Bombyx mori RPL12 Participates in UV-Induced DNA Damage Repair and Interacts with BmNPV Bm65 Protein Only After Ultraviolet Radiation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and Cells
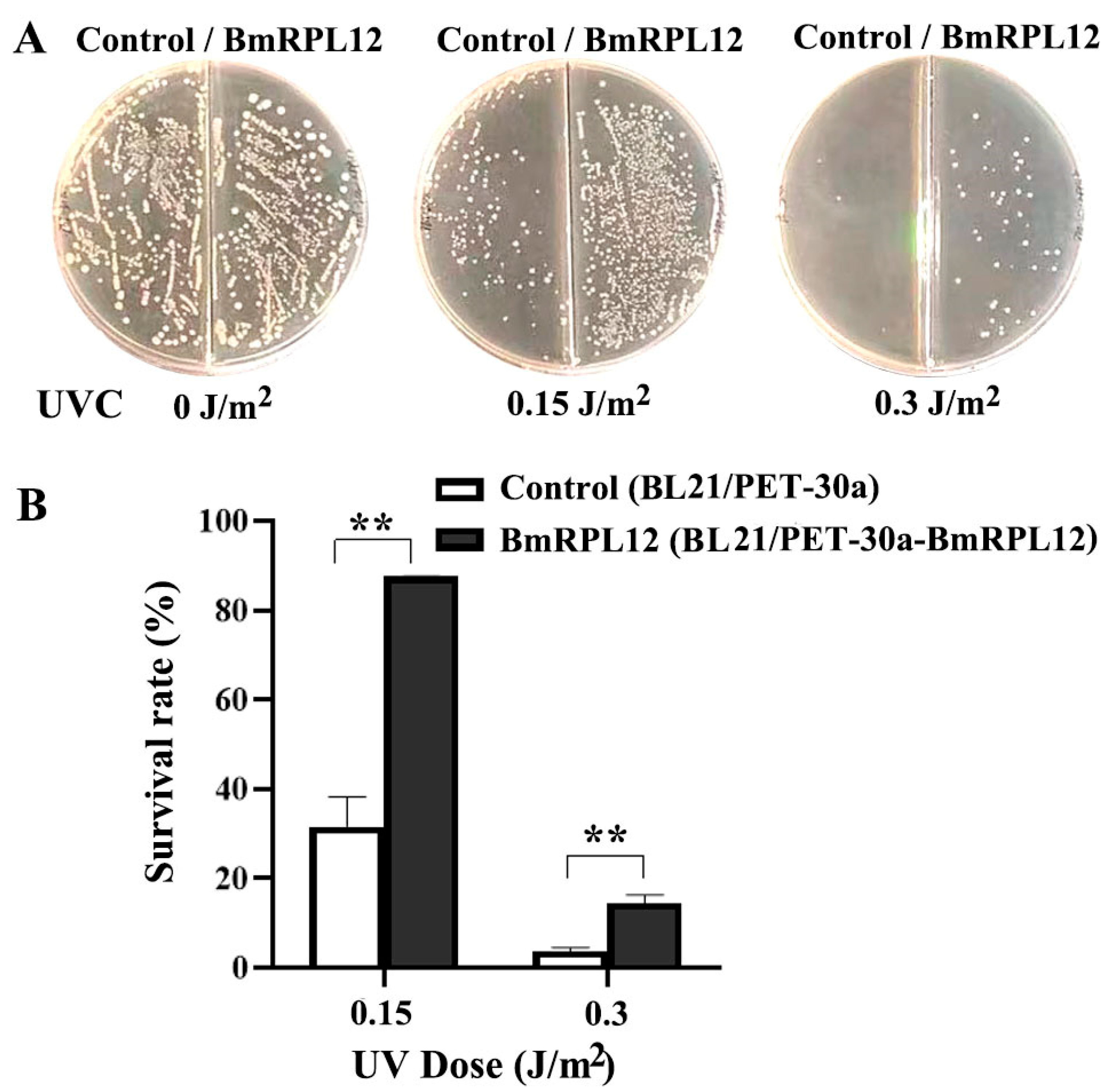
2.2. Effects of BmRPL12 on the UV Sensitivity of E. coli
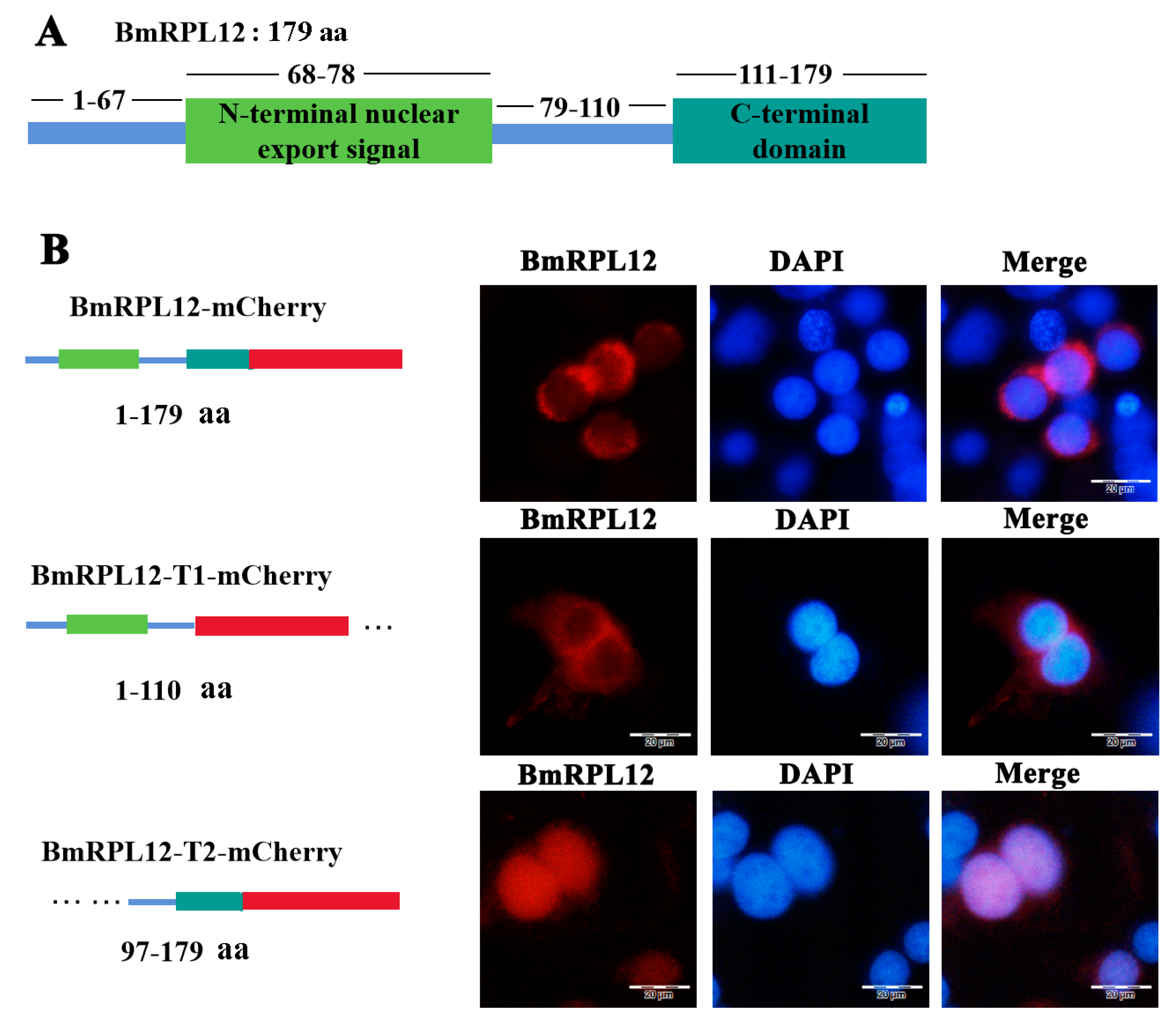
2.3. Localization Analysis of BmRPL12 in BmN Cells
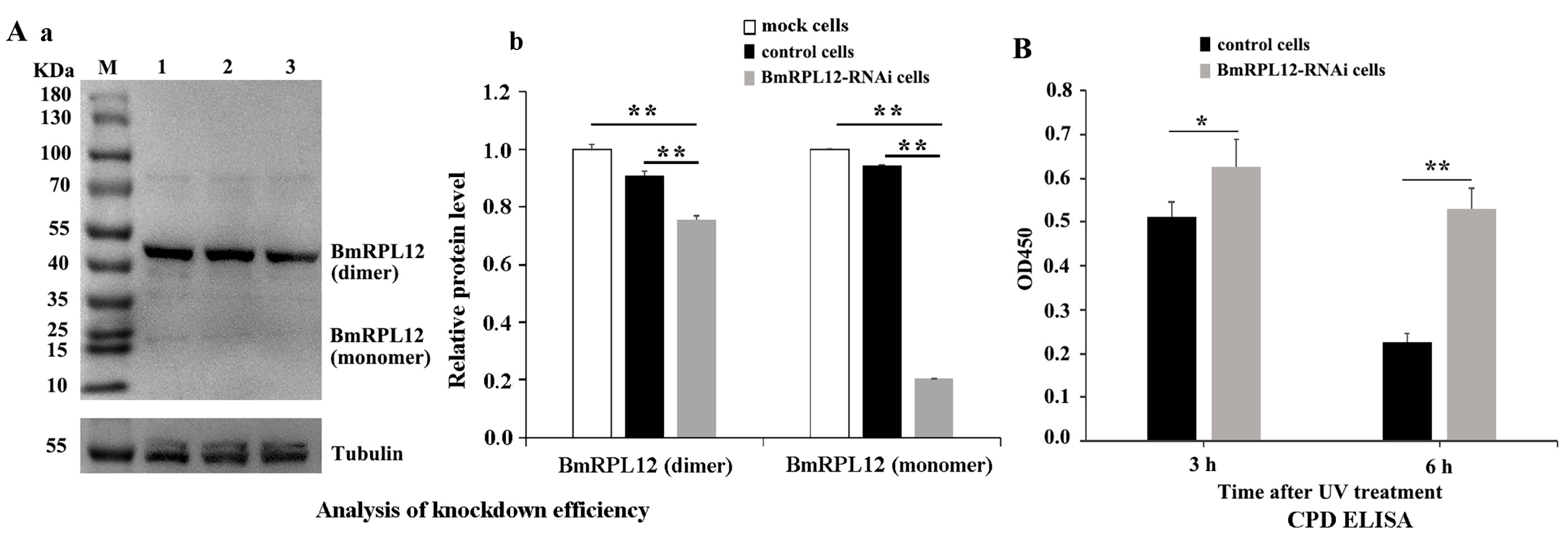
2.4. Knockdown of BmRPL12 in Silkworm Cells
2.5. Effect of BmRPL12 on UV-Induced DNA Damage Repair
2.6. Co-Immunoprecipitation (Co-IP) and Western Blotting Analysis
2.7. Colocalization Analysis of BmRPL12 and Bm65 with UV-Induced DNA Damage (CPD)
3. Results
3.1. Sequence Analysis of BmRPL12
3.2. BmRPL12 Improved the UV Survival Rate of E. coli
3.3. BmRPL12 Was Mainly Localized in the Cytoplasm of BmN Cells
3.4. Knocking Down the Expression of BmRPL12 Slowed Down the Speed of UV-Induced DNA Damage Repair
3.5. Reciprocal Interaction Between BmRPL12 and Bm65
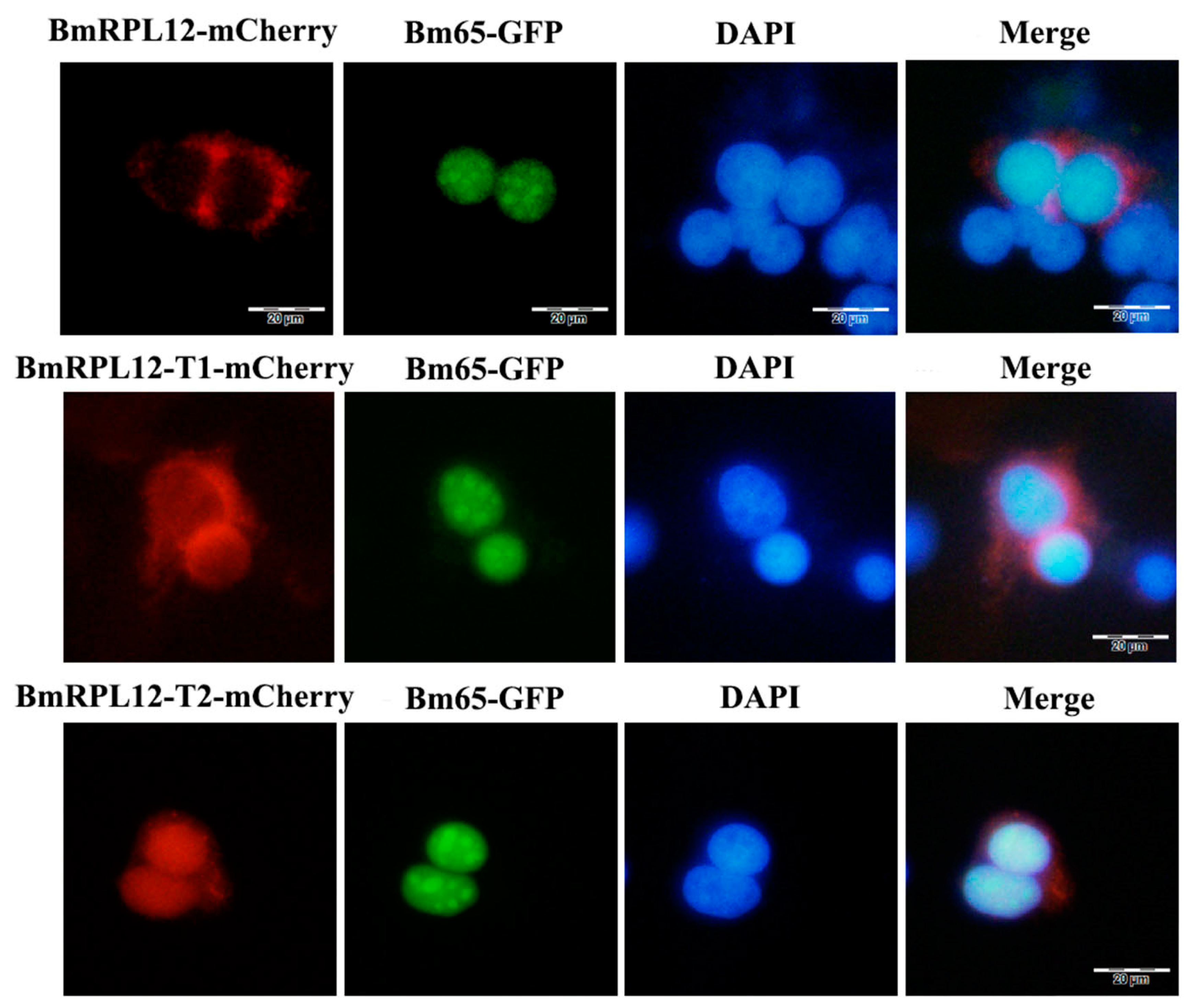
3.6. The Colocalization of BmRPL12 and Bm65 in BmN Cells
3.7. BmRPL12 Specifically Aggregated at the UV-Induced DNA Damage Sites Only in the Presence of Bm65
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Ishikawa-Fujiwara, T.; Shiraishi, E.; Fujikawa, Y.; Mori, T.; Tsujimura, T.; Todo, T. Targeted Inactivation of DNA Photolyase Genes in Medaka Fish (Oryzias latipes). Photochem. Photobiol. 