Morphogenetic Identification of a New Record Condica capensis (Lepidoptera: Noctuidae) in Yunnan, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Morphological Observation
2.3. Molecular Characterization of Condica capensis
2.3.1. Genomic DNA Extraction
2.3.2. PCR Conditions
2.3.3. Nucleotide Sequence and Phylogenetic Analysis
2.3.4. GenBank Accession Number
2.4. Biological Studies
2.4.1. Biology of Condica capensis
2.4.2. Natural Enemies of Condica capensis
2.5. Data Processing and Statistics
3. Results
3.1. Morphological Redescription
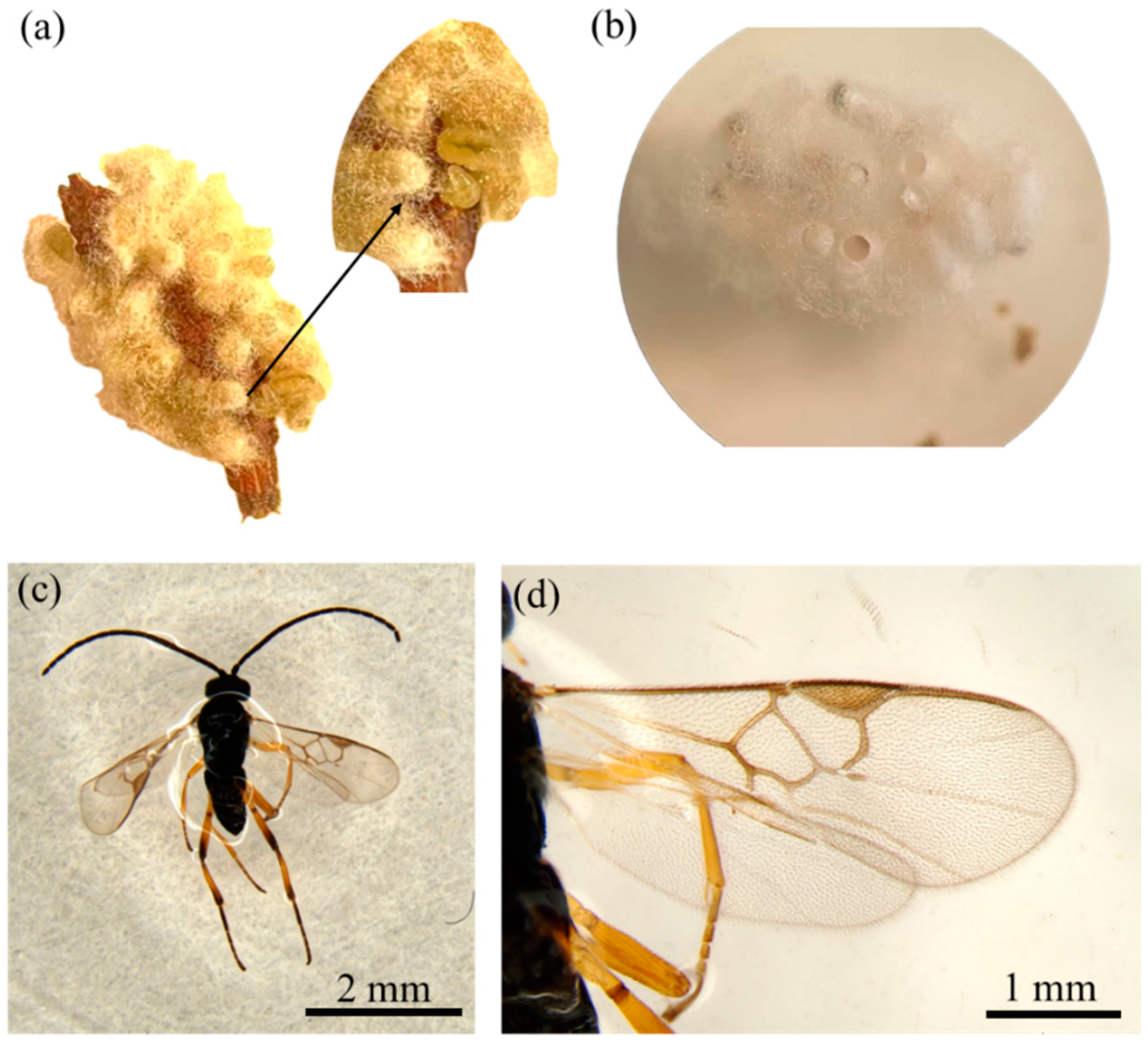
3.1.1. Egg
3.1.2. Larva
3.1.3. Pupa
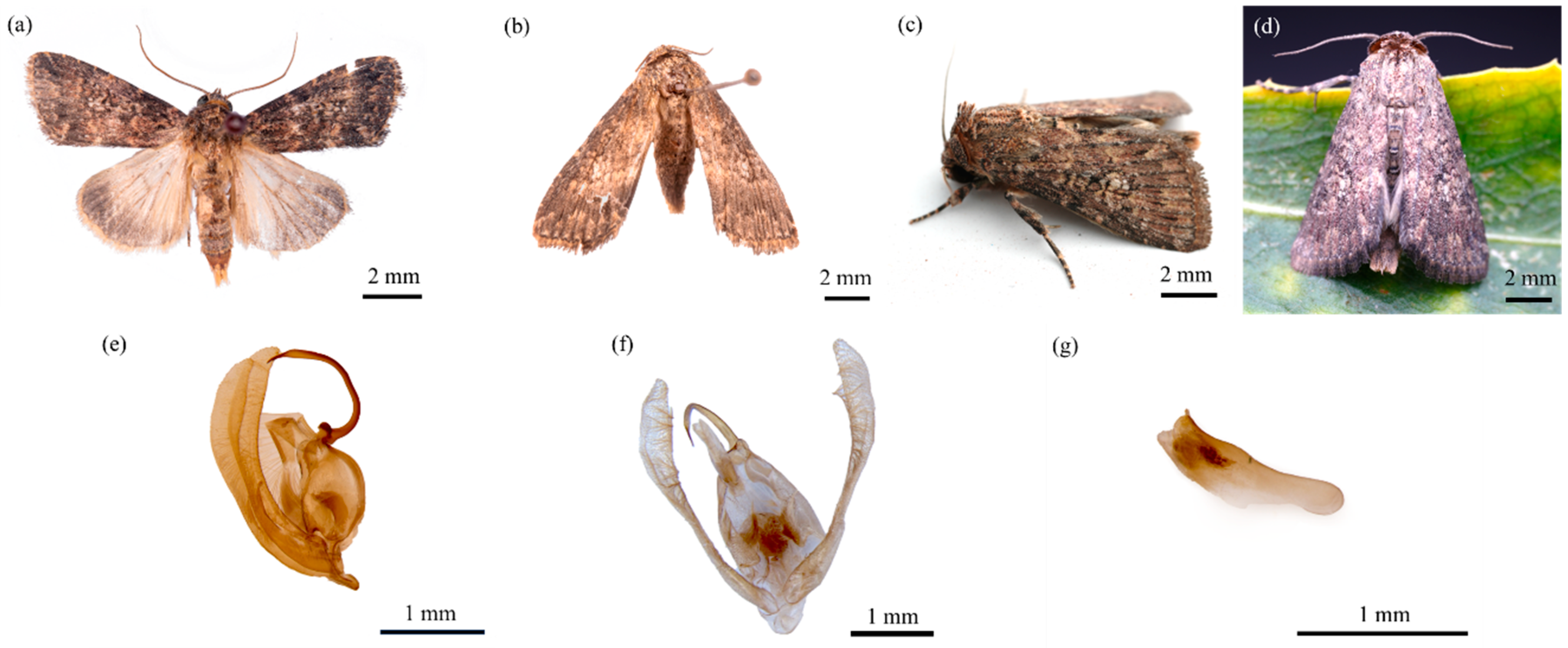
3.1.4. Adult
3.1.5. Male Genitalia
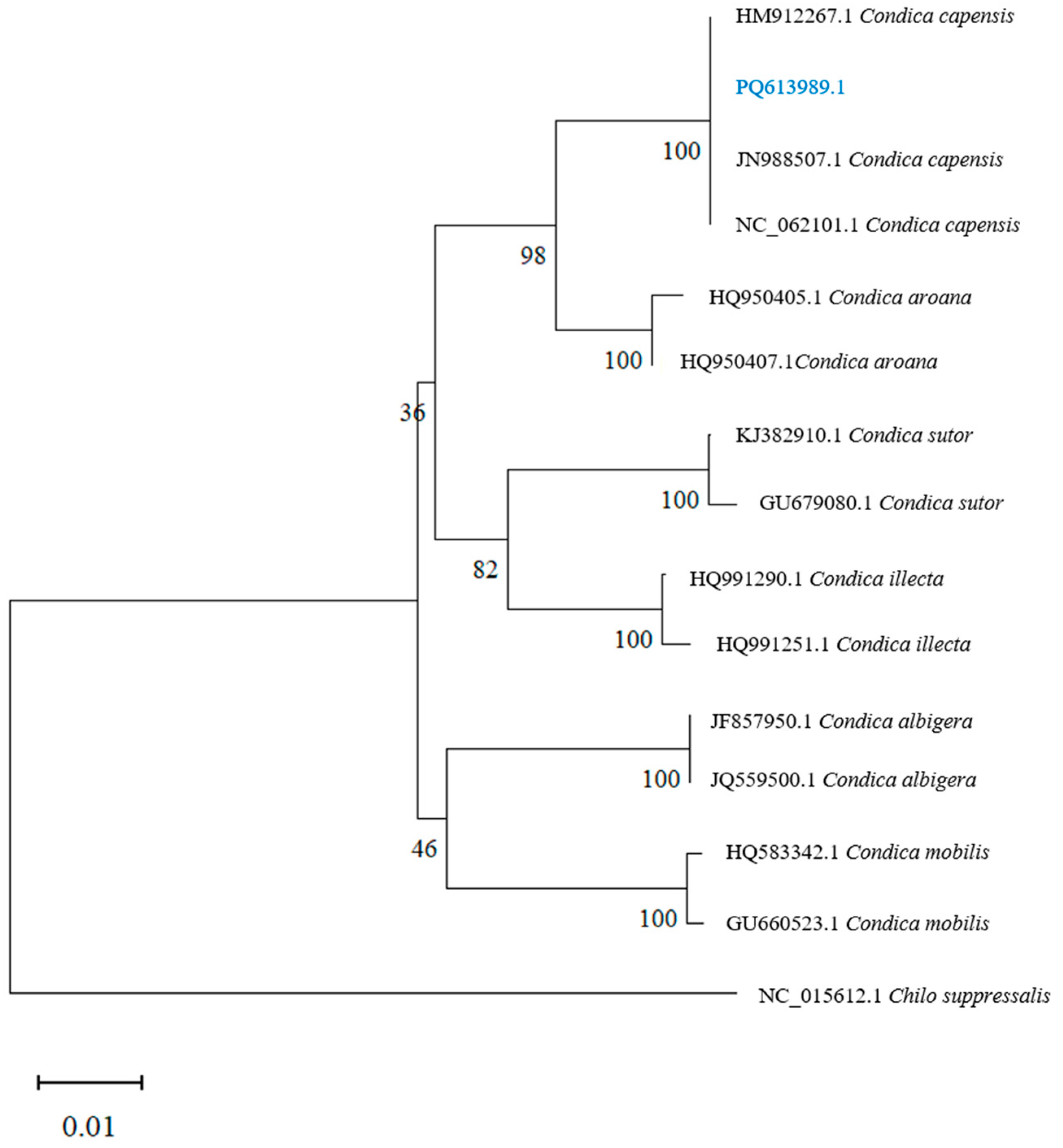
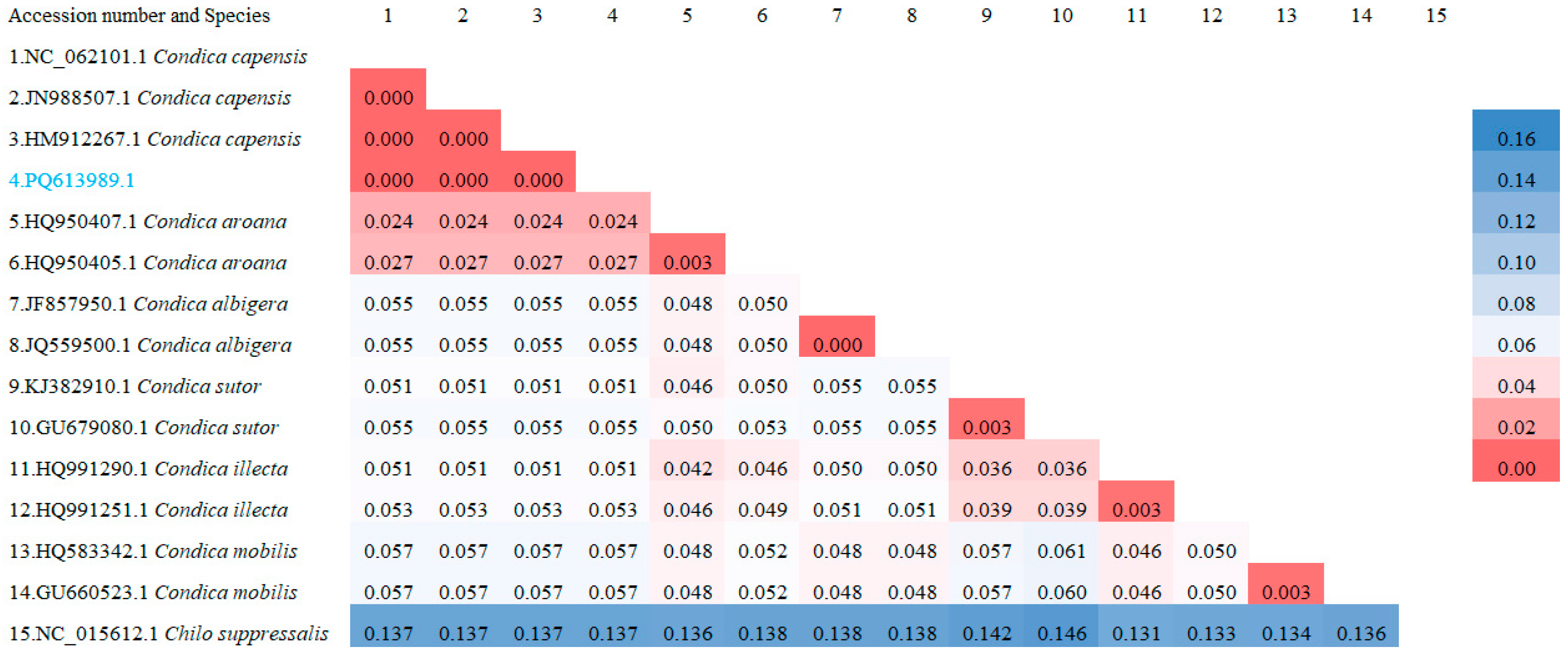
3.2. Molecular Identification
3.3. Phylogenetic Analysis
