Simple Summary
In response to herbivorous insect attacks, plants have developed the ability to rapidly modify the secondary metabolisms involved in their defense against insects. The spectrum specificity of plant secondary metabolisms plays a significant role in insect adaptation to each host. The fall armyworm (FAW) Spodoptera frugiperda is a serious agricultural pest that has invaded China. The FAW is a polyphagous insect that feeds on gramineous crops, such as maize, sorghum, and rice. However, the chemical basis of sorghum defenses against FAWs is not well understood in the current research. This study found that sorghum can reduce FAWs’ fitness, resulting in significantly lower selection and damage rates for sorghum, and reducing larval weight relative to maize. Responding to FAW attacks, both maize and sorghum rapidly alter their secondary metabolic profiles, which show species-specific changes. Gambogenic acid and chimonanthine, which are present and induced in sorghum, have a detrimental effect on larvae feeding and growth, deterring their feeding and lowering their weight increase. These findings indicate that the present and induced secondary compounds in sorghum have a role in chemical defense against FAWs and aid in the development of new pest control strategies.
Abstract
The fall armyworm (FAW), Spodoptera frugiperda, is one of the major agricultural pests that has invaded China. The FAW is a polyphagous insect with the gramineous crop sorghum being a key host plant. However, the basis of sorghum’s chemical defense against FAW feeding is still unclear. In this study, we investigated the potential defensive mechanism of sorghum against this insect species. It was found that FAW larvae preferred maize over sorghum, the selection and damage rates for sorghum plants by larvae were significantly lower than those of maize plants, and feeding on sorghum restricted larval weight. The non-target metabolomics revealed that the feeding of FAW larvae altered the plant secondary metabolite spectra in maize and sorghum, resulting in species-specific differential secondary metabolites (DSMs). Of these, 19 DSMs were specific in maize, and 51 in sorghum, and only 6 were found in both species. Two-choice and no-choice feeding assays found that gambogenic acid and chimonanthine, two DSMs unique to sorghum, were found to deter larval feeding and decrease the larval weight. These findings reveal that the defense of sorghum against FAW is regulated by changing the response spectra of secondary metabolites and that the induced metabolites have a defensive function by acting as antifeedants, which provides new insights into employing bioactive plant compounds against polyphagous insects.
1. Introduction
The co-evolution between herbivorous insects and host plants is considered an arms race [1]. To prevent insects from feeding, host plants develop and form multiple defense mechanisms, such as physical and chemical ones. In response to insect feeding, plants can quickly produce a large number of chemical defense components, such as chemical signals, biotoxins (secondary metabolites), defense proteins, and so forth. Among these, plant secondary metabolites, which are poisonous defensive compounds, are the main defenses against herbivorous insects [2,3].
Plant secondary metabolites mainly consist of terpenes, phenolic compounds (phenolic acids and flavonoids), and secondary nitrogen-containing compounds (alkaloids), which play an important role in plant defense against the feeding, growth and development, and reproduction of herbivorous insects [4,5,6]. In this plant–herbivore interaction, plant defenses against insects include indirect defense and direct defense. Based on their effects on insects, these compounds function as attractants, deterrents, digestive inhibitors, and toxins [7,8]. As a type of indirect defense, attractants are attractive plant volatiles that are appealing to natural enemies. The parasitic wasps Cotesia plutellae of the diamondback moth can be attracted to allyl isothiocyanate (AITC), and Dasineura brassicae parasitoids Omphale clypealis and Platygaster subuliformis are strongly attracted to AITC and phenylethyl isothiocyanate [9]. One plant direct defense is that secondary metabolites act as deterrents, digestive inhibitors, and toxins, which directly or negatively affect the feeding, growth and development, and reproduction of insects [10]. The effect of deterrents and toxins has been studied in depth in insects. Cucurbitacin acts as a feeding deterrent for Pieris rapae and strongly inhibits larval feeding [11]. Similarly, judaicin 7-O-glucoside, 2-methoxyjudaicin, judaicin, and maackiain are four isoflavonoids that have shown antifeedant activities, deterring larval feeding by H. armigera [12], and saponins act as feeding deterrents for the larvae of the diamondback moth [13,14]. Previous studies have proved that certain secondary compounds are poisonous to herbivorous insects, suppressing larval growth and development and reducing the female reproduction rate. Gossypol is a crucial component of pest defense in cotton due to its toxicity [15,16,17]. Naringenin and quercetin are toxic to Acyrthosiphon pisum, which leads to prolonged development times, increased mortality, and decreased fecundity after feeding [18]. Resveratrol and p-coumaric acid are two phenolic compounds that reduce the larval weight of Spodoptera litura and Amsacta albistriga [19]. Conversely, insect feeding can boost the amount of secondary defensive compounds in plants, enhancing their ability to defend against insects. Gallic acid, 4-cinnamic acid, p-coumaric acid, and salicylic acid accumulate in cotton plants as a result of feeding by H. armigera and S. litura, suppressing larval weight and raising mortality [20]. Ostrinia furnacalis feeding increases the content of benzoxazines in maize, which decreases the relative larval growth rate and increases the relative larval consumption rate [21]. Brown planthopper (BPH), Nilaparvata lugens, feeding has been shown to significantly cause the accumulation of sakuranetin in the leaf sheath and phloem of rice, which has shown a detrimental effect on BPH as a sucking deterrent [22]. Therefore, discovering new bioactive defense compounds is the key to understanding the underlying defensive adaptations of host plants against insects, and the control of pests using plant secondary compounds.
