Phylogenetics and Evolutionary Dynamics of Yunnan Acrididae Grasshoppers Inferred from 17 New Mitochondrial Genomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. DNA Quality Control and Genome Sequencing
2.3. Mitochondrial Genome Assembly and Annotation
2.4. Comparative Genomic and Phylogenetic Analyses
2.5. Statistical Analysis and Visualization
3. Results
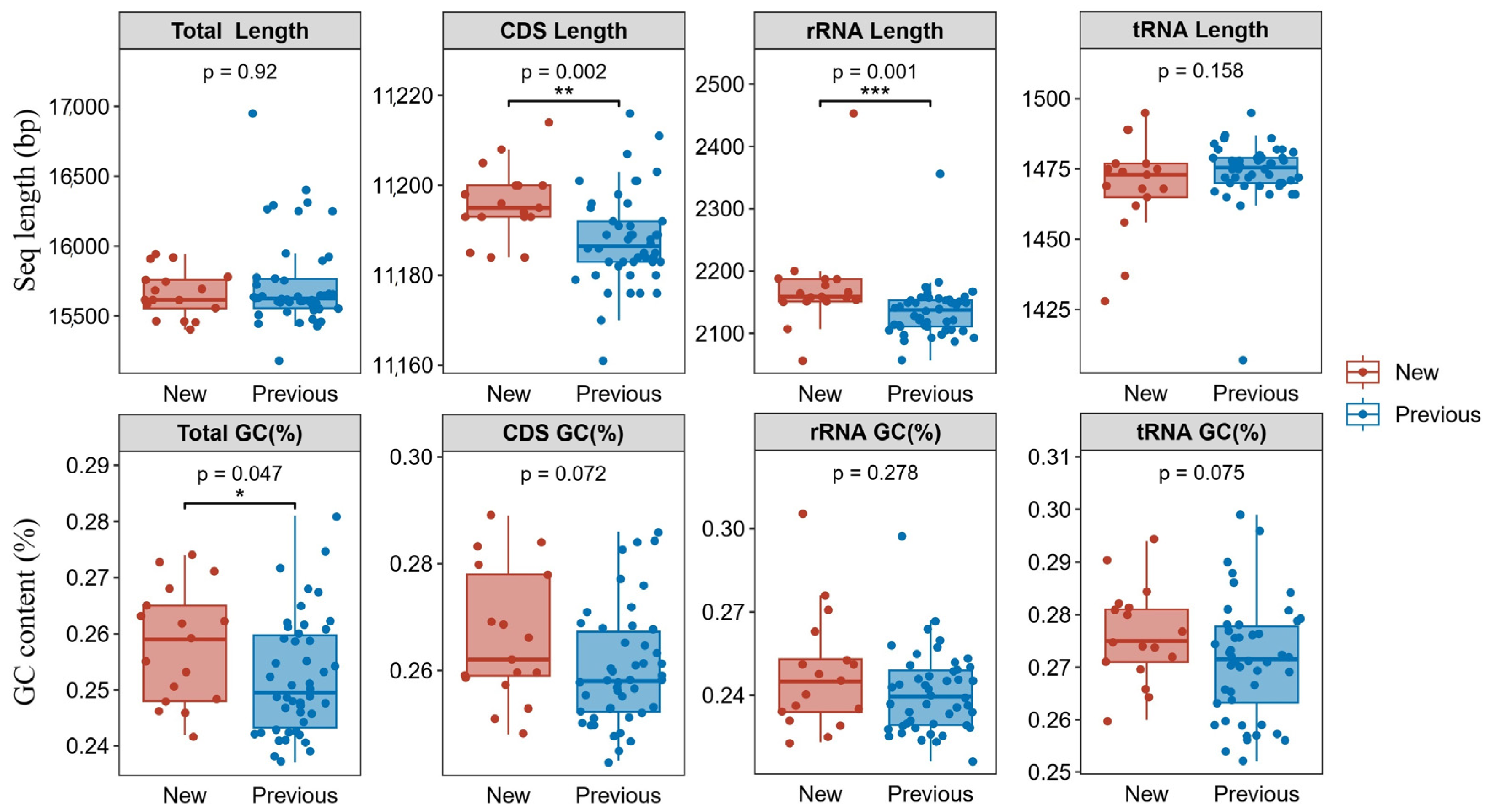
3.1. Mitochondrial Genome Organization and Features
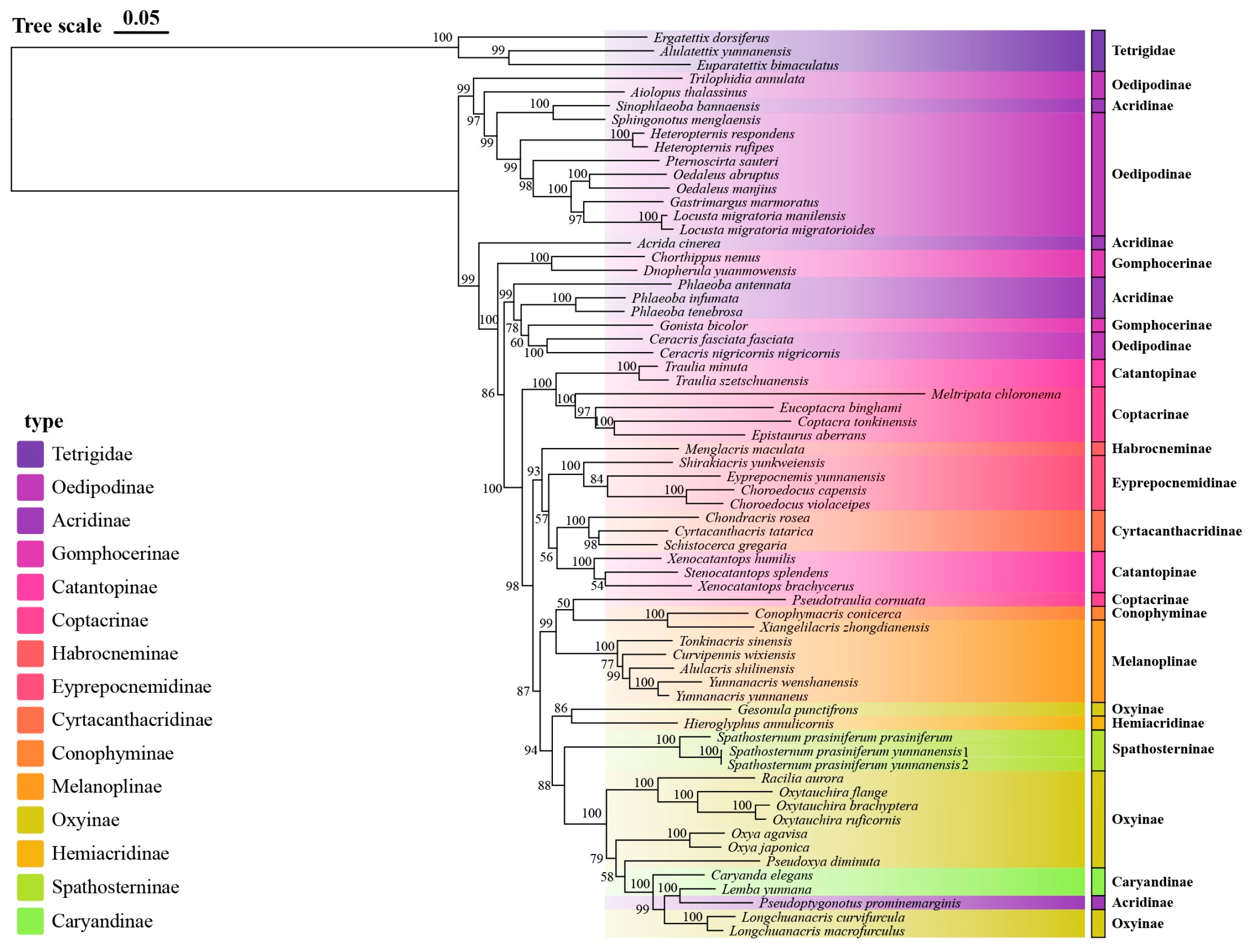
3.2. Phylogenetic Inference and Divergence Time Estimation
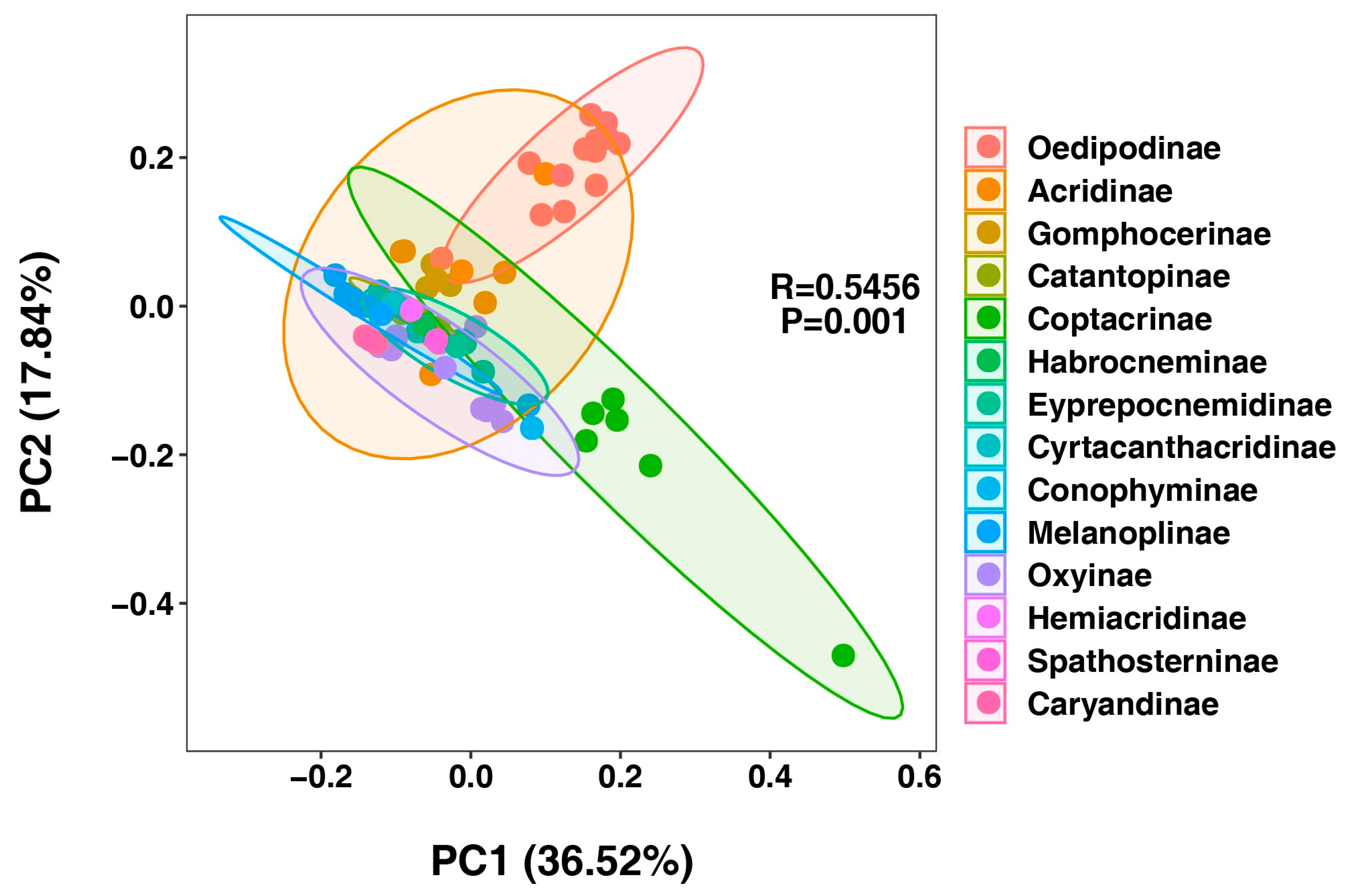
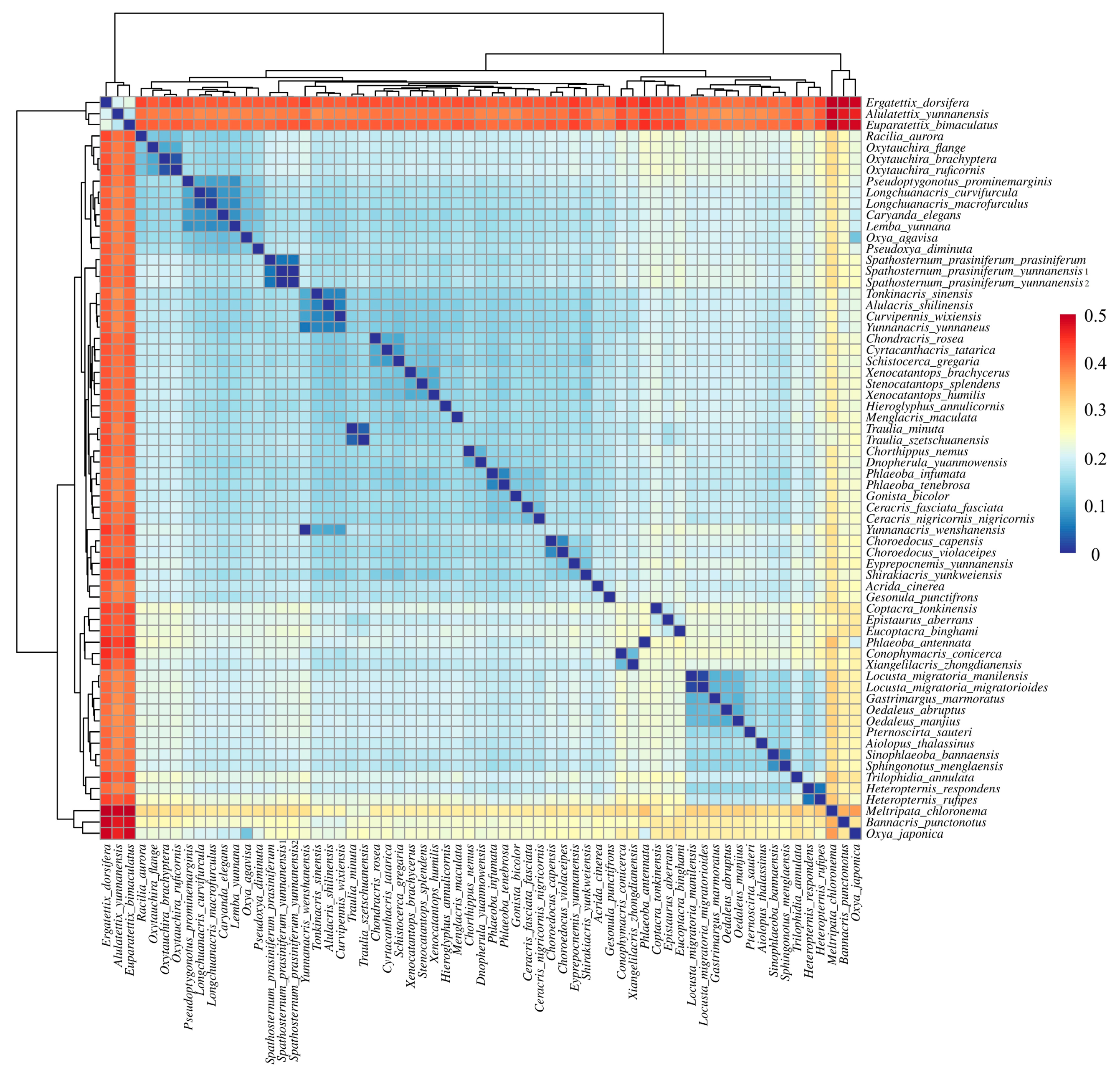
3.3. Phylogenetic Implications Through Analysis of Codon Usage and Genetic Distances
4. Discussion
4.1. Phylogenetic Relationships and Taxonomic Revisions
4.2. Divergence Times and Evolutionary Dynamics
4.3. Species Conservation Implications
4.4. Mitochondrial Genome Conservation and Variation
4.5. Implications for Future Studies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariño-Pérez, R.; Song, H. On the origin of the New World Pyrgomorphidae (Insecta: Orthoptera). Mol. Phylogenet. Evol. 2019, 139, 106537. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Amédégnato, C.; Cigliano, M.M.; Desutter-Grandcolas, L.; Heads, S.W.; Huang, Y.; Otte, D.; Whiting, M.F. 300 million years of diversification: Elucidating the patterns of orthopteran evolution based on comprehensive taxon and gene sampling. Cladistics Int. J. Willi Hennig Soc. 2015, 31, 621–651. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Qiu, Z.; Yuan, H.; Wang, X.; Li, X.; Sun, H.; Guo, X.; Lu, Y.; Feng, X.; Majid, M.; et al. Evolutionary rates of and selective constraints on the mitochondrial genomes of Orthoptera insects with different wing types. Mol. Phylogenet. Evol. 2020, 145, 106734. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Jiang, B.; Wang, H.; Wei, T.; Lin, L.; Huang, Y.; Huang, J. Phylogeny and species delimitation of the genus Longgenacris and Fruhstorferiola viridifemorata species group (Orthoptera: Acrididae: Melanoplinae) based on molecular evidence. PLoS ONE 2020, 15, e0237882. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Foquet, B.; Mariño-Pérez, R.; Woller, D.A. Phylogeny of locusts and grasshoppers reveals complex evolution of density-dependent phenotypic plasticity. Sci. Rep. 2017, 7, 6606. [Google Scholar] [CrossRef] [PubMed]
- Zhongying, Q.; Huihui, C.; Hao, Y.; Yuan, H.; Huimeng, L.; Xia, L.; Xingchun, G. Comparative mitochondrial genomes of four species of Sinopodisma and phylogenetic implications (Orthoptera, Melanoplinae). ZooKeys 2020, 969, 23–42. [Google Scholar] [CrossRef]
- Yang, J.; Lu, C.; Zhang, Z.B.; Huang, Y.; Lin, L.L. Mitochondrial Genomes of Two Pygmy Grasshoppers (Orthoptera: Tetrigoidea) and a Comparative Analysis of Caelifera Mitogenomes. Zool. Sci. 2017, 34, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhao, L.; Zheng, Z.M.; Huang, Y. Complete mitochondrial genome of Gomphocerus sibiricus (Orthoptera: Acrididae) and comparative analysis in four Gomphocerinae mitogenomes. Zool. Sci. 2013, 30, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wu, R.; Hua, C.; Ma, J.; Wang, W.; Yang, F.; Wang, J. Identifying local-scale wilderness for on-ground conservation actions within a global biodiversity hotspot. Sci. Rep. 2016, 6, 25898. [Google Scholar] [CrossRef]
- Liu, C.; Fischer, G.; Hita Garcia, F.; Yamane, S.; Liu, Q.; Peng, Y.Q.; Economo, E.P.; Guénard, B.; Pierce, N.E. Ants of the Hengduan Mountains: A new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 2020, 978, 1–171. [Google Scholar] [CrossRef]
- Liu, C.; Guénard, B.; Garcia, F.H.; Yamane, S.; Blanchard, B.; Yang, D.R.; Economo, E. New records of ant species from Yunnan, China. ZooKeys 2015, 477, 17–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Kallies, A.; Arita, Y.; He, J.; Li, H.; Yata, N.; Li, X. Two new species of the genus Taikona Arita & Gorbunov, 2001 (Lepidoptera, Sesiidae) from Yunnan, China. Zootaxa 2024, 5443, 135–140. [Google Scholar]