2017, 93, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Xin, D.; Wu, D.; Wang, X.; Chen, L.; Wang, Y.; Ma, K.; Li, Q.; Li, P.; Yu, X. Pre-ribosomal RNA reorganizes DNA damage repair factors in nucleus during meiotic prophase and DNA damage response. Cell Res. 2022, 32, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, Y.; Yu, X.Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.H.; Li, Y. Ribosome biogenesis in disease: New players and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhao, W.W.; Bai, S.M.; Ma, Y.; Yin, X.K.; Feng, L.L.; Zeng, G.D.; Wang, F.; Feng, W.X.; Zheng, J.; et al. DNA damage-induced paraspeckle formation enhances DNA repair and tumor radioresistance by recruiting ribosomal protein P0. Cell Death. Dis. 2022, 13, 709. [Google Scholar] [CrossRef]
- Falcon, K.T.; Watt, K.E.N.; Dash, S.; Zhao, R.; Sakai, D.; Moore, E.L.; Fitriasari, S.; Childers, M.; Sardiu, M.E.; Swanson, S.; et al. Dynamic regulation and requirement for ribosomal RNA transcription during mammalian development. Proc. Natl. Acad. Sci. USA 2022, 119, e2116974119. [Google Scholar] [CrossRef]
- Sadaie, M.; Shinmyozu, K.; Nakayama, J. A conserved SET domain methyltransferase, Set11, modifies ribosomal protein Rpl12 in fission yeast. J. Biol. Chem. 2008, 283, 7185–7195. [Google Scholar] [CrossRef]
- Nagaraj, S.; Senthil-Kumar, M.; Ramu, V.S.; Wang, K.; Mysore, K.S. Plant Ribosomal Proteins, RPL12 and RPL19, Play a Role in Nonhost Disease Resistance against Bacterial Pathogens. Front. Plant Sci. 2015, 6, 1192. [Google Scholar] [CrossRef]