The fall armyworm (FAW), Spodoptera frugiperda, is a notorious invasive pest in China [23,24]. The FAW is a polyphagous insect with a wide range of hosts and can feed on 353 plant species from 76 families [25]. The FAW is classed into two strains based on host preference: the maize strain and the rice strain [26,27]. The FAW found in China belongs to the maize strain [28]. In addition to maize, rice, and wheat, other gramineous grain crops, such as sorghum and millet, are the FAW’s host plants. Previous studies have demonstrated that FAW can transfer between hosts when there is a high population density or insufficient maize supplies [29]. To date, chemical control is still the main measure used to manage FAW; however, the frequent application of insecticides in a short period has led to the rapid development of insecticide resistance in this species [30]. Nonetheless, utilizing host plant resistance to defend against insects is an effective pest control strategy [31,32]. Hence, the study of the adaptation of FAW to host plants is very important for developing a new green and safe strategy to control FAWs. Plant secondary metabolites have been demonstrated to influence the feeding preferences and host adaptability of herbivorous insects [10,33]. In sorghum, some secondary compounds can reduce FAW fitness. For example, tannin and 3-deoxyanthocyanidin flavonoids in sorghum induce resistance to FAW [34,35], and the latter also contributes to resistance to Rhopalosiphum maidis [36]. Furthermore, it has been reported that some secondary compounds, such as cedrelone [37], carvacrol [38], essential oils from Hyptis marrubioides and Ocimum basilicum [39], and polyphenol extract present in purple maize pericarp extract [40], are toxic and/or inhibit its growth. The production of flavonoids in sorghum SC1345 is induced by FAW larvae [41]. Yet, how these compounds contribute to resistance against FAW is still unknown. Thus, plant secondary metabolites are essential for the host’s defense against herbivorous insects. However, further studies are needed to discover which secondary metabolites are present in sorghum and which secondary compounds, such as flavonoids, are involved in host defensive resistance to FAW.
Here, we investigate the underlying defense mechanism of sorghum against the invasive insect S. frugiperda using biological assays and non-target metabolomics. First, the preference of FAW larvae on maize and sorghum was studied by using a series of biological assays. Subsequently, non-target metabolomics was used to uncover the changes in the secondary metabolites in maize and sorghum before and after larvae infestation. Finally, the effects of sorghum-specific DSMs gambogenic acid, baohuoside II, and chimonanthine on the feeding preferences and growth and development of FAW larvae were analyzed. Our findings provide new insights into employing bioactive plant compounds against polyphagous insects and lay the foundation for the development of new strategies for pest control.
2. Materials and Methods
2.1. Insect and Plant Culture
The original fall armyworm Spodoptera frugiperda was collected from Dehong Dai and Jingpo Autonomous Prefecture, Yunnan Province, China. An artificial diet was prepared with 280 g maize powder, 90 g soybean powder, 35 g yeast powder, 25 g agar, 0.2 g vitamin B, 12 g cholesterol, 2 g sorbic acid, 12 g L-ascorbic acid, 5 g nipagin ester, 4 mL methanol, 0.2 g penicillin, and 1500 mL water according to the previous study [42]. The rearing conditions were a photoperiod of 16 h light–8 h darkness, a temperature of 25 ± 1 °C, and 60 ± 5% relative humidity. The pupae were separated by sex and then placed in a cage for emergence. Adults were fed with 10% honey water.
The seeds of the maize variety Zhongnongtian488 and sorghum variety Hongyingzi were planted into plastic pots (9 cm diameter × 10 cm height), respectively. All the pots were placed in a netting room under natural conditions. After germination and growth, 10 healthy and the same-sized seedlings were kept and allowed to grow for 3 weeks under natural conditions.
2.2. Chemicals
Gambogenic acid (≥98% purity) and baohuoside II (≥98% purity) were purchased from Yuanyeshengwu (Shanghai, China), and chimonanthine (≥98% purity) from Macklin (Shanghai, China).
2.3. Biological Assay
First, we used a two-choice test to evaluate the feeding preferences of the 5th-instar FAW larvae for maize and sorghum. Disks of maize and sorghum with 15 mm diameters were prepared. The maize leaf disks were used as control, and the sorghum leaf disks as the treatment. The two maize and sorghum leaf disks were alternately placed in a 9 cm diameter Petri dish. A wetting filter paper was used to cover each Petri dish to maintain humidity. Each healthy and selected larva was first starved for 2 h and then placed into the above Petri dish to feed for 2 h. The feeding area of larvae on the treated and control leaf disks was counted, and also the feeding preference index (PI) was calculated. PI = (the consumed area of the treated disk—the consumed area of the control disk)/(the consumed area of the treated disk + the consumed area of the control disk).
Second, a cage experiment was conducted to investigate the effects of different host plants on the selection preference of FAW larvae. Potted maize and sorghum seedlings were alternately placed at the four corners of a closed cage (length × width × height: 50 cm × 50 cm × 50 cm). Forty uniform 5th-instar larvae were placed in a central position at the bottom of the cage. The distance between the insect and the four corners of the cage was about 70 cm. The larvae were allowed to select host plants and to feed overnight (about 16 h) in natural conditions. The experiment was stopped after larvae feeding for 16 h, the number of maize and sorghum plants damaged by larvae and the number of larvae on the maize and sorghum plants were counted, respectively, calculating the plant damage rate and the number of larvae on the different plants. The plant damage rate (%) = (number of maize or sorghum plants damaged by larvae per pot)/(total number of maize or sorghum plants per pot) × 100.
Finally, we evaluated the effect of maize and sorghum on the growth and development of FAW larvae using a no-choice feeding assay. The newly hatched larvae were fed on fresh maize and sorghum leaves in plastic culture tubes (2.4 cm diameter × 9.5 cm height) for eight days. Larvae fed on maize leaves were used as the control group, and larvae fed on sorghum leaves as the treatment group. Each leaf was changed every day and each larva was weighed every two days. The changes in larval weight were used to evaluate the growth rate of the larvae. The control and treatment experiments were repeated five times, and five larvae were used in each replicate.
