Programmable LED Array for Evaluating Artificial Light Sources to Improve Insect Trapping
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
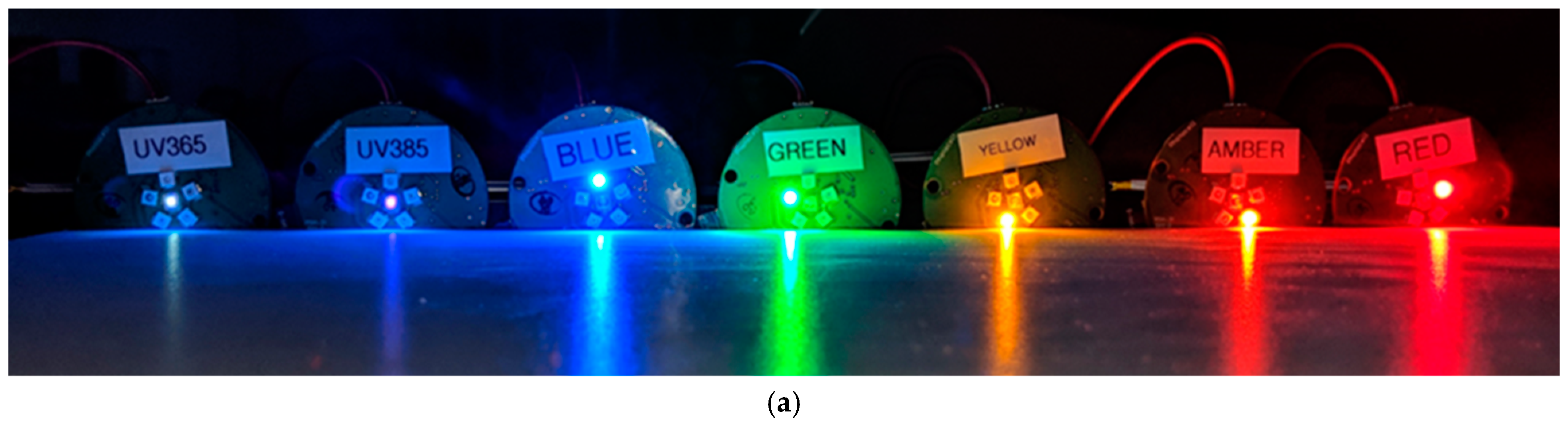
2.1. Hardware Design
2.2. Software Design
2.3. Artificial Lighting Effects in ACP Traps
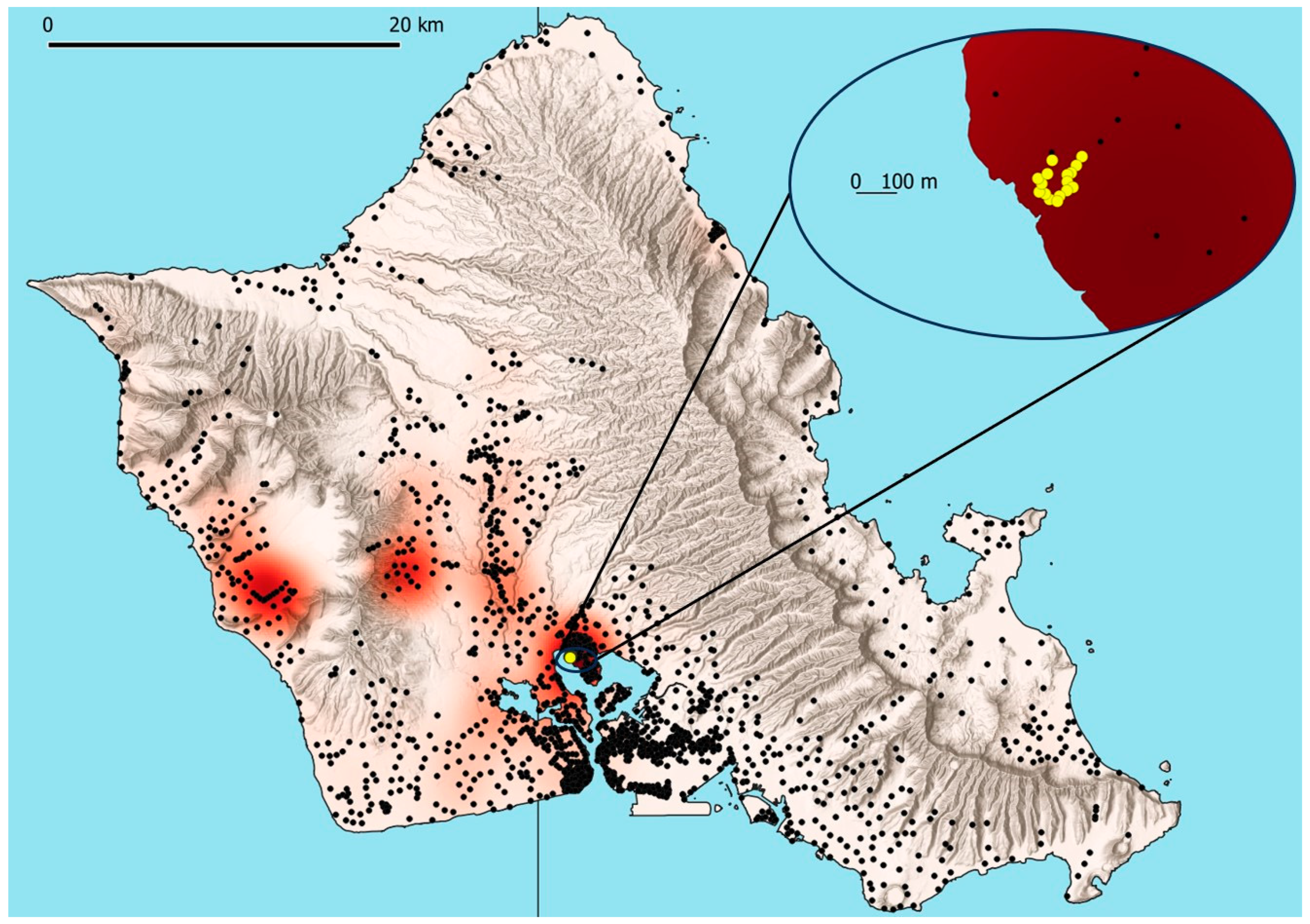
2.4. Artificial Lighting Effects in CRB Traps
3. Results
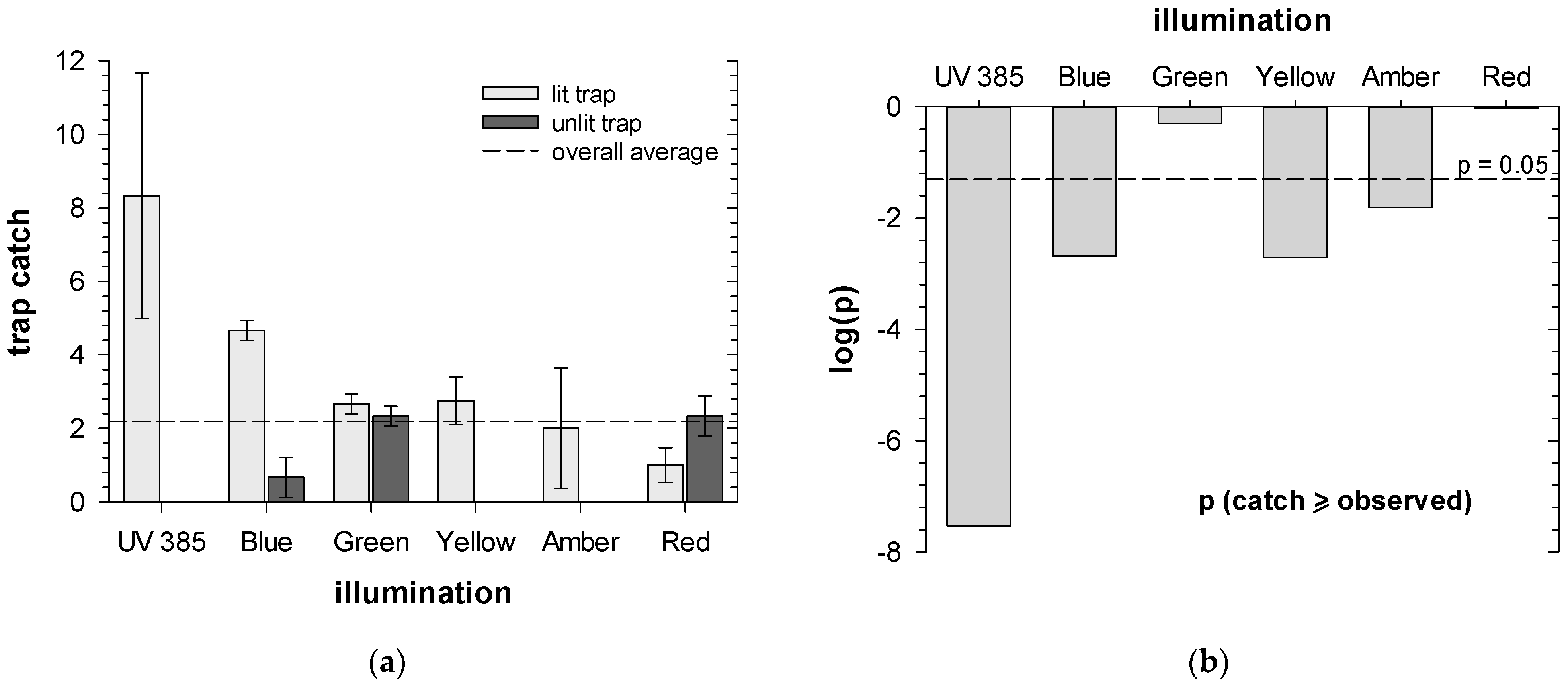
3.1. Artificial Lighting Effects in ACP Traps
3.2. Significance of Lighting, Trap Position, and Weekly Interval for CRB Capture
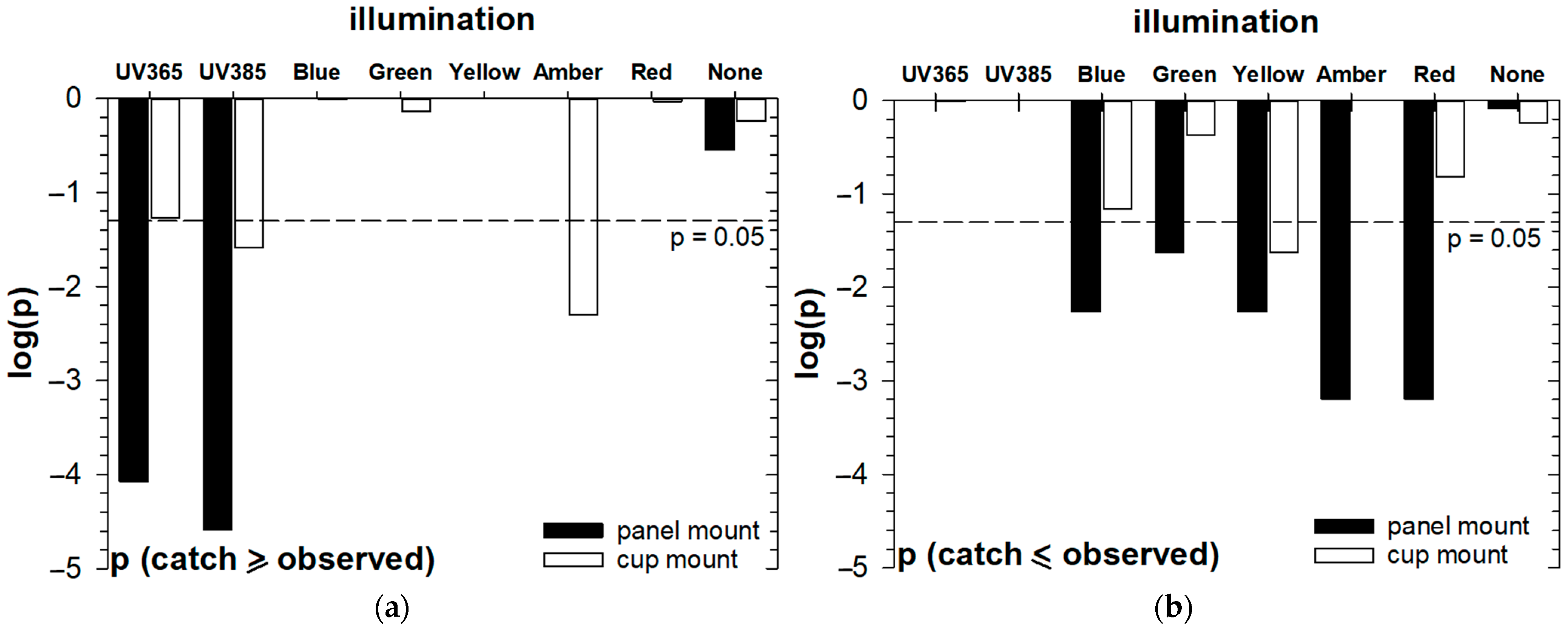
3.3. Evaluation of Lighting Effects for CRB Capture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forresi, C.; Gallinucci, E.; Golfarelli, M.; Maistrello, L.; Preti, M.; Vaccari, G. A Data Platform for Real-Time Monitoring and Analysis of the Brown Marmorated Stink Bug in Northern Italy. Ecol. Inform. 2024, 82, 102713. [Google Scholar] [CrossRef]
- Nisar, M.J.; Gogi, M.D.; Abbasi, A.; Atta, B.; Yousafi, Q.; Ul Haq, I.; Subhan, M.; Ali, H.M.; Alsakkaf, W.A.A.; Basiouny, M.S. Do Phagostimulants, Alone or Combined with Ammonium Acetate, Di-Ammonium Phosphate, and Acetic Acid, Effectively Attract Both Sexes of Peach Fruit Flies, Bactrocera zonata (Diptera: Tephritidae)?: Insights from Laboratory and Field Bioassays. Insects 2024, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.P.; Murugan, M.; Shanthi, M.; Elaiyabharathi, T.; Angappan, K.; Karthikeyan, G.; Arulkumar, G.; Manjari, P.; Ravishankar, M.; Sotelo-Cardona, P.; et al. Evaluation of Integrated Pest and Disease Management Combinations against Major Insect Pests and Diseases of Tomato in Tamil Nadu, India. Horticulturae 2024, 10, 766. [Google Scholar] [CrossRef]
- Marshall, S.D.G.; Moore, A.; Vaqalo, M.; Noble, A.; Jackson, T.A. A New Haplotype of the Coconut Rhinoceros Beetle, Oryctes rhinoceros, Has Escaped Biological Control by Oryctes rhinoceros nudivirus and Is Invading Pacific Islands. J. Invertebr. Pathol. 2017, 149, 127–134. [Google Scholar] [CrossRef]