- Song, H.; Yin, Z.L.; Storozhenko, S.Y.; Mao, B.Y. A review of the Caryanda tamdaoensis species-group (Orthoptera: Acrididae), with description of a new species from Yunnan, China. Zootaxa 2024, 5474, 412–426. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Dong, W.G.; Song, W.Y. An updated distribution and hosts: Trombiculid mites (Acari: Trombidiformes) associated with small mammals in Yunnan Province, southwest China. Parasitol. Res. 2016, 115, 1923–1938. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Song, W.Y.; Dong, W.G.; Fan, R. Species diversity of ectoparasitic chigger mites (Acari: Prostigmata) on small mammals in Yunnan Province, China. Parasitol. Res. 2016, 115, 3605–3618. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.L.; Mao, B.Y. A review of Caryanda viridis-species group (Orthoptera: Acrididae) with a new species. Zootaxa 2023, 5263, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Sun, H.; Zhao, Q.; Lin, C.; Sun, Y.; Gao, W.; Xu, J.; Zhou, J.; Ge, F.; Liu, N. Patterns of diversity, areas of endemism, and multiple glacial refuges for freshwater crabs of the genus Sinopotamon in China (Decapoda: Brachyura: Potamidae). PLoS ONE 2013, 8, e53143. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.L.; Colville, J.F.; Engelbrecht, A.; Couldridge, V.C.K. Ancient Grasshoppers: A revision of the genus Bullacris (Orthoptera: Pneumoridae). Zootaxa 2024, 5474, 301–354. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Fu, Y.; Zhou, X.; Yang, S.; Cao, Y.; Hou, F.; Liu, X.; Sun, T. Complete mitogenome of Calliptamus barbarus Costa (Orthoptera: Acrididae) and its phylogeny in Acridoidea. Zootaxa 2022, 5213, 427–440. [Google Scholar] [CrossRef]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.-T. The Silent Extinction of Species and Taxonomists—An Appeal to Science Policymakers and Legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Yang, R.; Guan, D.L.; Xu, S.Q. Complete mitochondrial genome of the Chinese endemic grasshopper Fruhstorferiola kulinga (Orthoptera: Acrididae: Podismini). Mitochondrial DNA. Part A DNA Mapp. Seq. Anal. 2016, 27, 3240–3241. [Google Scholar] [CrossRef]