2.4. Sample Collection and Metabolite Extraction
Here, the potted maize and sorghum were divided into two groups: one group of maize and sorghum seedlings served as the treatment group that was infested with larvae, and the other group was considered as the control that was un-infested with larvae. For the treatment group, each potted maize or sorghum seedling was infested by about 10–15 3rd-instar larvae. After infestation, each pot of seedlings was covered with a clean plastic cylinder (9 cm diameter × 20 cm length), and its top was sealed with gauze to prevent insect escape. The soil surface of the pot was also covered with plastic wrap to prevent larvae from getting into the soil. For the control group, each potted maize or sorghum seedling was covered with a clean plastic cylinder and sealed with gauze. After 24 h, the leaves of maize and sorghum belonging to the treatment and control groups were quickly cut out, wrapped in tin foil, and then frozen in liquid nitrogen. Each control and treatment experiment on the maize and sorghum was repeated six times.
The detections of non-target metabolomics for all samples were performed by Novogene Co., Ltd., Beijing, China. All chemicals and solvents used in this experiment were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Approximately 100 mg samples were individually ground with liquid nitrogen. The homogenates were resuspended with 500 μL 80% methanol–water solution by well vortexing, incubated on ice for 5 min, and then centrifuged at 4 °C and 15,000 rpm for 20 min. The supernatant was diluted with water to a final concentration containing 53% methanol and was centrifuged at 15,000 rpm for 20 min at 4 °C. The obtained supernatants were collected and filtered using a 0.22 μm filter membrane and the extracts were stored at −80 °C.
2.5. UHPLC-MS/MS Analysis
Ultra-high-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS) analyses were performed using a Vanquish UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) coupled to Q Exactive HF/Q Exactive HF-X Q Exactive (Thermo Fisher Scientific, Waltham, MA, USA). The binary mobile phases for the positive polarity mode were 0.1% formic acid (A) and methanol (B). The binary mobile phases for the negative polarity mode were 5 mM ammonium acetate (pH 9.0, A) and methanol (B). The solvent gradient was set as follows: 2% B, 1.5 min; 2–85% B, 3 min; 85–100% B, 10 min; 100–2% B, 10.1 min; 2% B, 12 min. The injection volume of the samples and standards was 5 µL. The column temperature was 40 °C, and the flow rate was set at 0.2 mL/min. A Q ExactiveTM HF mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.5 kV, capillary temperature of 320 °C, sheath gas flow rate of 35 psi, and aux gas flow rate of 10 L/min, as well as an S-lens RF level of 60 and aux gas heater temperature of 350 °C.
The raw data files obtained from UHPLC-MS/MS were processed using the software Compound Discoverer 3.1 (CD3.1, Thermo Fisher Scientific, Waltham, MA, USA) to integrate and correct the peaks. Then, the peaks were matched with the mzCloud (https://www.mzcloud.org/, accessed on 5 November 2023) and ChemSpider (http://www.chemspider.com/, accessed on 5 November 2023) databases to obtain the accurate qualitative and relative quantitative result for the corresponding metabolite. The metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/, accessed on 5 November 2023), HMDB database (https://hmdb.ca/, accessed on 5 November 2023), and Lipidmaps database (http://www.lipidmaps.org/, accessed on 5 November 2023). The identification of DSMs was performed by using principal components analysis (PCA), partial least squares discriminant analysis (PLS-DA), and univariate analysis (t-test). The metabolites with VIP (variable importance for the projection) > 1.0, FC (fold change) > 1.2, or FC < 0.833, p-value < 0.05, were considered to be DSMs. The heat maps of the abundance of DSMs were produced by using the heatmap tool in Hiplot Pro (https://hiplot.com.cn/, accessed on 28 October 2024).
2.6. Functional Assay of Candidate Compounds on S. frugiperda Larvae
The two-choice assay was carried out based on the previously reported feeding choice assay with a minor modification [43]. Gambogenic acid and baohuoside II were dissolved in acetone, and chimonanthine was dissolved in methyl alcohol. These compounds were prepared into a series of concentration gradient solutions at 20 μg/mL, 200 μg/mL, and 2000 μg/mL. Four maize leaf disks with an area of 1 cm2 were alternately placed in a 9 cm diameter Petri dish. The treated disk was coated with 20 μL of the candidate compounds. The concentration gradients for each compound were the same as above. The same volume of solvent (acetone or methanol) was applied as a control. Each Petri dish was covered with a humid filter paper to maintain humidity. Healthy fifth-instar larvae were first starved for 2 h and then larvae were placed in individual Petri dishes and allowed to feed on a leaf disk for 2 h. The area consumed by larvae on the treated and control leaf disks was counted for the calculation of the feeding preference index.
The effect of these three compounds on larval growth and development was evaluated by the following experiments. The artificial diet served as the substrate. Gambogenic acid, baohuoside II, and chimonanthine were prepared in 1000 μg/mL solutions. Fifty-microliter compounds were added to a 1 cm3 artificial diet and the same volume of acetone or methanol was added as a control. The control and treated artificial diets were put into a 24-well cell culture plate and after weighing, 2nd-instar larvae were placed in individual wells. At 3 and 5 days post-feeding, the larvae were weighed to evaluate the effect of these compounds on larval weight.