- Hawaii Department of Agriculture. Live Coconut Rhinoceros Beetles Detected in Waikoloa on Hawaii Island. 2024. Available online: https://hdoa.hawaii.gov/blog/main/nr24-13crb-waikoloa/ (accessed on 24 June 2024).
- Keremane, M.L.; McCollum, T.G.; Roose, M.L.; Lee, R.F.; Ramadugu, C. An Improved Reference Gene for Detection of “Candidatus Liberibacter asiaticus” Associated with Citrus Huanglongbing by qPCR and Digital Droplet PCR Assays. Plants 2021, 10, 2111. [Google Scholar] [CrossRef]
- Keremane, M.L.; Halbert, S.E.; Ramadugu, C.; Webb, S.; Lee, R.F. Detection of “Candidatus Liberibacter asiaticus” in Diaphorina citri and Its Importance in the Management of Citrus Huanglongbing in Florida. Phytopathology 2008, 98, 387–396. [Google Scholar] [CrossRef]
- Snyder, J.; Dickens, K.L.; Halbert, S.E.; Dowling, S.; Russell, D.; Henderson, R.; Rohrig, E.; Ramadugu, C. The Development and Evaluation of Insect Traps for the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Psyllidae), Vector of Citrus Huanglongbing. Insects 2022, 13, 295. [Google Scholar] [CrossRef]
- Wentz, K.M.; Cooper, W.R.; Horton, D.R.; Wohleb, C.H.; Waters, T.D.; Halbert, S.E.; Ramadugu, C.; Snyder, J.; Kao, R.M. Prototype 3D-Printed Traps Capture Bactericera cockerelli (Sulc) (Hemiptera: Triozidae) Directly into Preservative for Improved Detection of “Candidatus Liberibacter Solanacearum”. J. Entomol. Sci. 2020, 55, 147–155. [Google Scholar] [CrossRef]
- Keremane, M.; Singh, K.; Ramadugu, C.; Krueger, R.R.; Skaggs, T.H. Next Generation Sequencing, and Development of a Pipeline as a Tool for the Detection and Discovery of Citrus Pathogens to Facilitate Safer Germplasm Exchange. Plants 2024, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Keremane, M.L.; Ramadugu, C.; Rodriguez, E.; Kubota, R.; Shibata, S.; Hall, D.G.; Roose, M.L.; Jenkins, D.; Lee, R.F. A Rapid Field Detection System for Citrus Huanglongbing Associated ‘Candidatus Liberibacter asiaticus’ from the Psyllid Vector, Diaphorina citri Kuwayama and Its Implications in Disease Management. Crop Prot. 2015, 68, 41–48. [Google Scholar] [CrossRef]
