Widespread Distribution of chs-1 Mutations Associated with Resistance to Diflubenzuron Larvicide in Culex pipiens Across Italy, Reaching Virtual Fixation in the Venetian Lagoon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling Collections
2.2. Genotyping of Diflubenzuron Resistance Mutations
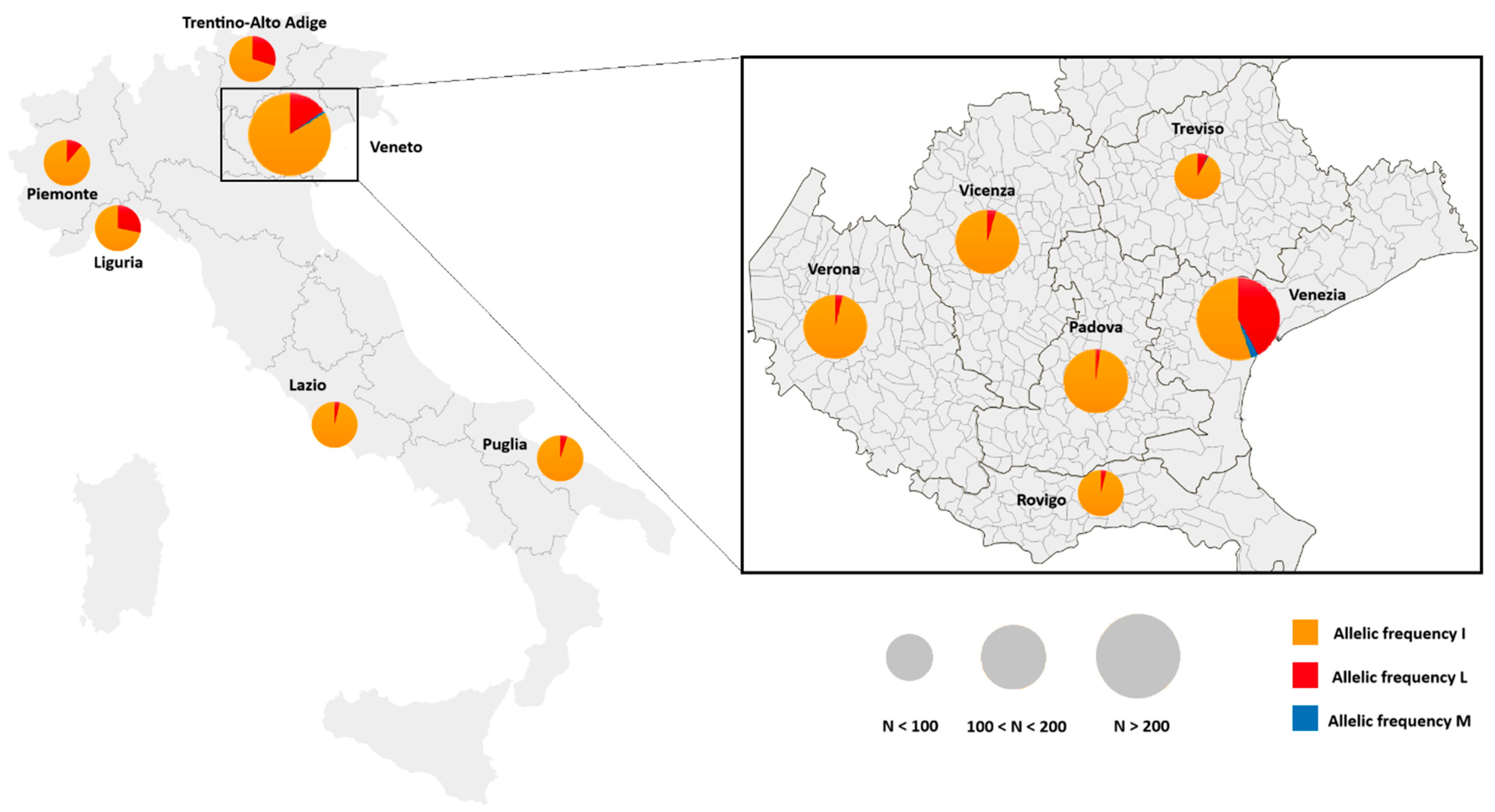
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Site | Date | N | Genotypic Frequencies | Allelic Freq | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gf II | Gf IL | Gf IM | Gf LL | Gf LM | Gf MM | Af L | Af M | Af I | |||
| Mezzolombardo | 2020 | 48 | 0.44 | 0.40 | 0 | 0.17 | 0 | 0 | 0.36 | 0 | 0.64 |
| San Michele All’adige | 2017 | 16 | 0.69 | 0.25 | 0 | 0.06 | 0 | 0 | 0.19 | 0 | 0.81 |
| Zambana | 2017 | 20 | 0.70 | 0.15 | 0 | 0.15 | 0 | 0 | 0.23 | 0 | 0.78 |
| Trento Province | 2017–2020 | 84 | 0.55 | 0.31 | 0 | 0.14 | 0 | 0 | 0.30 | 0 | 0.70 |
| Albignasego | 2022 | 10 | 0.90 | 0 | 0 | 0.10 | 0 | 0 | 0.10 | 0 | 0.90 |
| Battaglia Terme | 2022 | 2 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Borgoricco | 2022 | 5 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Campodarsego | 2021–2022 | 8 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Candiana | 2022 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Casale di Scodosia | 2022 | 5 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Codevigo | 2022 | 8 | 0.88 | 0.13 | 0 | 0 | 0 | 0 | 0.06 | 0 | 0.94 |
| Conselve | 2020 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Curtarolo | 2020–2022 | 19 | 0.84 | 0.05 | 0 | 0.11 | 0 | 0 | 0.13 | 0 | 0.87 |
| Due Carrare | 2022 | 8 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Maserà di Padova | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Padova | 2022 | 9 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Pernumia | 2021 | 21 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Piombino Dese | 2021 | 11 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Ponte San Nicolò | 2020 | 4 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Teolo | 2020–2022 | 13 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Trebaseleghe | 2020–2022 | 26 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Vigodarzere | 2022 | 3 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Villa Estense | 2020 | 7 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Padova Province | 2020–2022 | 181 | 0.97 | 0.01 | 0 | 0.02 | 0 | 0 | 0.02 | 0 | 0.98 |
| Ariano nel Polesine | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Castelmassa | 2022 | 9 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Ceneselli | 2022 | 9 | 0.89 | 0 | 0 | 0.11 | 0 | 0 | 0.11 | 0 | 0.89 |
| Ficarolo | 2022 | 9 | 0.89 | 0 | 0 | 0.11 | 0 | 0 | 0.11 | 0 | 0.89 |