2.7. Statistical Analysis
Statistical analyses were performed using SPSS (SPSS 20 software, Chicago, IL, USA), and GraphPad Prism 8.3.0 (GraphPad Software, San Diego, CA, USA). Data visualization was performed by using the GraphPad Prism software. The data from the two-choice feeding experiments were analyzed with the two-tailed Student’s t-test. The feeding preference indexes of compound-fed larvae were analyzed by one-way ANOVA and compared with the Tukey HSD test. The analysis of the change in larval weight was applied with the nonparametric test (Mann–Whitney U test). Data are presented as the mean ± standard error of the mean (SEM). Asterisks indicate statistical significance (* p < 0.05, ** p < 0.01, *** p < 0.001) and n.s. shows no significant differences (p > 0.05). Different letters indicate significant differences according to one-way ANOVA followed by the Tukey HSD test.
3. Results
3.1. Larvae of S. frugiperda Prefer to Feed on Maize over Sorghum
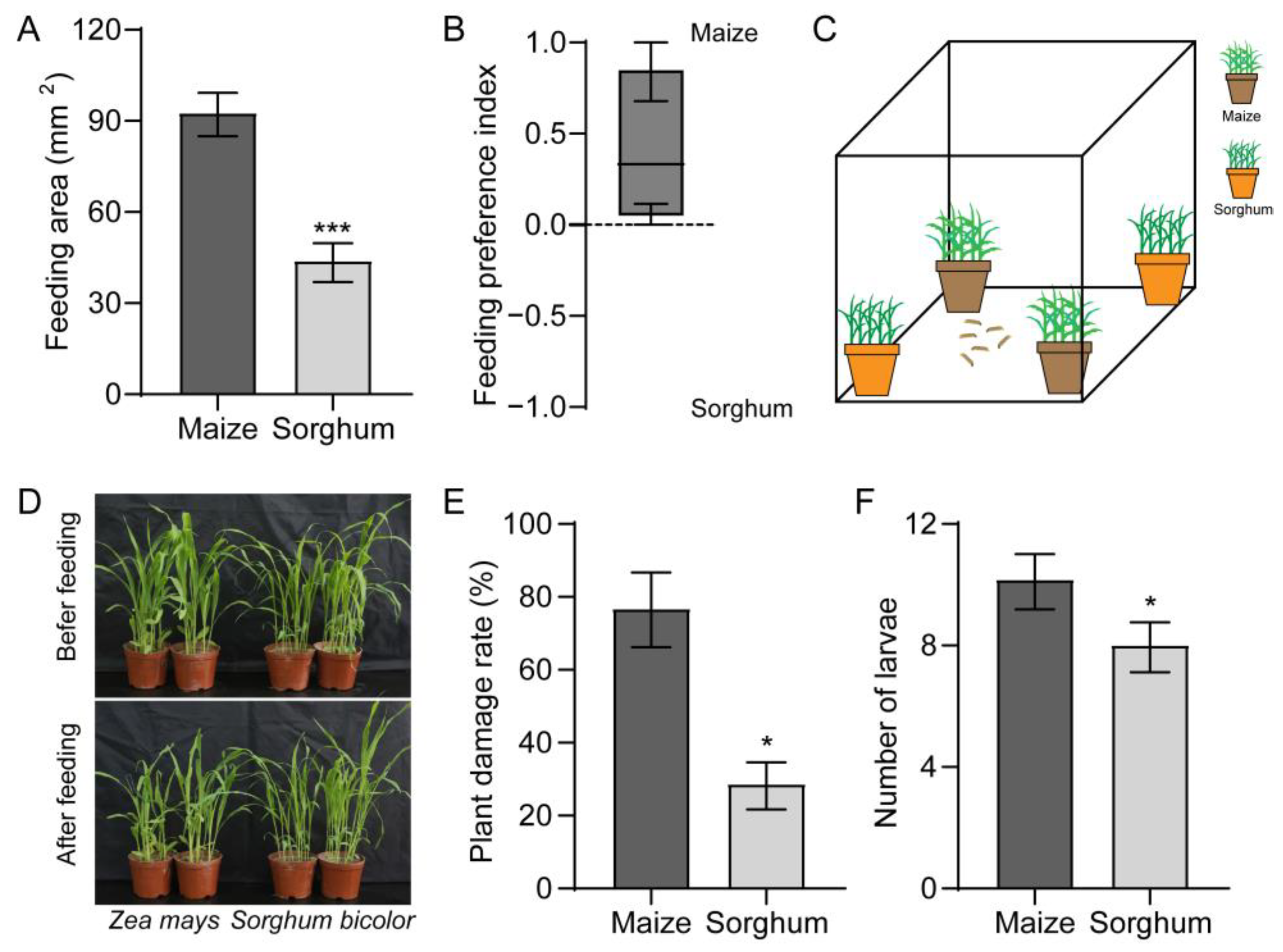
Using a two-choice test, we found that FAW larvae preferred maize over sorghum (Figure 1A). The FAW larvae displayed a stronger response to maize with a feeding preference index (PI) value of 0.4 (Figure 1B). We tested the preferences of the larvae towards the maize and sorghum plants (Figure 1C). Almost all maize plants were fed on by the FAW larvae, while the sorghum plants were fed on sparingly (Figure 1D). In contrast to maize, which had a 76.7% damage rate, the sorghum plants only had 28.6% damage (Figure 1E). The number of larvae collected from the sorghum plants was also lower than that collected from the maize (Figure 1F).
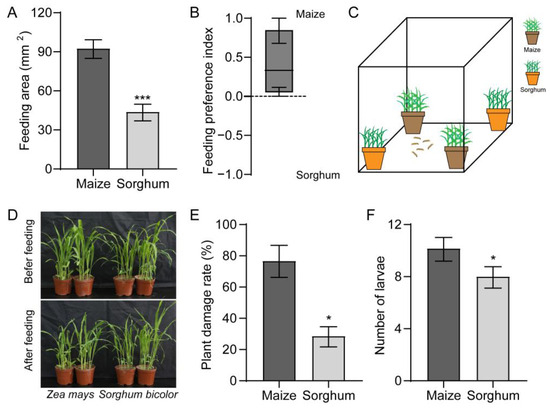
Figure 1.