- Babcock, B.A. Economic Impact of California’s Citrus Industry in 2020. J. Citrus Pathol. 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The Evolution of Color Vision in Insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Dearden, A.E.E.; Wood, M.J.J.; Frend, H.O.O.; Butt, T.M.; Allen, W.L.L. Visual Modelling Can Optimise the Appearance and Capture Efficiency of Sticky Traps Used to Manage Insect Pests. J. Pest Sci. 2024, 97, 469–479. [Google Scholar] [CrossRef]
- Dorin, A.; Shrestha, M.; Garcia, J.E.; Burd, M.; Dyer, A.G. Ancient Insect Vision Tuned for Flight among Rocks and Plants Underpins Natural Flower Colour Diversity. Proc. R. Soc. B. 2023, 290, 20232018. [Google Scholar] [CrossRef] [PubMed]
- Santer, R.D.; Allen, W.L. Optimising the Colour of Traps Requires an Insect’s Eye View. Pest Manag. Sci. 2024, 80, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.; Somanathan, H. Colour Vision in Nocturnal Insects. Philos. Trans. R. Soc. B. 2022, 377, 20210285. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, C.J.; Stavenga, D.G.; Arikawa, K.; Belusic, G.; Kelber, A. Evolution of Insect Color Vision: From Spectral Sensitivity to Visual Ecology. In Annual Review of Entomology; Douglas, A.E., Ed.; Annual Reviews: Palo Alto, CA, USA, 2021; Volume 66, pp. 435–461. ISBN 978-0-8243-0166-8. [Google Scholar]
- Fadzly, N.; Burns, K.C. What Weta Want: Colour Preferences of a Frugivorous Insect. Arthropod-Plant Interact. 2010, 4, 267–276. [Google Scholar] [CrossRef]
- Dacke, M.; Nordström, P.; Scholtz, C.H. Twilight Orientation to Polarised Light in the Crepuscular Dung Beetle Scarabaeus zambesianus. J. Exp. Biol. 2003, 206, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Dacke, M.; Nilsson, D.E.; Scholtz, C.H.; Byrne, M.; Warrant, E.J. Insect Orientation to Polarized Moonlight. Nature 2003, 424, 33. [Google Scholar] [CrossRef]