- Wang, M.Q.; Deng, Y.; Guan, D.L.; Mao, B.Y.; Li, M. A new species of the genus Tuberfemurus (Orthoptera: Tetrigoidea: Cladonotinae) with comments on the characters of mitochondrial genome. Zootaxa 2021, 5071, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, Z.; Wang, Q.; Guan, D.; Xu, S.; Robillard, T. A new species of the genus Hilethera Uvarov, 1923 (Orthoptera: Acrididae: Oedipodinae) from China and its complete mitochondrial genome. Zootaxa 2019, 4564, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.P.; Wang, X.Y.; Yin, Y.; Zhang, Y.X.; Rizvi, U.E.; Tan, S.Q.; Cao, C.; Yu, H.Y.; Ji, R. Dynamics of Aboveground Natural Enemies of Grasshoppers, and Biodiversity after Application of Paranosema locustae in Rangeland. Insects 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Theron, K.J.; Pryke, J.S.; Samways, M.J. Identifying managerial legacies within conservation corridors using remote sensing and grasshoppers as bioindicators. Ecol. Appl. A Publ. Ecol. Soc. Am. 2022, 32, e02496. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [PubMed]
- Blankenberg, D.; Coraor, N.; Von Kuster, G.; Taylor, J.; Nekrutenko, A. Integrating diverse databases into an unified analysis framework: A Galaxy approach. Database 2011, 2011, bar011. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.Y.; Gao, F.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Barido-Sottani, J.; Morlon, H. The ClaDS rate-heterogeneous birth-death prior for full phylogenetic inference in BEAST2. Syst. Biol. 2023, 72, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Baele, G.; Carvalho, L.M.; Brusselmans, M.; Dudas, G.; Ji, X.; McCrone, J.T.; Lemey, P.; Suchard, M.A.; Rambaut, A. HIPSTR: Highest independent posterior subtree reconstruction in TreeAnnotator X. bioRxiv 2024. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, B.; Wang, H.; Dai, L.; Huang, Y.; Chen, Z.; Huang, J. The Complete Mitogenomes of Three Grasshopper Species with Special Notes on the Phylogenetic Positions of Some Related Genera. Insects 2023, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Song, H. Annotated catalogue of Pakistani Acrididae (Orthoptera: Caelifera: Acridoidea). Zootaxa 2024, 5486, 1–47. [Google Scholar] [CrossRef]