Preferences of Spodoptera frugiperda larvae towards maize and sorghum. (A,B) Feeding area (A) and feeding preference index (B) of larvae on maize and sorghum leaves. n = 24. (C,D) Schematic diagram (C) and representative image (D) of the feeding effects of larvae on maize and sorghum plants. (E) Damage rate on maize and sorghum plants after being infested by larvae. n = 4. (F) Number of larvae collected on maize and sorghum. n = 3. Data are presented as mean ± SEM. * p < 0.05, *** p < 0.001.
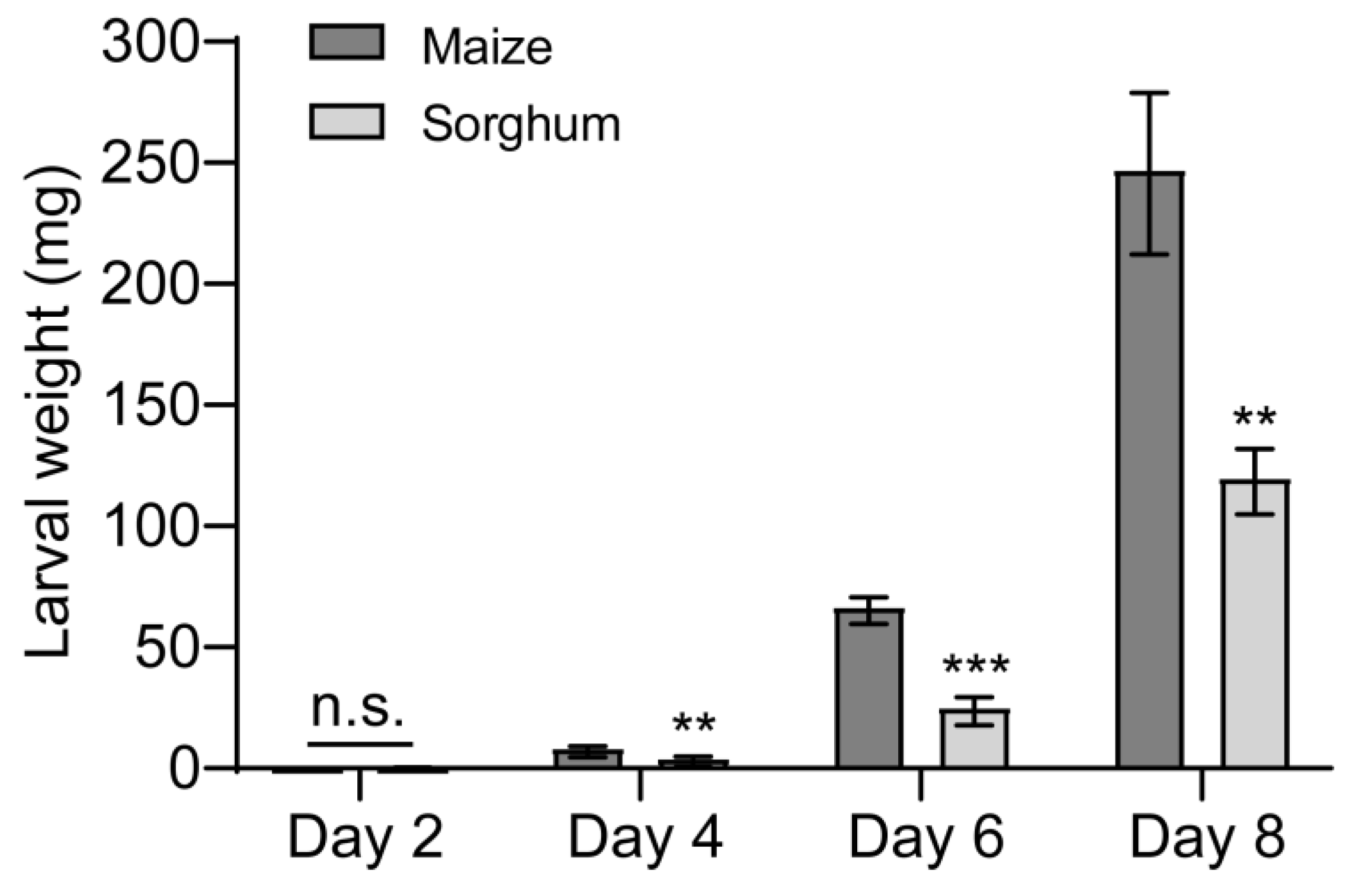
Next, we studied the effects of feeding on different host plants on the growth and development of FAW larvae. The no-choice feeding assay found that feeding on sorghum reduced larval weight (Figure 2). Compared to larvae reared on maize leaves, those raised on sorghum leaves gained less mass and grew more slowly over time (Figure 2), the weight of the sorghum-fed larvae decreased by 50.3%, 62.3%, and 51.5% at 4, 6, and 8 days, respectively, in comparison to the control (maize-fed larvae) (Figure 2).
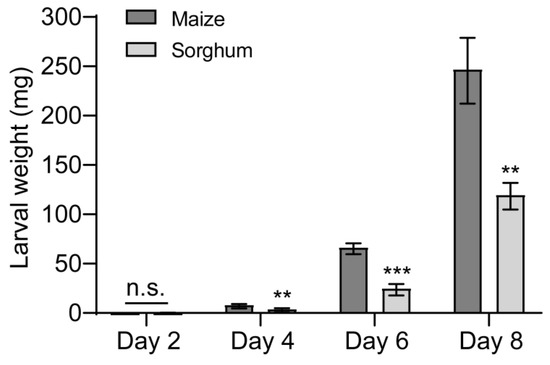
Figure 2.