- Yilmaz, A.; Belus, G.; Foster, J.J.; Tocco, C.; Khaldy, L.; Dacke, M. Polarisation Vision in the Dark: Green-Sensitive Photoreceptors in the Nocturnal Ball-Rolling Dung Beetle Escarabaeus satyrus. J. Exp. Biol. 2024, 227, jeb246374. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, A.; Hariyama, T.; Wilcockson, D.C.; Mercatelli, L. The Use of Polarized Light in the Zonal Orientation of the Sandhopper Talitrus saltator (Montagu). Zool. Lett. 2023, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Ensaldo-Cardenas, A.S.; Rocha-Ortega, M.; Schneider, D.; Robertson, B.A.; Cordoba-Aguilar, A. Ultraviolet Polarized Light and Individual Condition Drive Habitat Selection in Tropical Damselflies and Dragonflies. Anim. Behav. 2021, 180, 229–238. [Google Scholar] [CrossRef]
- Fraleigh, D.C.; Heitmann, J.B.; Robertson, B.A. Ultraviolet Polarized Light Pollution and Evolutionary Traps for Aquatic Insects. Anim. Behav. 2021, 180, 239–247. [Google Scholar] [CrossRef]
- Robertson, B.A.; Horvath, G. Color Polarization Vision Mediates the Strength of an Evolutionary Trap. Evol. Appl. 2019, 12, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Homberg, U.; Hensgen, R.; Jahn, S.; Pegel, U.; Takahashi, N.; Zittrell, F.; Pfeiffer, K. The Sky Compass Network in the Brain of the Desert Locust. J. Comp. Physiol. A 2023, 209, 641–662. [Google Scholar] [CrossRef]
- Massy, R.; Hawkes, W.L.S.; Doyle, T.; Troscianko, J.; Menz, M.H.M.; Roberts, N.W.; Chapman, J.W.; Wotton, K.R. Hoverflies Use a Time-Compensated Sun Compass to Orientate during Autumn Migration. Proc. R. Soc. B 2021, 288, 20211805. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Beetz, M.J.; Merlin, C.; Pfeiffer, K.; el Jundi, B. Weighting of Celestial and Terrestrial Cues in the Monarch Butterfly Central Complex. Front. Neural Circuits 2022, 16, 862279. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Shein-Idelson, M. Polarization Vision in Invertebrates: Beyond the Boundaries of Navigation. Curr. Opin. Insect Sci. 2021, 48, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fabian, S.T.; Sondhi, Y.; Allen, P.E.; Theobald, J.C.; Lin, H.-T. Why Flying Insects Gather at Artificial Light. Nat. Commun. 2024, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.L.; Preti, M.; Basoalto, E.; Fuentes-Contreras, E. Increasing Catches of Adult Moth Pests (Lepidoptera: Tortricidae) in Pome Fruit with Low-Intensity LED Lights Added to Sex Pheromone/Kairomone Lure-Baited Traps. J. Appl. Entomol. 2023, 147, 843–856. [Google Scholar] [CrossRef]