- Coléno-Costes, A.; Jang, S.M.; de Vanssay, A.; Rougeot, J.; Bouceba, T.; Randsholt, N.B.; Gibert, J.M.; Le Crom, S.; Mouchel-Vielh, E.; Bloyer, S.; et al. New partners in regulation of gene expression: The enhancer of Trithorax and Polycomb Corto interacts with methylated ribosomal protein l12 via its chromodomain. PLoS Genet. 2012, 8, e1003006. [Google Scholar] [CrossRef]
- Herniou, E.A.; Olszewski, J.A.; Cory, J.S.; O’Reilly, D.R. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 2003, 48, 211–234. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhao, S.; Wu, X. BmNPV-induced hormone metabolic disorder in silkworm leads to enhanced locomotory behavior. Dev. Comp. Immunol. 2021, 121, 104036. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ren, F.; Lu, Q.; Cao, Z.; Song, J.; Feng, M.; Liu, J.; Sun, J. An efficient method for multigene co-interference by recombinant Bombyx mori nucleopolyhedrovirus. Mol. Genet. Genom. 2019, 294, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wu, P.; Hu, Z.; Yang, Y.; Qiu, L.; Liu, H.; Zhu, S.; Guo, Z.; Xia, H.; Chen, K.; et al. Evidence for the role of BmNPV Bm65 protein in the repair of ultraviolet-induced DNA damage. J. Invertebr. Pathol. 2017, 149, 82–86. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, Y.; Tang, J.; Chen, F.; Qi, X.; Zhu, F.; Yu, Q.; Chen, H.; Wu, P.; Chen, L.; et al. BmNPV Orf 65 (Bm65) Is Identified as an Endonuclease Directly Facilitating UV-Induced DNA Damage Repair. J. Virol. 