Effects of maize and sorghum on Spodoptera frugiperda larval weight. The newly hatched S. frugiperda larvae were fed on the leaves of maize and sorghum, respectively, and the larvae were weighed every 2 days. n = 5. Five larvae were used for each replicate. Data are presented as mean ± SEM. ** p < 0.01, *** p < 0.001. n.s. indicates no significant difference (p > 0.05).
3.2. Screening of Differential Secondary Metabolites with Significant Changes in Maize and Sorghum
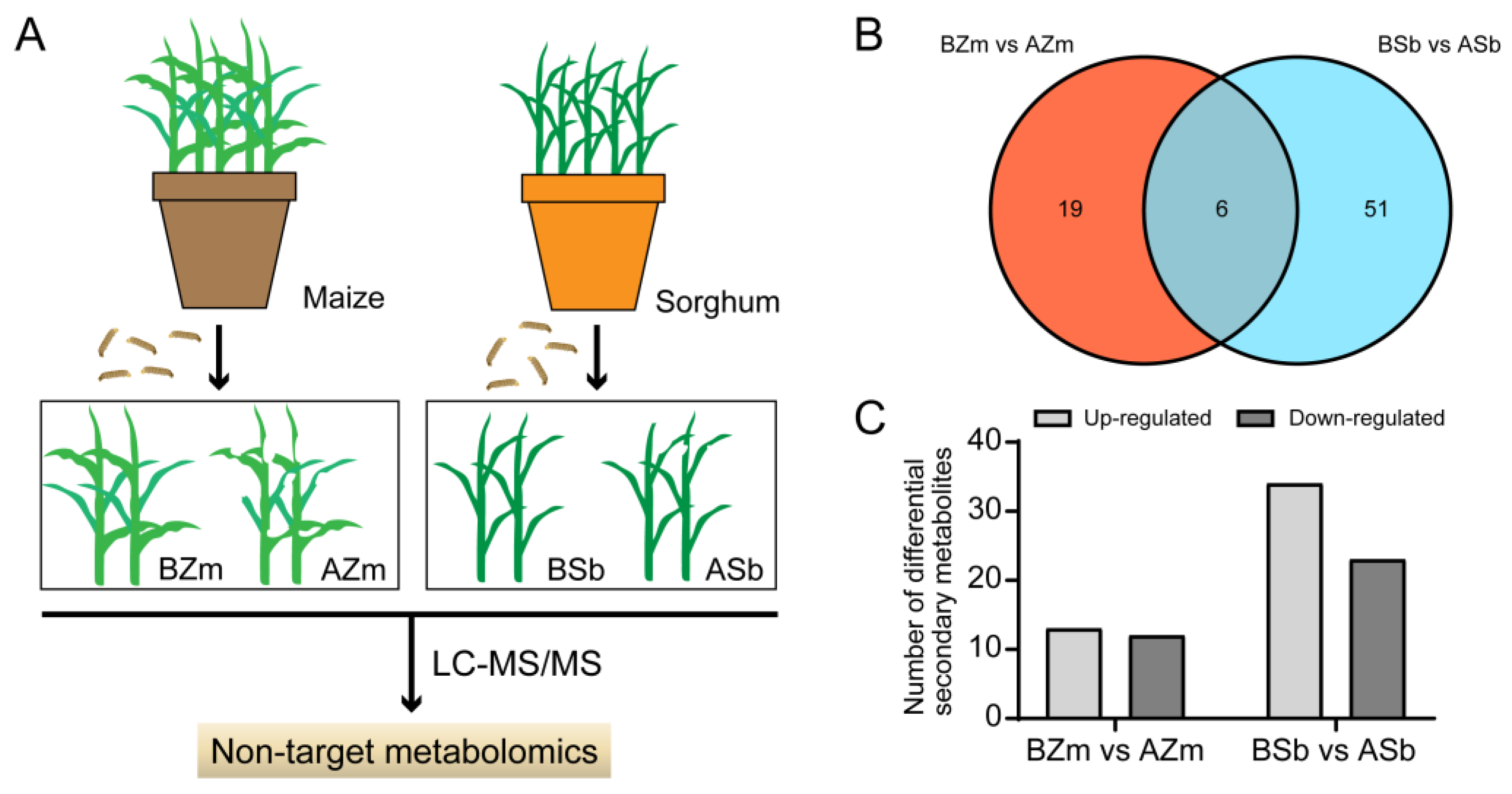
To explore the responses of host plants to the attack by insects, we detected changes in secondary metabolites in maize and sorghum both before (control) and after (treatment) FAW larvae infestation by using LC-MS/MS techniques (Figure 3A). The results showed that the response spectra of the plant secondary metabolites were altered by larvae fed on maize and sorghum. We analyzed the changes in maize secondary compounds before feeding by larvae (BZm) and after feeding by larvae (AZm) and found 25 DSMs in the BZm and AZm group, including 13 increased and 12 decreased ones (Figure 3B,C). And we discovered 57 DSMs with 34 increased and 23 decreased ones in sorghum before feeding by larvae (BSb) and after feeding by larvae (ASb) (Figure 3B,C). For these DSMs, the number of DSMs from the BZm vs. AZm group and BSb vs. ASb group were 19 and 51, respectively, and only 6 compounds were shared between them (Figure 3B and Table 1).