- Siderhurst, M.S.; Moore, A.; Quitugua, R.; Jang, E.B. Effects of Ultraviolet Light and Pheromone Release Rate in Trapping Coconut Rhinoceros Beetles, Oryctes rhinoceros (Coleoptera: Scarabaeidae), on Guam. Proc. Hawaii. Entomol. Soc. 2021, 53, 21–32. [Google Scholar]
- Crook, D.J.; Chiesa, S.G.; Warden, M.L.; Nadel, H.; Ioriatti, C.; Furtado, M. Electrophysiologically Determined Spectral Responses in Lobesia Botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2022, 115, 1499–1504. [Google Scholar] [CrossRef]
- Crook, D.J.; Francese, J.A.; Zylstra, K.E.; Fraser, I.; Sawyer, A.J.; Bartels, D.W.; Lance, D.R.; Mastro, V.C. Laboratory and Field Response of the Emerald Ash Borer (Coleoptera: Buprestidae), to Selected Regions of the Electromagnetic Spectrum. J. Econ. Entomol. 2009, 102, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gabarrella, A.; Gemeno, C.; Belusic, G. Spectral Sensitivity of Retinal Photoreceptors of Tortricid Moths Is Not Tuned to Diel Activity Period. J. Exp. Biol. 2023, 226, jeb245461. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Jikumaru, S.; Hoshino, S.; Ishikura, S.; Wada, M. Effects of Replacing Outdoor Lighting with White LEDs with Different Correlated Color Temperatures on the Attraction of Nocturnal Insects. Appl. Entomol. Zoolog. 2021, 56, 225–233. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurihara, K.; Saito, S.; Domae, M.; Masuya, N.; Shimura, Y.; Kajiyama, S.; Kanda, Y.; Sugizaki, K.; Ebina, K.; et al. Management of Flying Insects on Expressways through an Academic-Industrial Collaboration: Evaluation of the Effect of Light Wavelengths and Meteorological Factors on Insect Attraction. Zool. Lett. 2020, 6, 15. [Google Scholar] [CrossRef]