3.4. Biology of Condica capensis in Safflower
3.4.1. Life Cycle
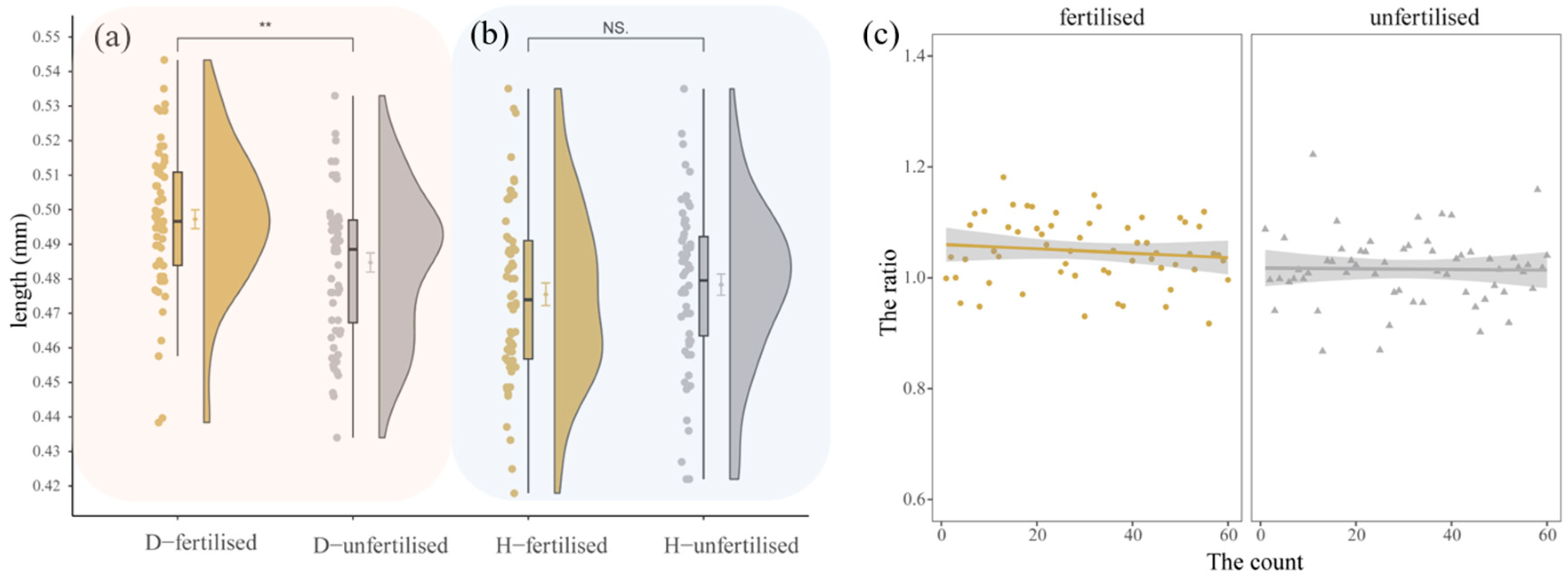
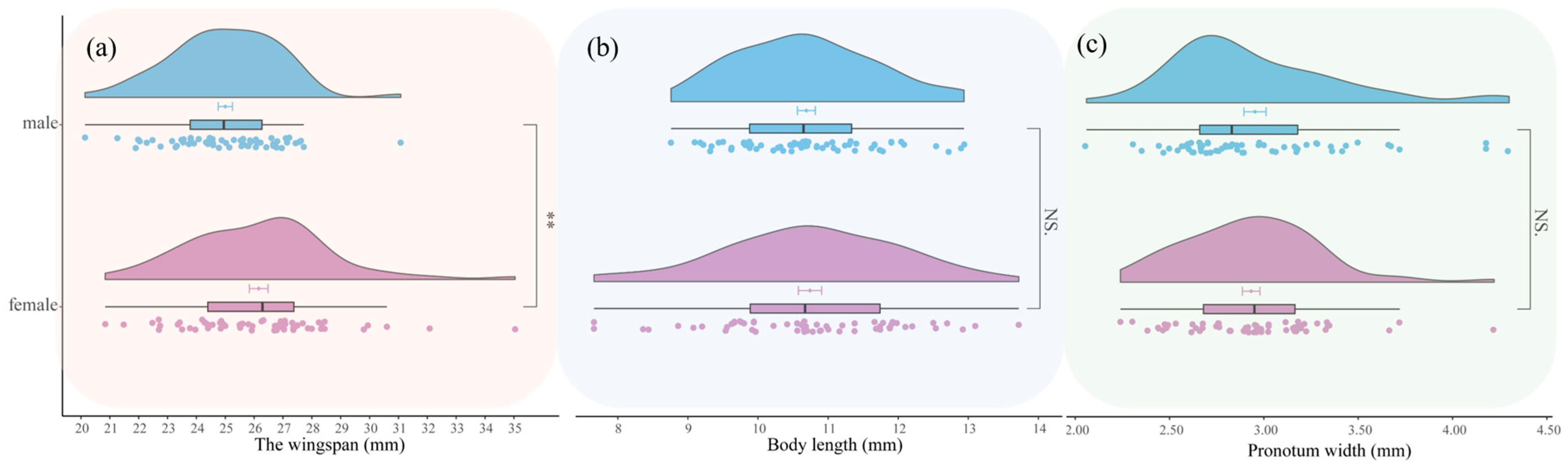
3.4.2. Morphometrics of Condica capensis
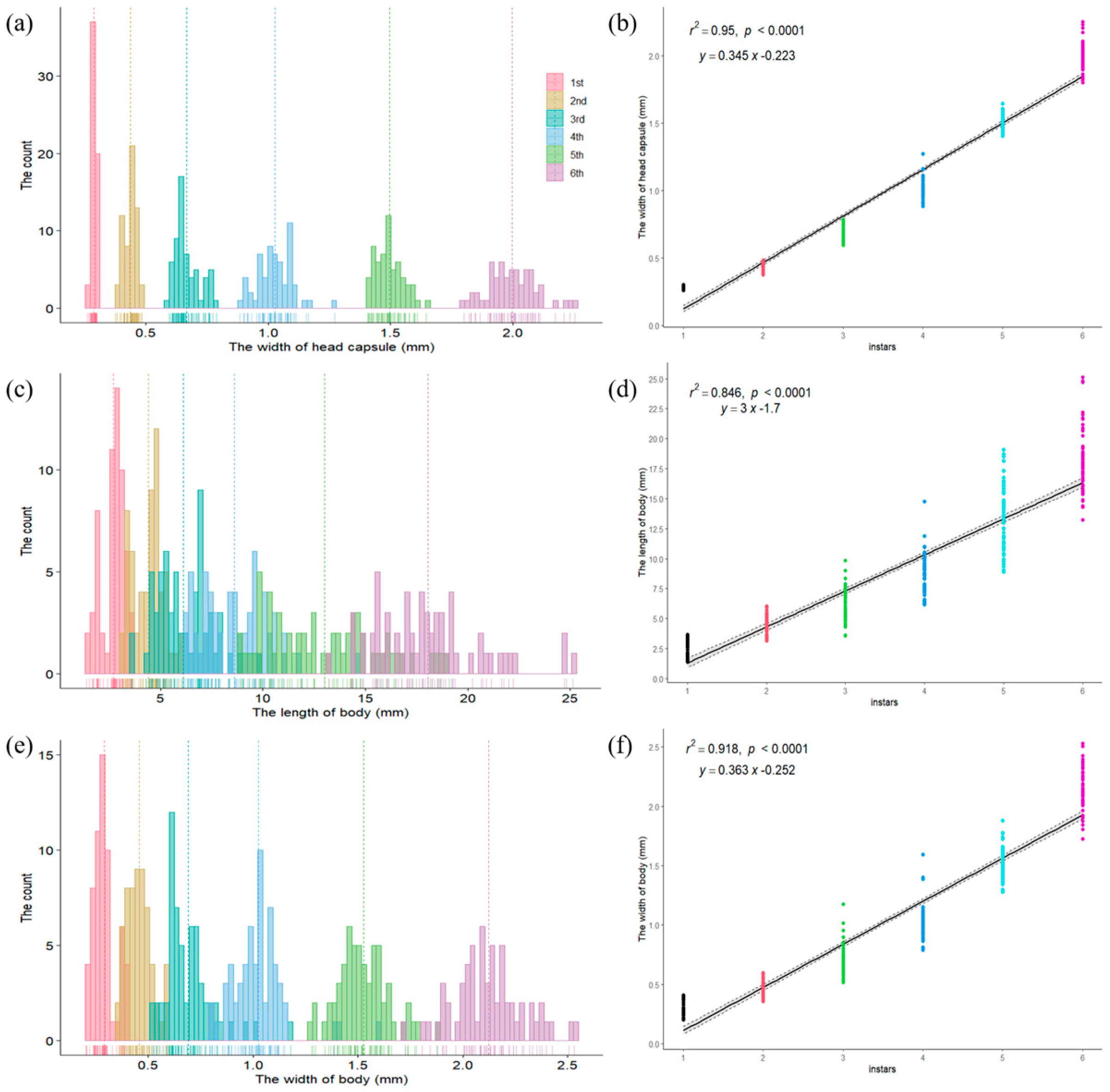
3.4.3. Instar Division
3.4.4. Reproductive Behavior
3.5. Natural Enemies of C. capensis in Safflower
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, S.C.; Yi, S.A.; Lee, B.S.; Yu, J.S.; Kim, J.-C.; Pang, C.; Jang, T.S.; Lee, J.; Kim, K.H. Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius). Biomedicines 2021, 9, 91. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Z.; Miao, L.; Wang, L. Effects of Extracts and Isolated Compounds from Safflower on Some Index of Promoting Blood Circulation and Regulating Menstruation. J. Ethnopharmacol. 2016, 191, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.S. Safflower; AOCS Press: Champaign, IL, USA, 1996; p. 2. [Google Scholar]
