials to Evaluate the Effectiveness of the Biogents®-Sweetscent Lure in Combination with Several Commercial Mosquito Traps and to Assess the Effectiveness of the Biogents-Mosquitaire Trap with and without Carbon Dioxide. J. Am. Mosq. Control Assoc. 2019, 35, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Li, T.; Liu, Q.; Gong, Z.; Hou, J. Effect of Different Carbon Dioxide (CO2) Flows on Trapping Aedes albopictus with BG Traps in the Field in Zhejiang Province, China. PLoS ONE 2020, 15, e0243061. [Google Scholar] [CrossRef]
- McPhatter, L.; Gerry, A.C. Effect of CO2 Concentration on Mosquito Collection Rate Using Odor-Baited Suction Traps. J. Vector Ecol. 2017, 42, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bazin, M.; Williams, C.R. Mosquito Traps for Urban Surveillance: Collection Efficacy and Potential for Use by Citizen Scientists. J. Vector Ecol. 2018, 43, 98–103. [Google Scholar] [CrossRef]
- Sukumaran, D.; Ponmariappan, S.; Sharma, A.K.; Jha, H.K.; Wasu, Y.H.; Sharma, A.K. Application of Biogenic Carbon Dioxide Produced by Yeast with Different Carbon Sources for Attraction of Mosquitoes towards Adult Mosquito Traps. Parasitol. Res. 2015, 115, 1453–1462. [Google Scholar]
- Burkett-Cadena, N.D.; Blosser, E.M.; Young, R.M.; Toé, L.D.; Unnasch, T.R. Carbon Dioxide Generated from Carbonates and Acids for Sampling Blood-Feeding Arthropods. Acta. Trop. 2015, 149, 254–261. [Google Scholar] [CrossRef]
- Rohe, D.L.; Fall, R.P. A Miniature Battery Powered CO2 Baited Light Trap for Mosquito Borne Encephalitis Virus Surveillance. Bull. Soc. Vector Ecol. 1979, 4, 24–27. [Google Scholar]
- Commonwealth of Australia Sydney. NSW—February 2024—Daily Weather Observations. Available online: http://www.bom.gov.au/climate/dwo/202402/html/IDCJDW2124.202402.shtml (accessed on 29 February 2024).
- Webb, C.E.; Doggett, S.L.; Russell, R.C. A Guide to Mosquitoes of Australia; CSIRO Publishing: Clayton, VIC, Australia, 2016; ISBN 9780643100305. [Google Scholar]
- Lee, H.S.; Noh, B.E.; Kim, S.Y.; Kim, H.; Lee, H.I. The Comparative Field Evaluation of Four Different Traps for Mosquito Surveillance in the Republic of Korea. Insects 2024, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Rossi da Silva, K.; Ribeiro da Silva, W.; Silva, B.P.; Arcos, A.N.; da Silva Ferreira, F.A.; Soares-da-Silva, J.; Pontes, G.O.; Roque, R.A.; Tadei, W.P.; Navarro-Silva, M.A.; et al. New Traps for the Capture of Aedes Aegypti (Linnaeus) and Aedes Albopictus (Skuse) (Diptera: Culicidae) Eggs and Adults. PLoS Negl. Trop. Dis. 2021, 15, e0008813. [Google Scholar]