- Mohammad, A.; Al-Deeb; Mahmoud, S.T.; Sharif, E.M. Use of Light Traps and Differing Light Color to Investigate Seasonal Abundance of the Date Palm Pest, Oryctes agamemnon arabicus (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2012, 105, 2062–2067. [Google Scholar] [CrossRef]
- Auf der Maur, M.; Pecchia, A.; Penazzi, G.; Rodrigues, W.; Di Carlo, A. Efficiency Drop in Green InGaN/GaN Light Emitting Diodes: The Role of Random Alloy Fluctuations. Phys. Rev. Lett. 2016, 116, 027401. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Jia, H.; Wang, W.; Yang, R.; Chen, H. Superior Optoelectronic Performance of N-Polar GaN LED to Ga-Polar Counterpart in the “Green Gap” Range. IEEE Access 2022, 10, 95565–95570. [Google Scholar] [CrossRef]
- Paryavi, M.; Weiser, K.; Melzer, M.; Ghorbani, R.; Jenkins, D.M. Autonomous Cellular-Networked Surveillance System for Coconut Rhinoceros Beetle. Comput. Electron. Agric. 2025. accepted pending minor revisions. [Google Scholar]
- Sétamou, M.; Sanchez, A.; Patt, J.M.; Nelson, S.D.; Jifon, J.; Louzada, E.S. Diurnal Patterns of Flight Activity and Effects of Light on Host Finding Behavior of the Asian Citrus Psyllid. J. Insect Behav. 2012, 25, 264–276. [Google Scholar] [CrossRef]
- Mangan, R.L.; Chapa, D. Evaluation of the Effects of Light Source and Plant Materials on Asian Citrus Psyllid (Hemiptera: Psyllidae) Trapping Levels in the Transtrap for Citrus Shipping Containers. Fla. Entomol. 2013, 96, 104–111. [Google Scholar] [CrossRef]
- Kalile, M.O.; Janssen, A.; Fancelli, M.; Magalhaes, D.G.; Cardoso, A.C.; Rosa, M.S.; Ledo, C.A.S.; Ragni, M. UV Light Attracts Diaphorina Citri and Its Parasitoid. Biol. Control 2022, 170, 104928. [Google Scholar] [CrossRef]
- Paris, T.M.; Allan, S.A.; Udell, B.J.; Stansly, P.A. Evidence of Behavior-Based Utilization by the Asian Citrus Psyllid of a Combination of UV and Green or Yellow Wavelengths. PLoS ONE 2017, 12, e0189228. [Google Scholar] [CrossRef]
- Battles, I.; Burkness, E.; Crossley, M.S.; Edwards, C.B.; Holmstrom, K.; Hutchison, W.; Ingerson-Mahar, J.; Owens, D.; Owens, A.C.S. Moths Are Less Attracted to Light Traps than They Used to Be. J. Insect Conserv. 2024, 28, 1007–1018. [Google Scholar] [CrossRef]