- Storozhenko, S.Y.; Kim, T. A new species in the genus Alectorolophus Brunner von Wattenwyl, 1898 from Indonesia with discussion on its position compared to allied genera in subfamily Catantopinae (Orthoptera: Acrididae). Zootaxa 2016, 4121, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Ren, G.; Ou, X. Yunnan Locust Fauna, Distribution Patterns and Adaptive Characteristics; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- Wang, Q.; Spicer, R.A.; Yang, J.; Wang, Y.F.; Li, C.S. The Eocene climate of China, the early elevation of the Tibetan Plateau and the onset of the Asian Monsoon. Glob. Change Biol. 2013, 19, 3709–3728. [Google Scholar] [CrossRef] [PubMed]
- Setty, S.; Cramwinckel, M.J.; van Nes, E.H.; van de Leemput, I.A.; Dijkstra, H.A.; Lourens, L.J.; Scheffer, M.; Sluijs, A. Loss of Earth system resilience during early Eocene transient global warming events. Sci. Adv. 2023, 9, eade5466. [Google Scholar] [CrossRef]
- Ni, X.; Li, Q.; Li, L.; Beard, K.C. Oligocene primates from China reveal divergence between African and Asian primate evolution. Science 2016, 352, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, N.V.; Pietrokovsky, S.M.; Cigliano, M.M.; Confalonieri, V.A. Unraveling the diversification history of grasshoppers belonging to the “Trimerotropis pallidipennis” (Oedipodinae: Acrididae) species group: A hotspot of biodiversity in the Central Andes. PeerJ 2017, 5, e3835. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Béthoux, O.; Shin, S.; Donath, A.; Letsch, H.; Liu, S.; McKenna, D.D.; Meng, G.; Misof, B.; Podsiadlowski, L.; et al. Phylogenomic analysis sheds light on the evolutionary pathways towards acoustic communication in Orthoptera. Nat. Commun. 2020, 11, 4939. [Google Scholar] [CrossRef]