2022, 96, e0055722. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, Q.; Hu, Z.; Wang, P.; Tang, Q.; Chen, K.; Yao, Q. Determination of the proteins encoded by BmBDV VD1-ORF4 and their interacting proteins in BmBDV-infected midguts. Curr. Microbiol. 2015, 70, 623–629. [Google Scholar] [CrossRef]
- Guo, Z.J.; Tao, L.X.; Dong, X.Y.; Yu, M.H.; Tian, T.; Tang, X.D. Characterization of aggregate/aggresome structures formed by polyhedrin of Bombyx mori nucleopolyhedrovirus. Sci. Rep. 2015, 5, 14601. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Chen, H.; Hu, Z.; Chen, F.; Deng, L.; Guo, Z.; Chen, K.; Tang, Q. The Motif of (76)KRKCSK in Bm65 Is an Efficient Nuclear Localization Signal Involved in Production of Infectious Virions. Front. Microbiol. 2019, 10, 2739. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhao, S.; Wu, X. Identification of A functional region in Bombyx mori nucleopolyhedrovirus VP39 that is essential for nuclear actin polymerization. Virology 2020, 550, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, S.H.; Kim, T.S.; Lee, S.M.; Cho, B.S.; Seo, C.I.; Kim, H.D.; Kim, J. Ribosomal protein S3 associates with the TFIIH complex and positively regulates nucleotide excision repair. Cell. Mol. Life Sci. 2021, 78, 3591–3606. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Kim, H.D.; Kim, J. The DNA repair domain of human rpS3 protects against photoaging by removing cyclobutane pyrimidine dimers. FEBS Lett. 2019, 593, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zang, W.; Ji, Y.; Li, T.; Yang, Y.; Zheng, X. Ribosomal protein L6 (RPL6) is recruited to DNA damage sites in a poly(ADP-ribose) polymerase-dependent manner and regulates the DNA damage response. J. Biol. Chem. 2019, 294, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.; Hu, J.; Gao, X.; Bian, P.; Liu, Y.; Wang, G. Cre-miR914-regulated RPL18 is involved with UV-B adaptation in Chlamydomonas reinhardtii. J. Plant Physiol. 2019, 232, 151–159. [Google Scholar] [CrossRef]
- Sang, W.; He, L.; Wang, X.P.; Zhu-Salzman, K.; Lei, C.L. Evaluation of Reference Genes for RT-qPCR in Tribolium castaneum (Coleoptera: Tenebrionidae) Under UVB Stress. Environ. Entomol. 2015, 44, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.H.; Siegfried, B.D. Selection of reference genes for normalization of RT-qPCR data in gene expression studies in Anthonomus eugenii Cano (Coleoptera: Curculionidae). Sci. Rep. 2020, 10, 5070. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, N.; Yang, Y.J.; Zhang, J.H.; Yin, H. Identification and Validation of Reference Genes for RT-qPCR Normalization in Mythimna separata (Lepidoptera: Noctuidae). BioMed Res. Int. 2018, 2018, 1828253. [Google Scholar] [CrossRef]