| Fiesso Umbertiano | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Gaiba | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Polesella | 2020 | 9 | 0.89 | 0 | 0 | 0.11 | 0 | 0 | 0.11 | 0 | 0.89 |
| Rovigo | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Rovigo Province | 2020–2022 | 76 | 0.96 | 0 | 0 | 0.04 | 0 | 0 | 0.04 | 0 | 0.96 |
| Cison di Valmarino | 2020 | 1 | 0 | 0 | 0 | 1.00 | 0 | 0 | 1.00 | 0 | 0 |
| Colle Umberto | 2021 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Gorgo al Monticano | 2022 | 1 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Loria | 2020 | 4 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Maser | 2022 | 8 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Meduna di Livenza | 2021–2022 | 19 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Nervesa della Battaglia | 2020 | 3 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Oderzo | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Pederobba | 2020 | 2 | 0.50 | 0.50 | 0 | 0 | 0 | 0 | 0.25 | 0 | 0.75 |
| Ponzano Veneto | 2022 | 5 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Quinto di Treviso | 2020 | 2 | 0 | 0 | 0 | 1.00 | 0 | 0 | 1.00 | 0 | 0 |
| Revine Lago | 2021 | 15 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| San Vendemiano | 2020 | 8 | 0.63 | 0.13 | 0 | 0.13 | 0.13 | 0 | 0.25 | 0.06 | 0.69 |
| Volpago del Montello | 2020 | 2 | 0.50 | 0 | 0 | 0.50 | 0 | 0 | 0.50 | 0 | 0.50 |
| Treviso Province | 2020–2022 | 86 | 0.91 | 0.02 | 0 | 0.06 | 0.01 | 0 | 0.08 | 0.01 | 0.92 |
| Campagna Lupia | 2022 | 5 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Camponogara | 2022 | 5 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Chioggia | 2020–2022 | 23 | 0.83 | 0.04 | 0.04 | 0 | 0 | 0.09 | 0.02 | 0.11 | 0.87 |
| Cona | 2020–2022 | 11 | 0.91 | 0.09 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Fiesso d’Artico | 2022 | 10 | 1.00 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Fossò | 2019–2020 | 42 | 0.57 | 0.33 | 0 | 0.10 | 0 | 0 | 0.26 | 0 | 0.74 |
| Lido di Venezia | 2019–2023 | 86 | 0.02 | 0.01 | 0 | 0.85 | 0.12 | 0 | 0.91 | 0.06 | 0.03 |
| Marcon | 2020 | 6 | 0.67 | 0 | 0 | 0.33 | 0 | 0 | 0.33 | 0 | 0.67 |
| Noale | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Pianiga | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Quarto d’Altino | 2020 | 6 | 0 | 0.83 | 0 | 0.17 | 0 | 0 | 0.58 | 0 | 0.42 |
| Salzano | 2022 | 10 | 0.90 | 0.10 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Scorzè | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Spinea | 2020 | 5 | 0.80 | 0 | 0 | 0.20 | 0 | 0 | 0.20 | 0 | 0.80 |
| Stra | 2021 | 3 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Venezia | 2022 | 10 | 0.20 | 0 | 0 | 0.80 | 0 | 0 | 0.80 | 0 | 0.20 |