- Khalaf, M.Z.; Shbar, A.K.; Al-Seria, M.H.; Sami, R.A.; Naher, F.H. Some Aspects of Biology and Control Methods of Fruit Stalk Borer Oryctes elegans Prell (Coleoptera: Scarabaeidae). J. Agric. Sci. Technol. A 2011, 2011, 142–147. [Google Scholar] [CrossRef]
- ATMEL Corporation. ATMEGA328P [Datasheet] 7810D–AVR–01/15. 2015. Available online: https://ww1.microchip.com/downloads/en/DeviceDoc/Atmel-7810-Automotive-Microcontrollers-ATmega328P_Datasheet.pdf (accessed on 7 August 2024).




| Source | SSE | ν/dof | MSE | F | p (>F) |
|---|---|---|---|---|---|
| light | 44.5 | 15 | 2.96667 | 3.62 | 1.07998 × 10−5 |
| error | 196.5 | 240 | 0.81875 | ||
| total | 241 | 255 |
| Source | SSE | ν/dof | MSE | F | p (>F) |
|---|---|---|---|---|---|
| week | 26.625 | 15 | 1.575 | 1.74 | 0.0445 |
| error | 217.375 | 240 | 0.90573 | ||
| total | 241 | 255 |
| Source | SSE | ν/dof | MSE | F | p (>F) |
|---|---|---|---|---|---|
| position | 13 | 15 | 0.86667 | 0.91 | 0.5511 |
| error | 228 | 240 | 0.95 | ||
| total | 241 | 255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paryavi, M.; Weiser, K.; Melzer, M.; Crook, D.; Ramadugu, C.; Jenkins, D.M. Programmable LED Array for Evaluating Artificial Light Sources to Improve Insect Trapping. Insects 2025, 16, 170. https://doi.org/10.3390/insects16020170
Paryavi M, Weiser K, Melzer M, Crook D, Ramadugu C, Jenkins DM. Programmable LED Array for Evaluating Artificial Light Sources to Improve Insect Trapping. Insects. 2025; 16(2):170. https://doi.org/10.3390/insects16020170
Chicago/Turabian StyleParyavi, Mohsen, Keith Weiser, Michael Melzer, Damon Crook, Chandrika Ramadugu, and Daniel M. Jenkins. 2025. "Programmable LED Array for Evaluating Artificial Light Sources to Improve Insect Trapping" Insects 16, no. 2: 170. https://doi.org/10.3390/insects16020170
APA StyleParyavi, M., Weiser, K., Melzer, M., Crook, D., Ramadugu, C., & Jenkins, D. M. (2025). Programmable LED Array for Evaluating Artificial Light Sources to Improve Insect Trapping. Insects, 16(2), 170. https://doi.org/10.3390/insects16020170