- Meza, F.C.; Tenywa, F.C.; Ashall, S.; Okumu, F.O.; Moore, S.J.; Tripet, F. Scalable Camera Traps for Measuring the Attractiveness of Sugar Baits for Controlling Malaria and Dengue Vectors. Parasites Vectors 2024, 17, 499. [Google Scholar] [CrossRef]
- Asfaw, N.; Hiruy, B.; Worku, N.; Massebo, F. Evaluating the Efficacy of Various Traps in Catching Tsetse Flies at Nech Sar and Maze National Parks, Southwestern Ethiopia: An Implication for Trypanosoma Vector Control. PLoS Negl. Trop. Dis. 2022, 16, e0010999. [Google Scholar] [CrossRef]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Night Time Extension of Aedes aegypti Human Blood Seeking Activity. Am. J. Trop. Med. Hyg. 2022, 107, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Golnar, A.J.; Meyers, J.I.; Slotman, M.A.; Hamer, G.L. Plasmodium Relictum Infection in Culex quinquefasciatus (Culicidae) Decreases Diel Flight Activity but Increases Peak Dusk Flight Activity. Malar. J. 2022, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Loner, C.; Acquisto, N.M.; Lenhardt, H.; Sensenbach, B.; Purick, J.; Jones, C.M.C.; Cushman, J.T. Accuracy of Intravenous Infusion Flow Regulators in the Prehospital Environment. Prehosp. Emerg. Care 2018, 22, 645–649. [Google Scholar] [CrossRef] [PubMed]
- İpci, K.; Öktemer, T.; Birdane, L.; Altıntoprak, N.; Bayar Muluk, N.; Passali, D.; Lopatin, A.; Bellussi, L.; Mladina, R.; Pawankar, R.; et al. Effervescent Tablets: A Safe and Practical Delivery System for Drug Administration. ENT Updates 2016, 6, 46–50. [Google Scholar] [CrossRef]
- Webb, C.E.; Porigneaux, P.G.; Durrheim, D.N. Assessing the Risk of Exotic Mosquito Incursion through an International Seaport, Newcastle, NSW, Australia. Trop. Med. Infect. Dis. 2021, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Cooperband, M.F.; Carde, R.T. Comparison of Plume Structures of Carbon Dioxide Emitted from Different Mosquito Traps. Med. Vet. Entomol. 2006, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Govella, N.J.; Maliti, D.F.; Mlwale, A.T.; Masallu, J.P.; Mirzai, N.; Johnson, P.C.D.; Ferguson, H.M.; Killeen, G.F. An Improved Mosquito Electrocuting Trap That Safely Reproduces Epidemiologically Relevant Metrics of Mosquito Human-Feeding Behaviours as Determined by Human Landing Catch. Malar. J. 2016, 15, 465. [Google Scholar] [CrossRef]