- Hu, Z.; Han, Y.P.; Guan, D.L.; Mao, B.Y. Characterization of the complete mitochondrial genome of the Yunnan endemic grasshopper Longchuanacris curvifurculus (Insecta: Orthoptera: Catantopidae). Mitochondrial DNA. Part B Resour. 2018, 3, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, G.; Yue, C.; Zhang, L.; Duan, Y.; Liu, Y.; Yang, S.; Wang, Z.; Chen, P. Distribution patterns and potential suitable habitat prediction of Ceracris kiangsu (Orthoptera: Arcypteridae) under climate change—A case study of China and Southeast Asia. Sci. Rep. 2024, 14, 20580. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Zhang, X.; Mao, B.; Yang, G.; Shi, F.; Bi, J.; Ma, Z.; Tang, G. Effects of Environmental Factors on the Diversity of Grasshopper Communities along Altitude Gradients in Xizang, China. Insects 2024, 15, 671. [Google Scholar] [CrossRef]
- Mao, B.Y.; Huang, Z.P. Taxonomy on three allied genera within Arcypterini (Orthoptera: Acrididae) from Qinghai-Xizang Plateau, China. Zootaxa 2023, 5239, 265–279. [Google Scholar] [CrossRef]
- Li, R.; Jiao, M.; Li, Y.; Jiang, L. The complete mitochondrial genome of Sinopodisma hengshanica (Orthoptera: Acrididae) and its phylogenetic implication. Mitochondrial DNA. Part B Resour. 2022, 7, 616–618. [Google Scholar] [CrossRef]
- Gaugel, S.M.; Hawlitschek, O.; Dey, L.S.; Husemann, M. Evolution of mitogenomic gene order in Orthoptera. Insect Mol. Biol. 2023, 32, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Xue, X.; Qin, G.; Gao, Y.; Li, K.; Zhang, Y.; Li, X.J. Comparative analysis of mitogenomes among three species of grasshoppers (Orthoptera: Acridoidea: Gomphocerinae) and their phylogenetic implications. PeerJ 2023, 11, e16550. [Google Scholar] [CrossRef]
- Schmidt, R.; Dufresnes, C.; Krištín, A.; Künzel, S.; Vences, M.; Hawlitschek, O. Phylogenetic insights into Central European Chorthippus and Pseudochorthippus (Orthoptera: Acrididae) species using ddRADseq data. Mol. Phylogenet. Evol. 2024, 193, 108012. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huang, Y. Complete Mitochondrial Genome Sequence of Acrida cinerea (Acrididae: Orthoptera) and Comparative Analysis of Mitochondrial Genomes in Orthoptera. Comp. Funct. Genom. 