| Venezia Province | 2019–2023 | 252 | 0.50 | 0.09 | 0.004 | 0.35 | 0.04 | 0.01 | 0.42 | 0.03 | 0.55 |
| Albaredo d’Adige | 2020 | 5 | 0.20 | 0.40 | 0 | 0.40 | 0 | 0 | 0.60 | 0 | 0.40 |
| Arcole | 2022 | 9 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Bardolino | 2022 | 7 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Bussolengo | 2022 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Caldiero | 2021 | 1 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Castel d’Azzano | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Illasi | 2021 | 2 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Malcesine | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Monteforte d’Alpone | 2020 | 4 | 0.75 | 0.25 | 0 | 0 | 0 | 0 | 0.13 | 0 | 0.88 |
| Mozzecane | 2020–2022 | 18 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Pescantina | 2020 | 9 | 0.89 | 0 | 0 | 0.11 | 0 | 0 | 0.11 | 0 | 0.89 |
| Peschiera del Garda | 2022 | 8 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Ronco All’Adige | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| San Giovanni Ilarione | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Sona | 2020 | 1 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Veronella | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Verona Province | 2020–2022 | 120 | 0.95 | 0.03 | 0 | 0.03 | 0 | 0 | 0.04 | 0 | 0.96 |
| Alonte | 2020–2021 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Bassano del Grappa | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Bressanvido | 2022 | 10 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Camisano Vicentino | 2022 | 4 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Chiampo | 2022 | 10 | 0.90 | 0.10 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Gambugliano | 2021 | 3 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Lonigo | 2020 | 7 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Marostica | 2020 | 7 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Montecchio Precalcino | 2020 | 2 | 0 | 0 | 0 | 1.00 | 0 | 0 | 1.00 | 0 | 0 |
| Monticello Conte Otto | 2020–2021 | 15 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Sossano | 2021 | 6 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Torri di Quartesolo | 2021 | 7 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Trissino | 2020–2022 | 15 | 0.73 | 0.13 | 0 | 0.13 | 0 | 0 | 0.20 | 0 | 0.80 |
| Vicenza | 2021–2022 | 22 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Vicenza Province | 2020–2022 | 124 | 0.77 | 0.02 | 0 | 0.03 | 0 | 0 | 0.04 | 0 | 0.78 |
| Torino | 2017 | 18 | 0.78 | 0.22 | 0 | 0 | 0 | 0 | 0.11 | 0 | 0.89 |
| Torino Province | 2017 | 18 | 0.78 | 0.22 | 0 | 0 | 0 | 0 | 0.11 | 0 | 0.89 |