- Jin, J.; Liu, J.; Wang, D.; Gao, S.; Zhao, F.; Wang, Y. Reconstruction of Traditional Safflower (Carthamus tinctorius L.) Dyeing and Red Colors in the Qing Dynasty (17th–19th Century). Dye Pigment. 2022, 207, 110697. [Google Scholar] [CrossRef]
- Pearl, S.A.; Burke, J.M. Genetic Diversity in Carthamus tinctorius (Asteraceae; Safflower), an Underutilized Oilseed Crop. Am. J. Bot. 2014, 101, 1640–1650. [Google Scholar] [CrossRef]
- Kammili, A.; Yadav, P. Enhancing Oleic Acid and Oil Content in Low Oil and Oleic Type Indian Safflower (Carthamus tinctorius L.). Ind. Crops Prod. 2022, 175, 114254. [Google Scholar] [CrossRef]
- Khalid, N.; Khan, R.S.; Hussain, M.I.; Farooq, M.; Ahmad, A.; Ahmed, I. A Comprehensive Characterisation of Safflower Oil for Its Potential Applications as a Bioactive Food Ingredient—A Review. Trends. Food. Sci. Technol. 2017, 66, 176–186. [Google Scholar] [CrossRef]
- Chapman, M.A.; Hvala, J.; Strever, J.; Burke, J.M. Population Genetic Analysis of Safflower (Carthamus tinctorius; Asteraceae) Reveals a near Eastern Origin and Five Centers of Diversity. Am. J. Bot. 2010, 97, 831–840. [Google Scholar] [CrossRef]
- Wu, X.; Cai, X.; Ai, J.; Zhang, C.; Liu, N.; Gao, W. Extraction, Structures, Bioactivities and Structure-Function Analysis of the Polysaccharides from Safflower (Carthamus tinctorius L.). Front. Pharmacol. 2021, 12, 767947. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, K.; Azura, A.N.; Omar, D.; Abood, F. Pests of Safflower (Carthamus tinctorious L.) and Their Natural Enemies in Gachsara, Iran. South Asian J. Exp. Biol. 2012, 1, 286–291. [Google Scholar] [CrossRef]
- Androcioli, H.G.; Hoshino, A.T.; Pastório, M.A.; Cardoso, P.C.; de Araújo, P.M.; Fernandes, T.A.P.; Menezes, A.O. First Record of Euphoria Lurida Fabricius (Coleoptera: Scarabaeidae) Injurious to Safflower (Carthamus tinctorius L.) (Asterales: Asteraceae) in Brazil. Neotrop. Entomol. 2017, 46, 130–132. [Google Scholar] [CrossRef]
- Yucel, C.; Ozdemir, I.; Coral, D. DNA Barcoding Data of Aphids (Hemiptera: Aphidomorpha) in Safflower (Carthamus tinctorius L.) with New Host Plant Records in Turkey. J. Entomol. Res. Soc. 2022, 24, 47–61. [Google Scholar] [CrossRef]
- Condica capensis in Catalogue of Life China: 2024 Annual Checklist, Beijing, China. Available online: http://www.sp2000.org.cn/species/show_species_details/1CE8B40F-2F79-4EC9-93A4-059E5349ED80 (accessed on 11 December 2024).