2010, 2010, 319486. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Guan, D.L.; Xu, S.Q. The complete mitochondrial genome of a grasshopper endemic to the Qinghai-Tibet plateau, Uvaroviola multispinosa (Acrididae: Oedipodinae). Mitochondrial DNA. Part B Resour. 2019, 4, 2817–2818. [Google Scholar] [CrossRef]
- Guan, D.L.; Xu, S.Q. Complete mitochondrial genome of the geophilous grasshopper Trilophidia annulata (Acrididae: Oedipodinae: Trilophidia). Mitochondrial DNA. Part A DNA Mapp. Seq. Anal. 2016, 27, 3143–3144. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.L.; Huang, C.M.; Deng, W.A. Reassessment of the Phylogenetics of Two Pygmy Grasshopper Generic Groups Tetrix and Systolederus through Mitochondrial Phylogenomics Using Four New Mitochondrial Genome Assemblies. Insects 2024, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P. Putting the genome in insect phylogenomics. Curr. Opin. Insect Sci. 2019, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L. Insect Mitochondrial Genomics: A Decade of Progress. Annu. Rev. Entomol. 2025, 70, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.K.; Manu, S.; Chandrakumaran, N.K.; Vasudevan, K. Nuclear and Mitochondrial Genome Assemblies of the Beetle, Zygogramma bicolorata, a Globally Important Biocontrol Agent of Invasive Weed Parthenium hysterophorus. Genome Biol. Evol. 2023, 15, evad188. [Google Scholar] [CrossRef] [PubMed]





| Subfamily | Species | Authority | Collecting Data |
|---|---|---|---|
| Acrididae | |||
| Acridinae | Pseudoptygonotus prominemarginis | Zheng and Mao, 1996 | China: Yunnan: Dali (from Orthoptera Species File) |
| Caryandinae | Lemba yunnana | Ma and Zheng, 1994 | China: Yunnan |
| Spathosterninae | Spathosternum prasiniferum yunnanensis1 | Wei and Zheng, 2005 | China: Yunnan: Jinping |
| Catantopinae | Xenocatantops humilis | (Serville, 1838) | Baie de Palabaun |
| Conophyminae | Conophymacris conicerca | Bi and Xia, 1984 | China: Yunnan: Baoshan |
| Coptacrinae | Pseudotraulia cornuata | Laosinchai and Jago, 1980 | Thailand |