| Imperia | 2018 | 16 | 0.63 | 0.19 | 0 | 0.19 | 0 | 0 | 0.28 | 0 | 0.72 |
| Imperia Province | 2018 | 16 | 0.63 | 0.19 | 0 | 0.19 | 0 | 0 | 0.28 | 0 | 0.72 |
| Anzio | 2017 | 18 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Roma | 2016 | 18 | 0.94 | 0.06 | 0 | 0 | 0 | 0 | 0.03 | 0 | 0.97 |
| Roma Province | 2016–2017 | 36 | 0.97 | 0.03 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0.99 |
| Strangolagalli | 2017 | 19 | 0.89 | 0.05 | 0 | 0.05 | 0 | 0 | 0.08 | 0 | 0.92 |
| Frosinone Province | 2017 | 19 | 0.89 | 0.05 | 0 | 0.05 | 0 | 0 | 0.08 | 0 | 0.92 |
| Bari | 2017–2018 | 20 | 0.90 | 0.10 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Bari Province | 2017–2018 | 20 | 0.90 | 0.10 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Region | Province | Site | Date | N | Gf II | Gf IL | Gf IM | Gf LL | Gf LM | Gf MM | Af L | Af M | Af I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veneto | Venezia | Lido di Venezia | 2019 | 55 | 0 | 0 | 0 | 0.95 | 0.05 | 0 | 0.97 | 0.03 | 0 |
| Veneto | Venezia | Lido di Venezia | 2020 | 21 | 0.10 | 0 | 0 | 0.57 | 0.33 | 0 | 0.74 | 0.17 | 0.10 |
| Veneto | Venezia | Lido di Venezia | 2023 | 10 | 0 | 0.10 | 0 | 0.90 | 0 | 0 | 0.95 | 0 | 0.05 |
| Cod | Region | Province | Municipality | Date | N | Af I | Af L | Af M | Af F | Paper |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Veneto | Venezia | Scorzè | 2022 | 10 | 1.00 | 0 | 0 | 0 | Present paper |
| 2 | Veneto | Venezia | Noale | 2022 | 10 | 1.00 | 0 | 0 | 0 | Present paper |
| 3 | Veneto | Venezia | Salzano | 2022 | 10 | 0.95 | 0.05 | 0 | 0 | Present paper |
| 4 | Veneto | Venezia | Mirano | 2018 | 33 | 0.90 | 0.03 | 0.07 | 0 | Porretta 2022 |
| 5 | Veneto | Venezia | Venezia | 2022 | 10 | 0.20 | 0.80 | 0 | 0 | Present paper |
| 6 | Veneto | Venezia | Lido di Venezia | 2019–2023 | 86 | 0.03 | 0.91 | 0.06 | 0 | Present paper |
| 7 | Veneto | Venezia | Pianiga | 2022 | 10 | 1.00 | 0 | 0 | 0 | Present paper |
| 8 | Veneto | Venezia | Fiesso d’Artico | 2022 | 10 | 1.00 | 0 | 0 | 0 | Present paper |
| 9 | Veneto | Venezia | Fossò | 2019–2020 | 42 | 0.74 | 0.26 | 0 | 0 | Present paper |
| 10 | Veneto | Venezia | Chioggia | 2020–2022 | 23 | 0.87 | 0.02 | 0.11 | 0 | Present paper |
| 11 | Veneto | Venezia | Cona | 2020–2022 | 11 | 0.95 | 0.05 | 0 | 0 | Present paper |
| 12 | Veneto | Rovigo | Rovigo | 2018–2022 | 46 | 0.95 | 0.02 | 0.03 | 0 | Porretta 2022; Present paper |
| 13 | Emilia-Romagna | Ferrara | Comacchio | 2018 | 30 | 0 | 0.07 | 0.93 | 0 | Fotakis 2020 |
| 14 | Emilia-Romagna | Ferrara | Ferrara | 2017–2020 | 87 | 0.73 | 0.26 | 0 | 0 | Porretta 2019; Porretta 2022 |
| 15 | Emilia-Romagna | Ferrara | Poggio Renatico | 2017 | 47 | 0.88 | 0.12 | 0 | 0 | Porretta 2019 |