- Helton, L.G.; Soliman, A.; von Zweydorf, F.; Kentros, M.; Manschwetus, J.T.; Hall, S.; Gilsbach, B.; Ho, F.Y.; Athanasopoulos, P.S.; Singh, R.K.; et al. Allosteric Inhibition of Parkinson’s-Linked LRRK2 by Constrained Peptides. ACS Chem. Biol. 2021, 16, 2326–2338. [Google Scholar] [CrossRef]
- Rodriguez-Peña, R.; Mounadi, K.E.; Garcia-Ruiz, H. Changes in Subcellular Localization of Host Proteins Induced by Plant Viruses. Viruses 2021, 13, 677. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, F.; Wu, P.; Qiu, L.; Chen, H.; Chen, K.; Li, G. BmNPV infection correlates with the enhancement of the resistance of Bombyx mori cells to UV radiation. Arch. Insect Biochem. Physiol. 2019, 102, e21598. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Kong, X.; Chen, N.; Zeng, X.; Wu, X. Bombyx mori nucleopolyhedrovirus Bm46 is essential for efficient production of infectious BV and nucleocapsid morphogenesis. Virus Res. 2020, 289, 198145. [Google Scholar] [CrossRef]




| Primer Name | Primer Sequence (5′→3′) | Enzyme Digestion Sites |
|---|---|---|
| BmRPL12-F | CGGAATTCATGAACGGAATCAGAATAATTAAAAAT | EcoR I |
| BmRPL12-R | CGCTCGAGTTCAATTTCTATAATGGCTCCAACTTT | Xho I |
| BmRPL12-gfp-F | CGGAATTCATGAACGGAATCAGAATAATTAAAAAT | EcoR I |
| BmRPL12-gfp-R | CGCTCGAGTTCAATTTCTATAATGGCTCCAACTTT | Xho I |
| BmRPL12-mCherry-F | CGGAATTCATGAACGGAATCAGAATAATTAAAAAT | Pst I |
| BmRPL12-mCherry-R | CGCTCGAGTTCAATTTCTATAATGGCTCCAACTTT | Xho I |
| BmRPL12-T2-mCherry-F | ATGAATTCATGGTTGATGAAGAAGAGGCAGCCCCGAAA | EcoR I |