- Rijllo, G.; La Cava, S.; Zucco, G.; Scalercio, S. Gone with the Wind? Condica capensis (Guenée 1852), a Migrant Species New for Italy (Lepidoptera: Noctuidae). Nat. Hist. Sci. 2024, 11, 61–64. [Google Scholar] [CrossRef]
- Amer, A. Revision of Family Noctuidae (4) Subfamilies “Bryophilinae, Condicinae, Cuculliinae, Eriopinae and Eustrotiinae” of Egypt (Lepidoptera, Noctuidae). Egypt. Acad. J. Biol. Sci. A Entomol. 2020, 13, 1–34. [Google Scholar] [CrossRef]
- Rangarajan, A.V.; Mahadevan, N.R.; Iyemperumal, S. Pest Complex of Sunflower (Helianthus annus Linn.) in Tamil Nadu. Indian J. Entomol. 1977, 37, 188–191. [Google Scholar]
- Balikai, R.A. Insect Pests of Safflower and Their Natural Enemies in Northern Karnataka. Karnataka J. Agric. Sci. 2000, 13, 737–740. [Google Scholar]
- Kranthi, S.; Kranthi, K.R.; Rishi, K.; Udikeri, S.S.; Rao, G.M.V.P.; Zanwar, P.R.; Nagrare, V.N.; Naik, C.B.; Singh, V.; Ramamurthy, V.V.; et al. Emerging and key insect pests on Bt cotton-their identification, taxonomy, genetic diversity and management. In Proceedings of the World Cotton Research Conference-5, Mumbai, India, 7–11 November 2011; Excel India Publishers: New Delhi, India, 2011; Volume 47, pp. 281–286. [Google Scholar]
- Chen, L.; Pan, Q.; Waqas, M.S.; Liu, T. Morphological Traits for Sex Identification of the Oriental Armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Integr. Agric. 2020, 19, 1458–1463. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Loerch, C.R.; Cameron, E.A. Determination of Larval Instars of the Bronze Birch Borer, Agrilus anxius (Coleoptera: Buprestidae)1. Ann. Entomol. Soc. Am. 1983, 76, 948–952. [Google Scholar] [CrossRef]
- AR6 Synthesis Report: Climate Change 2023—IPCC. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 10 December 2024).
- Sinelnikov, I.G.; Siedhoff, N.E.; Chulkin, A.M.; Zorov, I.N.; Schwaneberg, U.; Davari, M.D.; Sinitsyna, O.A.; Shcherbakova, L.A.; Sinitsyn, A.P.; Rozhkova, A.M. Expression and Refolding of the Plant Chitinase from Drosera Capensis for Applications as a Sustainable and Integrated Pest Management. Front. Bioeng. Biotechnol. 2021, 9, 728501. [Google Scholar] [CrossRef] [PubMed]
- Walker, F. List of the Specimens of Lepidopterous Insects in the Collection of the British Museum; Part IX—Noctuidae; British Museum (Natural History): London, UK, 1856; pp. 214–239. [Google Scholar]
- Browse Taxonomic Tree in Catalogue of Life China: 2024 Annual Checklist, Beijing, China. Available online: http://www.sp2000.org.cn/browse/browse_this_taxa/ee2b3e1d-5eaf-4841-a579-eb580c239491 (accessed on 11 December 2024).