| 16 | Emilia-Romagna | Ferrara | Cento | 2017 | 49 | 0.89 | 0.11 | 0 | 0 | Porretta 2019 |
| 17 | Emilia-Romagna | Ravenna | Massa Lombarda | 2018 | 27 | 0.35 | 0.65 | 0 | 0 | Fotakis 2020 |
| 18 | Emilia-Romagna | Ravenna | Lugo | 2018 | 30 | 0.82 | 0.10 | 0.05 | 0.03 | Fotakis 2020 |
| 19 | Emilia-Romagna | Ravenna | Ravenna | 2016–2018 | 171 | 0.44 | 0.08 | 0.48 | 0 | Grigoraki 2017; Porretta 2019; Porretta 2022 |
| 20 | Emilia-Romagna | Ravenna | Faenza | 2017 | 47 | 0.94 | 0.06 | 0 | 0 | Porretta 2019 |
| 21 | Emilia-Romagna | Ravenna | Cervia | 2017 | 87 | 0.60 | 0.18 | 0.20 | 0.02 | Porretta 2019; Fotakis 2020 |
| 22 | Emilia-Romagna | Forlì-Cesena | Forlì | 2017–2018 | 113 | 0.52 | 0.26 | 0.18 | 0.04 | Porretta 2019; Fotakis 2020; Porretta 2022 |
| 23 | Emilia-Romagna | Forlì-Cesena | Forlimpopoli | 2017 | 48 | 0.84 | 0.16 | 0 | 0 | Porretta 2019 |
| 24 | Emilia-Romagna | Forlì-Cesena | Bertinoro | 2018 | 30 | 0.68 | 0.20 | 0.12 | 0 | Fotakis 2020 |
| 25 | Emilia-Romagna | Forlì-Cesena | Cesena | 2018–2020 | 65 | 0.50 | 0.26 | 0.18 | 0.06 | Fotakis 2020; Porretta 2022 |
| 26 | Emilia-Romagna | Forlì-Cesena | Cesenatico | 2017 | 50 | 0.66 | 0.26 | 0.08 | 0 | Porretta 2019 |
| 27 | Emilia-Romagna | Rimini | Santarcangelo di Romagna | 2017–2018 | 80 | 0.58 | 0.24 | 0.19 | 0 | Porretta 2019; Fotakis 2020 |
| 28 | Emilia-Romagna | Rimini | Riccione | 2017–2018 | 77 | 0.43 | 0.31 | 0.25 | 0.01 | Porretta 2019; Fotakis 2020 |
| 29 | Emilia-Romagna | Rimini | Rimini | 2017–2018 | 123 | 0.37 | 0.16 | 0.46 | 0 | Porretta 2019; Fotakis 2020 |
References
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 2017, 53, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Severini, F.; Toma, L.; Di Luca, M.; Romi, R. Italian mosquitoes: General information and identification of adults (Diptera, Culicidae)/Le zanzare italiane: Generalità e identificazione degli adulti (Diptera, Culicidae). Fragm. Entomol. 2009, 41, 213–372. [Google Scholar] [CrossRef]
- Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The Role of Culex pipiens L. (Diptera: Culicidae) in Virus Transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control (ECDC). West Nile Virus Infection—AER Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Barzon, L.; Pacenti, M.; Franchin, E.; Squarzon, L.; Lavezzo, E.; Cattai, M.; Cusinato, R.; Palù, G. The complex epidemiological scenario of West Nile virus in Italy. Int. J. Environ. Res. Public Health 2013, 10, 4669–4689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control (ECDC). Historical Data by Year—West Nile Virus Seasonal Surveillance; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed on 7 November 2024).