| Coptacrinae | Coptacra tonkinensis | Willemse, 1939 | Vietnam: Son La: Than-Moi |
| Coptacrinae | Epistaurus aberrans | Brunner von Wattenwyl, 1893 | Myanmar: Kachin: Bhamó |
| Coptacrinae | Eucoptacra binghami | Uvarov, 1921 | Myanmar |
| Coptacrinae | Meltripata chloronema | Zheng, 1982 | China: Yunnan: Jinghong |
| Eyprepocnemidinae | Choroedocus violaceipes | Miller, 1934 | Negri Sembilan, Tampin |
| Eyprepocnemidinae | Eyprepocnemis yunnanensis | Zheng, Lian and Xi, 1982 | China: Yunnan: Jinghong |
| Oedipodinae | Heteropternis rufipes | (Shiraki, 1910) | Japan |
| Oedipodinae | Pternoscirta sauteri | (Karny, 1915) | Taiwan: Taiwan: Nantou: Kosempo |
| Oxyinae | Longchuanacris macrofurculus | Zheng and Fu, 1989 | China: Yunnan: Ruili |
| Oxyinae | Racilia aurora | (Brunner von Wattenwyl, 1893) | Myanmar |
| Spathosterninae | Spathosternum prasiniferum yunnanensis2 | Wei and Zheng, 2005 | China: Yunnan: Jinping, Mengla |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Song, J.; Lu, J.; Zhao, L.; Deng, W.; Guan, D.; Mao, B. Phylogenetics and Evolutionary Dynamics of Yunnan Acrididae Grasshoppers Inferred from 17 New Mitochondrial Genomes. Insects 2025, 16, 151. https://doi.org/10.3390/insects16020151
Zhang K, Song J, Lu J, Zhao L, Deng W, Guan D, Mao B. Phylogenetics and Evolutionary Dynamics of Yunnan Acrididae Grasshoppers Inferred from 17 New Mitochondrial Genomes. Insects. 2025; 16(2):151. https://doi.org/10.3390/insects16020151
Chicago/Turabian StyleZhang, Keyao, Jing Song, Junhui Lu, Lu Zhao, Weian Deng, Delong Guan, and Benyong Mao. 2025. "Phylogenetics and Evolutionary Dynamics of Yunnan Acrididae Grasshoppers Inferred from 17 New Mitochondrial Genomes" Insects 16, no. 2: 151. https://doi.org/10.3390/insects16020151
APA StyleZhang, K., Song, J., Lu, J., Zhao, L., Deng, W., Guan, D., & Mao, B. (2025). Phylogenetics and Evolutionary Dynamics of Yunnan Acrididae Grasshoppers Inferred from 17 New Mitochondrial Genomes. Insects, 16(2), 151. https://doi.org/10.3390/insects16020151