- Marzal, A.; Magallanes, S. Stimuli Followed by Avian Malaria Vectors in Host-Seeking Behaviour. Biology 2022, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.L.; Lau, C.L.; Leder, K. The Evolving Japanese Encephalitis Situation in Australia and Implications for Travel Medicine. J. Travel Med. 2023, 30, taad029. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Williams, D.T.; van den Hurk, A.F.; Smith, D.W.; Currie, B.J. Japanese Encephalitis Virus: The Emergence of Genotype IV in Australia and Its Potential Endemicity. Viruses 2022, 14, 2480. [Google Scholar] [CrossRef] [PubMed]



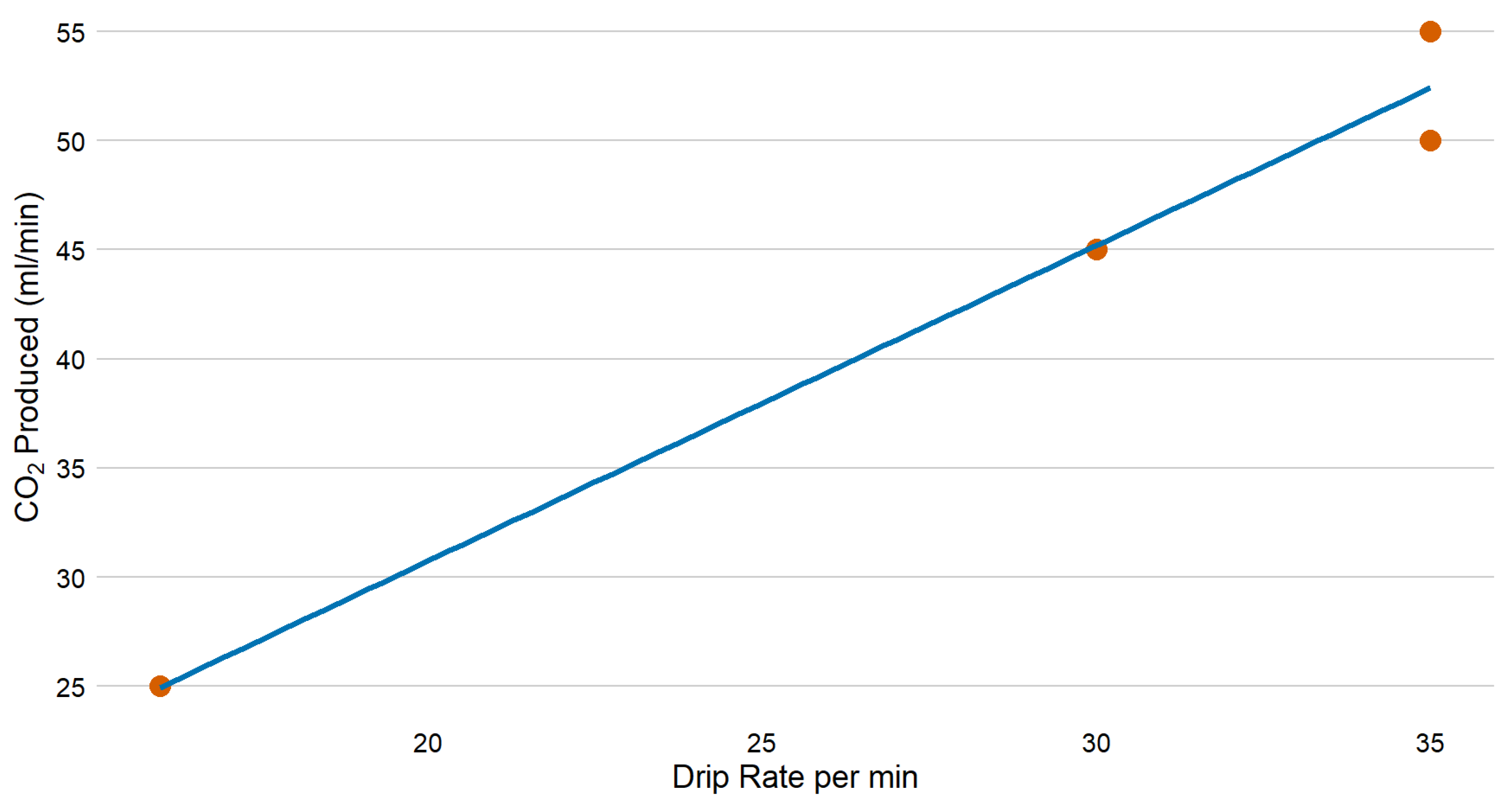
| Trials | Citric Acid Solution | Sodium Bicarbonate (g) | Flow Rate (Drops/min) | Amount of Citric Acid Solution (mL/min) | CO2 Production Rate (mL/min)—Starting Amount |
|---|---|---|---|---|---|
| Trial 1 | 300 g/1000 mL | 200 | 16 | 0.8 | 25 |
| Trial 2 | 300 g/1000 mL | 200 | 30 | 1.5 | 45 |
| Trial 3 | 300 g/1000 mL | 200 | 35 | 1.8 | 55 |
| Trial 4 | 300 g/1000 mL | 200 | 35 | 1.8 | 50 |
| Trial 5 | 500 g/550 mL | 500 | 38 | 1.9 | 180 |
| Trial 6 | 500 g/550 mL | 500 | 48 | 2.4 | 210 |
| Trial 7 | 500 g/550 mL | 500 | 14 | 0.7 | 50 |
| Trial 8 | 500 g/550 mL | 500 | 34 | 1.7 | 180 |
| Trial 9 | 500 g/550 mL | 500 | 30 | 1.5 | 150 |
| Trial 10 | 500 g/550 mL | 500 | 30 | 1.5 | 170 |
| Trial 11 | 500 g/550 mL | 500 | 30 | 1.5 | 140 |
| Trial 12 | 500 g/550 mL | 500 | 30 | 1.5 | 135 |
| Trial 13 | 500 g/550 mL | 500 | 30 | 1.5 | 140 |
| Trial 14 | 500 g/550 mL | 500 | 30 | 1.5 | 140 |
| Trial 15 | 500 g/550 mL | 500 | 30 | 1.5 | 140 |
| Species | Dry Ice (Mean, SE) | Citric Acid (Mean, SE) | Percentage in Dry Ice and Citric Acid Treatment (%/%) | p-Value (MWU) | Total |
|---|---|---|---|---|---|
| University of Sydney | |||||
| Aedes notoscriptus | 50.6 ± 26.0 | 28.2 ± 7.8 | 74.2/49.5 | 0.9 | 631 |
| Culex annulirostris | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.2/0.0 | NA | 1 |
| Culex molestus | 0.1 ± 0.1 | 0.6 ± 0.3 | 0.2/1.1 | NA | 6 |
| Culex quinquefasciatus | 17.4 ± 7.2 | 28.2 ± 12.1 | 25.5/49.5 | 0.02 | 365 |