| BmRPL12-T1-mCherry-R | ATCTCGAGACTGGTCTTCACAGCTTTCGGGGCTGC | Xho I |
| BmRPL12 siRNA | GUGGCGAUCUUUAUCAAGATT UCUUGAUAAAGAUCGCCACTT | |
| siRNA (negative control) | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Chen, C.; Huang, J.; Li, G.; Zhu, F.; Yu, Q.; Sun, L.; Chen, H.; Chen, L.; Ma, S.; et al. Bombyx mori RPL12 Participates in UV-Induced DNA Damage Repair and Interacts with BmNPV Bm65 Protein Only After Ultraviolet Radiation. Insects 2025, 16, 187. https://doi.org/10.3390/insects16020187
Tang Q, Chen C, Huang J, Li G, Zhu F, Yu Q, Sun L, Chen H, Chen L, Ma S, et al. Bombyx mori RPL12 Participates in UV-Induced DNA Damage Repair and Interacts with BmNPV Bm65 Protein Only After Ultraviolet Radiation. Insects. 2025; 16(2):187. https://doi.org/10.3390/insects16020187
Chicago/Turabian StyleTang, Qi, Ceru Chen, Jiaying Huang, Guohui Li, Feifei Zhu, Qian Yu, Lindan Sun, Huiqing Chen, Liang Chen, Shangshang Ma, and et al. 2025. "Bombyx mori RPL12 Participates in UV-Induced DNA Damage Repair and Interacts with BmNPV Bm65 Protein Only After Ultraviolet Radiation" Insects 16, no. 2: 187. https://doi.org/10.3390/insects16020187
APA StyleTang, Q., Chen, C., Huang, J., Li, G., Zhu, F., Yu, Q., Sun, L., Chen, H., Chen, L., Ma, S., Liu, X., & Chen, K. (2025). Bombyx mori RPL12 Participates in UV-Induced DNA Damage Repair and Interacts with BmNPV Bm65 Protein Only After Ultraviolet Radiation. Insects, 16(2), 187. https://doi.org/10.3390/insects16020187