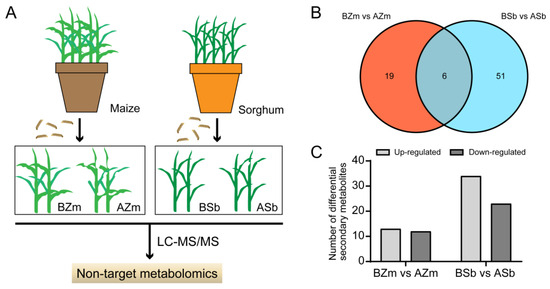
Figure 3.
Detection of differential secondary metabolites of maize and sorghum after feeding by Spodoptera frugiperda larvae. (A) Flow diagram of detection of differential secondary metabolites (DSMs) in maize and sorghum. (B) Venn diagrams of DSMs among different maize and sorghum samples. (C) Numbers of induced and reduced DSMs in the maize and sorghum groups. BZm, maize plant before feeding by larvae; AZm, maize plant after feeding by larvae; BSb, sorghum plant before feeding by larvae; ASb, sorghum plant after feeding by larvae.

Table 1.
The fold change and VIP value of differential secondary metabolites in maize and sorghum groups.
Phenols mainly include simple phenols and flavonoid compounds, and alkaloids are representative of secondary nitrogen-containing compounds [5]. Therefore, we went on to describe these differential compounds as terpenes, simple phenols, flavonoids, and alkaloids. In the BZm vs. AZm group, all DSMs included nine terpenes, eight simple phenols, four flavonoids, and four alkaloids, and the DSMs present in the BSb vs. ASb group were composed of twenty-eight terpenes, ten simple phenols, fourteen flavonoids, and five alkaloids (Table 1). We then further subdivided these compounds according to the classification characteristics of each class. The detailed classification of these compounds is shown in Table S1.
3.3. Change in the Abundance of Differential Secondary Metabolites in Maize and Sorghum Groups
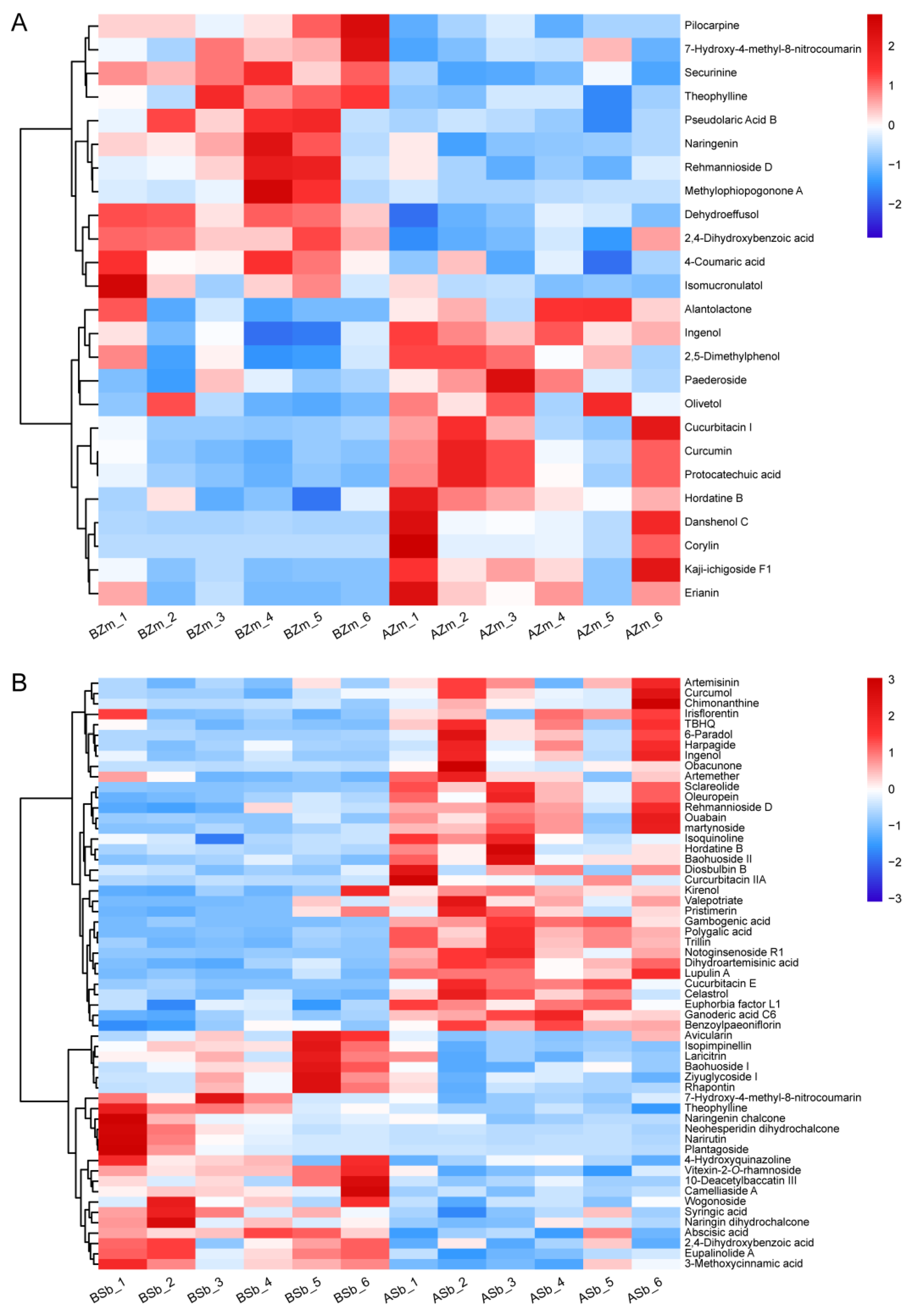
We constructed a heatmap to show the significantly induced or reduced DSMs in both the maize and sorghum groups. In the BZm vs. AZm group, six terpenes were induced in response to FAW larvae attack, with the highest degree of induction being for danshenol C (12.58 folds), followed by kaji-ichigoside F1 (4.93 folds) and cucurbitacin I (Figure 4A, Table 1). Three terpenes (pseudolaric acid B, dehydroeffusol, and rehmannioside D) showed a similar reduction pattern (Figure 4A, Table 1). However, in the BSb vs. ASb group, up to 24 terpene abundances were significantly increased, and notoginsenoside R1 showed the highest induction (12.89 folds), followed by cucurbitacin E (5.35 folds) and sclareolide (4.77 folds) (Figure 4B, Table 1) and only four terpenoids were inhibited (Figure 4B, Table 1). For the common DSMs, ingenol was significantly elevated in both groups; however, the level of rehmannioside D was reduced in the BZm vs. AZm group, but induced in the BSb vs. ASb group (Figure 4).