- European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 7 November 2024).
- European Centre for Disease Prevention and Control (ECDC). Guidelines for the Surveillance of Invasive Mosquitoes in Europe; ECDC: Stockholm, Sweden, 2012; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf (accessed on 15 December 2024). [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Guidelines for the Surveillance of Native Mosquitoes in Europe; ECDC: Stockholm, Sweden, 2014; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/surveillance-of%20native-mosquitoes%20-guidelines.pdf (accessed on 15 December 2024). [CrossRef]
- Ministero della Salute. Piano Nazionale di Prevenzione, Sorveglianza e Risposta alle Arbovirosi (PNA) 2020–2025; Ministero della Salute: Rome, Italy, 2019. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 15 December 2024).
- Regulation Eu N° 528/2012—EN—EUR Lex. Available online: https://eur-lex.europa.eu/eli/reg/2012/528/oj (accessed on 15 December 2024).
- Pusztai, M.; Fast, P.; Gringorten, L.; Kaplan, H.; Lessard, T.; Carey, P.R. The mechanism of sunlight-mediated inactivation of Bacillus thuringiensis crystals. Biochem. J. 1991, 273, 43–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Zhang, X.; Zhang, Y.; Wu, S.; Gelbič, I.; Xu, L.; Guan, X. A new formulation of Bacillus thuringiensis: UV protection and sustained release mosquito larvae studies. Sci. Rep. 2016, 6, 39425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Douris, V.; Steinbach, D.; Panteleri, R.; Livadaras, I.; Pickett, J.A.; Van Leeuwen, T.; Nauen, R.; Vontas, J. Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 14692–14697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellini, R.; Zeller, H.; Van Bortel, W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit. Vectors 2014, 7, 323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Grigoraki, L.; Puggioli, A.; Mavridis, K.; Douris, V.; Montanari, M.; Bellini, R.; Vontas, J. Striking diflubenzuron resistance in Culex pipiens, the prime vector of West Nile Virus. Sci Rep. 2017, 7, 11699, Erratum in Sci Rep. 2018, 8, 6122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fotakis, E.A.; Mastrantonio, V.; Grigoraki, L.; Porretta, D.; Puggioli, A.; Chaskopoulou, A.; Osório, H.; Weill, M.; Bellini, R.; Urbanelli, S.; et al. Identification and detection of a novel point mutation in the Chitin Synthase gene of Culex pipiens associated with diflubenzuron resistance. PLoS Negl. Trop. Dis. 2020, 14, e0008284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucchesi, V.; Grimaldi, L.; Mastrantonio, V.; Porretta, D.; Di Bella, L.; Ruspandini, T.; Di Salvo, M.L.; Vontas, J.; Bellini, R.; Negri, A.; et al. Cuticle Modifications and Over-Expression of the Chitin-Synthase Gene in Diflubenzuron-Resistant Phenotype. Insects 2022, 13, 1109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mastrantonio, V.; Porretta, D.; Lucchesi, V.; Güz, N.; Çağatay, N.S.; Bellini, R.; Vontas, J.; Urbanelli, S. Evolution of Adaptive Variation in the Mosquito Culex pipiens: Multiple Independent Origins of Insecticide Resistance Mutations. Insects 2021, 12, 676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porretta, D.; Fotakis, E.A.; Mastrantonio, V.; Chaskopoulou, A.; Michaelakis, A.; Kioulos, I.; Weill, M.; Urbanelli, S.; Vontas, J.; Bellini, R. Focal distribution of diflubenzuron resistance mutations in Culex pipiens mosquitoes from Northern Italy. Acta Trop. 2019, 193, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Porretta, D.; Mastrantonio, V.; Lucchesi, V.; Bellini, R.; Vontas, J.; Urbanelli, S. Historical samples reveal a combined role of agriculture and public-health applications in vector resistance to insecticides. Pest Manag. Sci. 2022, 78, 1567–1572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fotakis, E.A.; Mavridis, K.; Kampouraki, A.; Balaska, S.; Tanti, F.; Vlachos, G.; Gewehr, S.; Mourelatos, S.; Papadakis, A.; Kavalou, M.; et al. Mosquito population structure, pathogen surveillance and insecticide resistance monitoring in urban regions of Crete, Greece. PLoS Negl. Trop. Dis. 2022, 16, e0010186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guz, N.; Cagatay, N.S.; Fotakis, E.A.; Durmusoglu, E.; Vontas, J. Detection of diflubenzuron and pyrethroid resistance mutations in Culex pipiens from Muğla, Turkey. Acta Trop. 2020, 203, 105294. [Google Scholar] [CrossRef] [PubMed]
- Paronyan, L.; Babayan, L.; Vardanyan, H.; Manucharyan, A.; Papapostolou, K.M.; Balaska, S.; Vontas, J.; Mavridis, K. Molecular monitoring of insecticide resistance in major disease vectors in Armenia. Parasit. Vectors 2024, 17, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.P.; Rhaiem, A.; Brunhes, J. The Mosquitoes of Europe. An Identification and Training Programme; IRD Editions & EID MeÂditerrane Âe: Montpellier, France, 2001. [Google Scholar]
- Rider, M.A.; Byrd, B.D.; Keating, J.; Wesson, D.M.; Caillouet, K.A. PCR detection of malaria parasites in desiccated Anopheles mosquitoes is uninhibited by storage time and temperature. Malar. J. 2012, 11, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drago, A. (Entostudio srl, Ponte San Nicolò, Padua, Italy). Personal communication, 2024.
- Pichler, V.; Giammarioli, C.; Bellini, R.; Veronesi, R.; Arnoldi, D.; Rizzoli, A.; Lia, R.P.; Otranto, D.; Ballardini, M.; Cobre, P.; et al. First evidence of pyrethroid resistance in Italian populations of West Nile virus vector Culex pipiens. Med. Vet. Entomol. 2022, 36, 390–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pichler, V.; Mancini, E.; Micocci, M.; Calzetta, M.; Arnoldi, D.; Rizzoli, A.; Lencioni, V.; Paoli, F.; Bellini, R.; Veronesi, R.; et al. A Novel Allele Specific Polymerase Chain Reaction (AS-PCR) Assay to Detect the V1016G Knockdown Resistance Mutation Confirms Its Widespread Presence in Aedes albopictus Populations from Italy. Insects 2021, 12, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Liverani, A.; Venturi, G.; Gavaudan, S.; Riccardo, F.; Salvoni, G.; Fortuna, C.; Marinelli, K.; Marsili, G.; Pesaresi, A.; et al. Autochthonous dengue outbreak in Marche Region, Central Italy, August to October 2024. Eurosurveillance 2024, 29, 2400713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pichler, V.; Malandruccolo, C.; Serini, P.; Bellini, R.; Severini, F.; Toma, L.; Di Luca, M.; Montarsi, F.; Ballardini, M.; Manica, M.; et al. Phenotypic and genotypic pyrethroid resistance of Aedes albopictus, with focus on the 2017 chikungunya outbreak in Italy. Pest Manag. Sci. 2019, 75, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.S.; Hadjichristodoulou, C.; Kyritsi, M.A.; Papadopoulos, N.T. Short-Term Selection to Diflubenzuron and Bacillus thuringiensis Var. Israelensis Differentially Affects the Winter Survival of Culex pipiens f. Pipiens and Culex pipiens f. Molestus (Diptera: Culicidae). Insects 2021, 12, 527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Virgillito, C. (Sapienza University of Rome, Rome, Italy). Personal communication, 2024.