| Newington Nature Reserve | |||||
| Aedes aculeatus | 0.6 ± 0.3 | 0.8 ± 0.4 | 0.2/0.4 | NA | 11 |
| Aedes alternans | 15.5 ± 3.8 | 8.1 ± 2.0 | 3.9/3.7 | 0.2 | 189 |
| Aedes multiplex | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0/0.1 | NA | 2 |
| Aedes notoscriptus | 10.1 ± 3.8 | 9.9 ± 3.6 | 2.6/4.4 | 0.6 | 160 |
| Aedes procax | 2.5 ± 0.5 | 0.8 ± 0.4 | 0.6/0.4 | NA | 11 |
| Aedes vigilax | 254.1 ± 44.1 | 128.8 ± 20.8 | 65.3/57.9 | 0.01 | 3063 |
| Anopheles annulipes | 23.1 ± 7.2 | 2.8 ± 0.7 | 5.9/1.2 | 0.0009 | 207 |
| Coquillettidia linealis | 22.2 ± 6.7 | 11.4 ± 2.0 | 5.7/5.1 | 0.2 | 269 |
| Coquillettidia xanthogaster | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.1/0.1 | NA | 4 |
| Culex annulirostris | 36.0 ± 5.0 | 25.0 ± 5.2 | 9.3/11.3 | 0.01 | 488 |
| Culex molestus | 0.6 ± 0.3 | 0.0 ± 0.0 | 0.2/0.0 | NA | 5 |
| Culex orbostiensis | 0.8 ± 0.4 | 0.3 ± 0.2 | 0.2/0.1 | NA | 8 |
| Culex quinquefasciatus | 0.9 ± 0.5 | 0.0 ± 0.0 | 0.2/0.0 | NA | 7 |
| Culex sitiens | 21.4 ± 5.8 | 12.8 ± 1.6 | 5.5/5.7 | 0.1 | 273 |
| Mansonia uniformis | 0.8 ± 0.5 | 0.3 ± 0.2 | 0.2/0.1 | NA | 8 |
| Tripteroides atripes | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0/0.0 | NA | 1 |
| Verrallina funerea | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0/0.1 | NA | 1 |
| Sites | Dry Ice (SDI, ME) | Citric Acid (SDI, ME) |
|---|---|---|
| University of Sydney | 0.397 ± 0.086 | 0.516 ± 0.095 |
| Newington Nature Reserve | 2.522 ± 0.036 | 2.103 ± 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.; Brookes, V.J.; Zadoks, R.N.; Webb, C.E. Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance. Insects 2025, 16, 90. https://doi.org/10.3390/insects16010090
Hong C, Brookes VJ, Zadoks RN, Webb CE. Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance. Insects. 2025; 16(1):90. https://doi.org/10.3390/insects16010090
Chicago/Turabian StyleHong, Christine, Victoria J. Brookes, Ruth N. Zadoks, and Cameron E. Webb. 2025. "Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance" Insects 16, no. 1: 90. https://doi.org/10.3390/insects16010090
APA StyleHong, C., Brookes, V. J., Zadoks, R. N., & Webb, C. E. (2025). Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance. Insects, 16(1), 90. https://doi.org/10.3390/insects16010090