- Hussain, Z.; Sarwar, Z.M.; Akbar, A.; Alhag, S.K.; Ahmed, N.; Alam, P.; Almadiy, A.A.; Zouidi, F.; Jawalkar, N.B. Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem. Agriculture 2022, 12, 2003. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Feng, H.; Liu, Z.; Wu, K. Trans-Regional Migration of Agrotis ipsilon (Lepidoptera: Noctuidae) in North-East Asia. Ann. Entomol. Soc. Am. 2015, 108, 519–527. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Zhao, S.; Guo, J.; Wyckhuys, K.A.G.; Wu, K. Migratory Helicoverpa armigera (Lepidoptera: Noctuidae) Exhibits Marked Seasonal Variation in Morphology and Fitness. Environ. Entomol. 2019, 48, 755–763. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Huang, L.; Liu, J.; Zhang, H.-B.; Qiu, K.; Lu, F.; Hu, G. Migration Dynamics of Fall Armyworm Spodoptera frugiperda (Smith) in the Yangtze River Delta. Insects 2023, 14, 127. [Google Scholar] [CrossRef]
- Cang, X.; Zhao, S.; Yang, X.; Yuan, H.; Liu, J.; Liu, D.; Yang, X.; Wu, K. Migration Monitoring and Route Analysis of the Oriental Armyworm Mythimna separata (Walker) in Northeast China. Agriculture 2023, 13, 172. [Google Scholar] [CrossRef]
- Song, Y.; Cang, X.; He, W.; Zhang, H.; Wu, K. Migration Activity of Spodoptera litura (Lepidoptera: Noctuidae) between China and the South-Southeast Asian Region. Inscets 2024, 15, 335. [Google Scholar] [CrossRef]
- Wang, X.; Du, Z.; Duan, Y.; Liu, S.; Liu, J.; Li, B.; Ma, L.; Wu, Y.; Tian, L.; Song, F.; et al. Population Genomics Analyses Reveal the Role of Hybridization in the Rapid Invasion of Fall Armyworm. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Idrees, A.; Haider, M.U.; Rasool, B.; Mehmood, R.; Afzal, A.; Javaid, A.; Shad, N.; Ashrafl, S.; Liz, J. COI-Gene Based Molecular Identification of Chilo partellus Swinhoe (Lepidoptera: Pyralidae) Infesting Maize in Lahore, Pakistan. Philipp. Agric. Sci. 2022, 105, 303–308. [Google Scholar] [CrossRef]
- Abdel-Galil, F.A.; Mousa, S.E.; Abou-Elhagag, G.H.; Ahmed, A.M.M.; Al-Farga, A.; Allam, M.; Mahmoud, M.A.B. Morphogenetic Identification of a New Record Deudorix Livia (Lepidoptera: Lycaenidae) in Assiut Governorate of Northern Upper Egypt. Sci. Rep. 2023, 13, 20009. [Google Scholar] [CrossRef]
- Sekar, S. A Meta-Analysis of the Traits Affecting Dispersal Ability in Butterflies: Can Wingspan Be Used as a Proxy? J. Anim. Ecol. 2012, 81, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Perrard, A. Wasp Waist and Flight: Convergent Evolution in Wasps Reveals a Link between Wings and Body Shapes. Am. Nat. 2020, 195, 181–191. [Google Scholar] [CrossRef]
- Galaschi-Teixeira, J.S.; Veiga, J.C.; Tavares, V.D.C.; Imperatriz-Fonseca, V.L. Geometric Morphometrics of the Wings of Amazonian Species of Melipona (Illiger, 1806) (Hymenoptera: Apidae). Zootaxa 2024, 5404, 124–133. [Google Scholar] [CrossRef]
- Queiroz-Santos, L.; Casagrande, M.M.; Specht, A. Morphological Characterization of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae: Heliothinae). Neotrop. Entomol. 2018, 47, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Williams, T.; Escribano, A.; Caballero, P.; Cave, R.D.; Goulson, D. Age-Related Cannibalism and Horizontal Transmission of a Nuclear Polyhedrosis Virus in Larval Spodoptera frugiperda. Ecol. Entomol. 1999, 24, 268–275. [Google Scholar] [CrossRef]
- Elvira, S.; Williams, T.; Caballero, P. Juvenile Hormone Analog Technology: Effects on Larval Cannibalism and the Production of Spodoptera Exigua (Lepidoptera: Noctuidae) Nucleopolyhedrovirus. J. Econ. Entomol. 2010, 103, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.T. Circadian Rhythms of Sexual Activities in Moths: A Review. Front. Ecol. Evol. 2014, 2, 00043. [Google Scholar] [CrossRef]
- Ulmer, B.; Gillott, C.; Erlandson, M. Conspecific Eggs and Bertha Armyworm, Mamestra configurata (Lepidoptera: Noctuidae), Oviposition Site Selection. Environ. Entomol. 2003, 32, 529–534. [Google Scholar] [CrossRef]
- Volp, T.M.; Zalucki, M.P.; Furlong, M.J. What Defines a Host? Oviposition Behavior and Larval Performance of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Five Putative Host Plants. J. Econ. Entomol. 2022, 115, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; North, H.L.; Peng, Y.; Liu, H.; Liu, B.; Pan, R.; Zhou, Y.; Zheng, W.; Liu, K.; Yang, B.; et al. Adaptive Evolution to the Natural and Anthropogenic Environment in a Global Invasive Crop Pest, the Cotton Bollworm. Innov. 2023, 4, 100454. [Google Scholar] [CrossRef] [PubMed]