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]


| Region-Province | Date | N° Sites | N° Samples | Genotypic Frequencies | Allelic Freq | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gf II | Gf IL | Gf IM | Gf LL | Gf LM | Gf MM | Af L | Af M | Af I | ||||
| Trentino-Alto Adige-Trento | 2017–2020 | 3 | 84 | 0.55 | 0.31 | 0 | 0.14 | 0 | 0 | 0.30 | 0 | 0.70 |
| Veneto-Padova | 2020–2022 | 19 | 181 | 0.97 | 0.01 | 0 | 0.02 | 0 | 0 | 0.02 | 0 | 0.98 |
| Veneto-Rovigo | 2020–2022 | 8 | 76 | 0.96 | 0 | 0 | 0.04 | 0 | 0 | 0.04 | 0 | 0.96 |
| Veneto-Treviso | 2020–2022 | 14 | 86 | 0.91 | 0.02 | 0 | 0.06 | 0.01 | 0 | 0.08 | 0.01 | 0.92 |
| Veneto-Venezia | 2019–2023 | 16 | 252 | 0.50 | 0.09 | 0.004 | 0.35 | 0.04 | 0.01 | 0.42 | 0.03 | 0.55 |
| Veneto-Verona | 2020–2022 | 16 | 120 | 0.95 | 0.03 | 0 | 0.03 | 0 | 0 | 0.04 | 0 | 0.96 |
| Veneto-Vicenza | 2020–2022 | 14 | 124 | 0.94 | 0.02 | 0 | 0.03 | 0 | 0 | 0.04 | 0 | 0.96 |
| Piemonte-Torino | 2017 | 1 | 18 | 0.78 | 0.22 | 0 | 0 | 0 | 0 | 0.11 | 0 | 0.89 |
| Liguria-Imperia | 2018 | 1 | 16 | 0.63 | 0.19 | 0 | 0.19 | 0 | 0 | 0.28 | 0 | 0.72 |
| Lazio-Frosinone | 2017 | 1 | 19 | 0.89 | 0.05 | 0 | 0.05 | 0 | 0 | 0.08 | 0 | 0.92 |
| Lazio-Roma | 2016–2017 | 2 | 36 | 0.97 | 0.03 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0.99 |
| Puglia-Bari | 2017–2018 | 1 | 20 | 0.90 | 0.10 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0.95 |
| Total | 2016–2023 | 96 | 1032 | 0.80 | 0.07 | 0 | 0.12 | 0.01 | 0 | 0.16 | 0.01 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micocci, M.; Pichler, V.; Serini, P.; Giammarioli, C.; Malandruccolo, C.; Virgillito, C.; Ballardini, M.; Lia, R.P.; Arnoldi, D.; Vettore, S.; et al. Widespread Distribution of chs-1 Mutations Associated with Resistance to Diflubenzuron Larvicide in Culex pipiens Across Italy, Reaching Virtual Fixation in the Venetian Lagoon. Insects 2025, 16, 204. https://doi.org/10.3390/insects16020204
Micocci M, Pichler V, Serini P, Giammarioli C, Malandruccolo C, Virgillito C, Ballardini M, Lia RP, Arnoldi D, Vettore S, et al. Widespread Distribution of chs-1 Mutations Associated with Resistance to Diflubenzuron Larvicide in Culex pipiens Across Italy, Reaching Virtual Fixation in the Venetian Lagoon. Insects. 2025; 16(2):204. https://doi.org/10.3390/insects16020204
Chicago/Turabian StyleMicocci, Martina, Verena Pichler, Paola Serini, Carola Giammarioli, Chiara Malandruccolo, Chiara Virgillito, Marco Ballardini, Riccardo Paolo Lia, Daniele Arnoldi, Stefano Vettore, and et al. 2025. "Widespread Distribution of chs-1 Mutations Associated with Resistance to Diflubenzuron Larvicide in Culex pipiens Across Italy, Reaching Virtual Fixation in the Venetian Lagoon" Insects 16, no. 2: 204. https://doi.org/10.3390/insects16020204
APA StyleMicocci, M., Pichler, V., Serini, P., Giammarioli, C., Malandruccolo, C., Virgillito, C., Ballardini, M., Lia, R. P., Arnoldi, D., Vettore, S., Bonetto, D., Martini, S., Drago, A., della Torre, A., & Caputo, B. (2025). Widespread Distribution of chs-1 Mutations Associated with Resistance to Diflubenzuron Larvicide in Culex pipiens Across Italy, Reaching Virtual Fixation in the Venetian Lagoon. Insects, 16(2), 204. https://doi.org/10.3390/insects16020204