- Hervet, V.A.D.; Murillo, H.; Fernández-Triana, J.L.; Shaw, M.R.; Laird, R.A.; Floate, K.D. First Report of Cotesia vanessae (Hymenoptera: Braconidae) in North America. Can. Entomol. 2014, 146, 560–566. [Google Scholar] [CrossRef]
- Matioli, T.F.; Zanardi, O.Z.; Yamamoto, P.T. Impacts of Seven Insecticides on Cotesia flavipes (Cameron) (Hymenoptera: Braconidae). Ecotoxicology 2019, 28, 1210–1219. [Google Scholar] [CrossRef]
- Song, W.; Liu, L.; Li, P.; Sun, H.; Qin, Y. Analysis of the Mating and Reproductive Traits of Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2014, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.J.; Gray, H.L.; Heimpel, G.E. High Hyperparasitism of Cotesia rubecula (Hymenoptera: Braconidae) in Minnesota and Massachusetts. J. Kans. Entomol. Soc. 2016, 89, 385–389. [Google Scholar] [CrossRef]




| Life Stages | Mean ± SE (days) | Variation (days) | |
|---|---|---|---|
| Eggs period | 3.92 ± 0.28 | 3.00–4.00 | |
| Larval period | 1st | 4.03 ± 0.18 | 4.00–5.00 |
| 2nd | 2.02 ± 0.34 | 1.00–3.00 | |
| 3rd | 2.55 ± 0.57 | 1.00–3.00 | |
| 4th | 2.90 ± 1.04 | 1.00–5.00 | |
| 5th | 3.72 ± 1.37 | 2.00–10.00 | |
| 6th | 6.42 ± 1.68 | 4.00–14.00 | |
| Pupal period | 9.63 ± 1.54 | 6.00–14.00 | |
| Male | 13.17 ± 3.83 | 4.00–20.00 | |
| Female | 14.33 ± 3.17 | 5.00–20.00 | |
| Variable | Instars | Mean ± SE (mm) | Variation (mm) | Coefficient of Variance (%) | Brooks Index | Crosby Index |
|---|---|---|---|---|---|---|
| Head capsule width | 1 | 0.2870 ± 0.0087 f | 0.2600–0.3000 | 3.0161 | ||
| 2 | 0.4363 ± 0.0260 e | 0.3740–0.4840 | 5.9598 | 1.5199 | ||
| 3 | 0.6662 ± 0.0495 d | 0.5940–0.7870 | 7.4241 | 1.5271 | 0.0048 | |
| 4 | 1.0270 ± 0.0711 c | 0.8850–1.2730 | 6.9240 | 1.5416 | 0.0095 | |
| 5 | 1.4942 ± 0.0571 b | 1.4060–1.6450 | 3.8230 | 1.4549 | −0.0562 | |
| 6 | 1.9956 ± 0.0989 a | 1.8010–2.2540 | 4.9547 | 1.3355 | −0.0821 | |
| Body Width | 1 | 0.2903 ± 0.0515 f | 0.2020–0.4090 | 17.7495 | ||
| 2 | 0.4570 ± 0.0610 e | 0.3590–0.5970 | 13.3539 | 1.5742 | ||
| 3 | 0.6902 ± 0.1191 d | 0.5160–1.1770 | 17.2499 | 1.5103 | −0.0406 | |
| 4 | 1.0265 ± 0.1343 c | 0.7910–1.5920 | 13.0806 | 1.4872 | −0.0153 | |
| 5 | 1.5257 ± 0.1245 b | 1.2800–1.8800 | 8.1585 | 1.4863 | −0.0006 | |
| 6 | 2.1234 ± 0.1673 a | 1.7260–2.5310 | 7.8810 | 1.3918 | −0.0636 | |
| Body Length | 1 | 2.6906 ± 0.5643 f | 1.3770–3.6670 | 20.9745 | ||
| 2 | 4.4065 ± 0.7545 e | 3.1410–6.0270 | 17.1232 | 1.6378 | ||
| 3 | 6.1109 ± 1.3043 d | 3.5920–9.8510 | 21.3444 | 1.3868 | −0.1532 | |
| 4 | 8.6005 ± 1.6687 c | 6.1490–14.7500 | 19.4025 | 1.4074 | 0.0149 | |
| 5 | 13.0174 ± 2.7303 b | 8.9060–19.0730 | 20.9745 | 1.5136 | 0.0754 | |
| 6 | 18.0580 ± 2.6158 a | 13.2260–25.1350 | 14.4854 | 1.3872 | −0.0835 |
| Treatment | Number of Observations (ind.) | Pre-Copulation Period (days) | Pre-Oviposition Period (days) | Number of Eggs (Grains) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Variation | Mean ± SE | Variation | Mean ± SE | Variation | ||
| Mated female | 18 | 1.61 ± 0.78 | 0.00–3.00 | 3.22 ± 1.22 b | 1.00–6.00 | 364.44 ± 216.30 a | 36.00–913.00 |
| Virgin female | 12 | / | / | 5.00 ± 1.35 a | 3.00–7.00 | 309.42 ± 164.30 a | 88.00–662.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, P.; Fan, J.; Zhang, X.; Zeng, M.; Han, X.; Li, Y.; Luo, X. Morphogenetic Identification of a New Record Condica capensis (Lepidoptera: Noctuidae) in Yunnan, China. Insects 2025, 16, 130. https://doi.org/10.3390/insects16020130
Qian P, Fan J, Zhang X, Zeng M, Han X, Li Y, Luo X. Morphogenetic Identification of a New Record Condica capensis (Lepidoptera: Noctuidae) in Yunnan, China. Insects. 2025; 16(2):130. https://doi.org/10.3390/insects16020130
Chicago/Turabian StyleQian, Pengfan, Jiayin Fan, Xiaoyuan Zhang, Minfang Zeng, Xiaolong Han, Yonghe Li, and Xulu Luo. 2025. "Morphogenetic Identification of a New Record Condica capensis (Lepidoptera: Noctuidae) in Yunnan, China" Insects 16, no. 2: 130. https://doi.org/10.3390/insects16020130
APA StyleQian, P., Fan, J., Zhang, X., Zeng, M., Han, X., Li, Y., & Luo, X. (2025). Morphogenetic Identification of a New Record Condica capensis (Lepidoptera: Noctuidae) in Yunnan, China. Insects, 16(2), 130. https://doi.org/10.3390/insects16020130