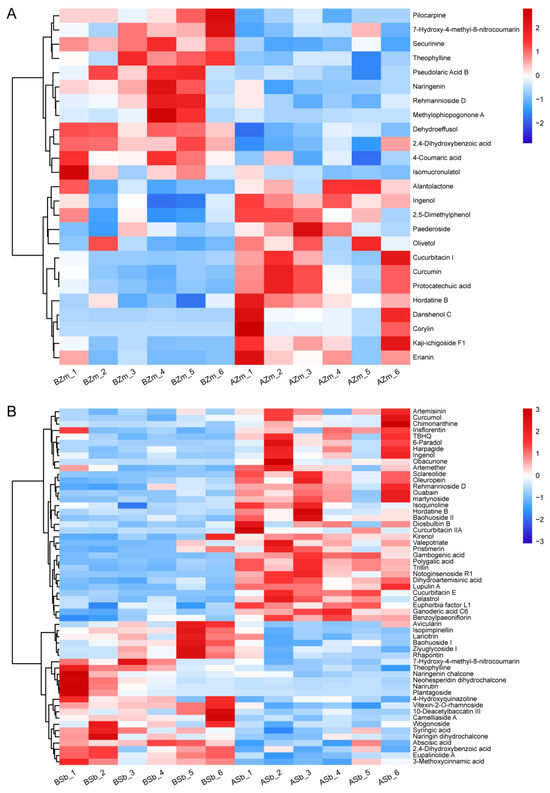
Figure 4.
Hierarchical cluster analysis heatmap for the differential secondary metabolites of maize and sorghum. The heatmap of changes in DSMs from maize (A) and sorghum (B) before and after being fed on by S. frugiperda larvae was made by using non-target metabolomics. BZm, maize plant before feeding by larvae; AZm, maize plant after feeding by larvae; BSb, sorghum plant before feeding by larvae; ASb, sorghum plant after feeding by larvae. The horizontal axis represents the samples. The abundance of DSMs in each sample is shown in different colors. Red indicates high abundance, and blue indicates low abundance.
Larvae feeding on maize almost equally increased the abundance of five simple phenols (erianin, 2,5-dimethylphenol, protocatechuic acid, curcumin, olivetol) and decreased three (Figure 4A, Table 1). In terms of flavonoids, the only one induced was corylin. It increased by 29.11 times, and the remaining three flavonoids were inhibited by larvae feeding on the maize (Figure 4A, Table 1). In the BSb vs. ASb group, four simple phenols were increased and eight were decreased, where the abundance of martynoside was elevated by 6.44 folds, followed by 6-paradol (5.22 folds), and lupulin A (4.94 folds), and the highest degree of inhibition of a simple phenol was 7-hydroxy-4-methyl-8-nitrocoumarin (Figure 4B, Table 1). Three differential flavonoids were significantly induced and twelve were inhibited, where the abundance of gambogenic acid was increased by 6.73 folds, followed by baohuoside II (3.55 folds), and plantagoside (0.09 fold) had the most significant inhibition (Figure 4B, Table 1). In addition, the common DSMs (7-hydroxy-4-methyl-8-nitrocoumarin and 2,4-dihydroxybenzoic acid) were all reduced in both maize and sorghum groups (Figure 4).
In alkaloids, only the abundance of hordatine B was significantly increased and the remaining three alkaloids decreased in the larvae-fed maize (Figure 4A, Table 1). Conversely, in the BSb vs. ASb group, three alkaloids were significantly increased and two were inhibited, of which hordatine B (4.08 folds) displayed the highest induction degree, followed by chimonanthine (3.57 folds), and the compound reduced to the highest degree was theophylline (0.36 fold) (Figure 4B, Table 1). The common alkaloid hordatine B was induced, and theophylline was inhibited in maize and sorghum fed on by larvae (Figure 4).
Flavonoids and alkaloids have a wide range of biological functions in plants, and are important barriers for plants to defend against herbivorous insects [35,44,45]. When the larvae ingested maize and sorghum, feeding maize only caused an increase in hordatine B, but resulted in the induction of seven compounds, irisflorentin, gambogenic acid, baohuoside II, isoquinoline, chimonanthine, and hordatine B, in sorghum (Figure 4, Table 1). Therefore, we hypothesize that the DSMs are specific to sorghum and are brought on by FAW larvae feeding, particularly those that exhibit drastic alterations (FC > 3), including gambogenic acid, baohuoside II, and chimonanthine, which are involved in the defense of sorghum against S. frugiperda.
3.4. The Inhibiting Effect of Sorghum Defensive Compounds on the Larval Feeding and Growth of S. frugiperda
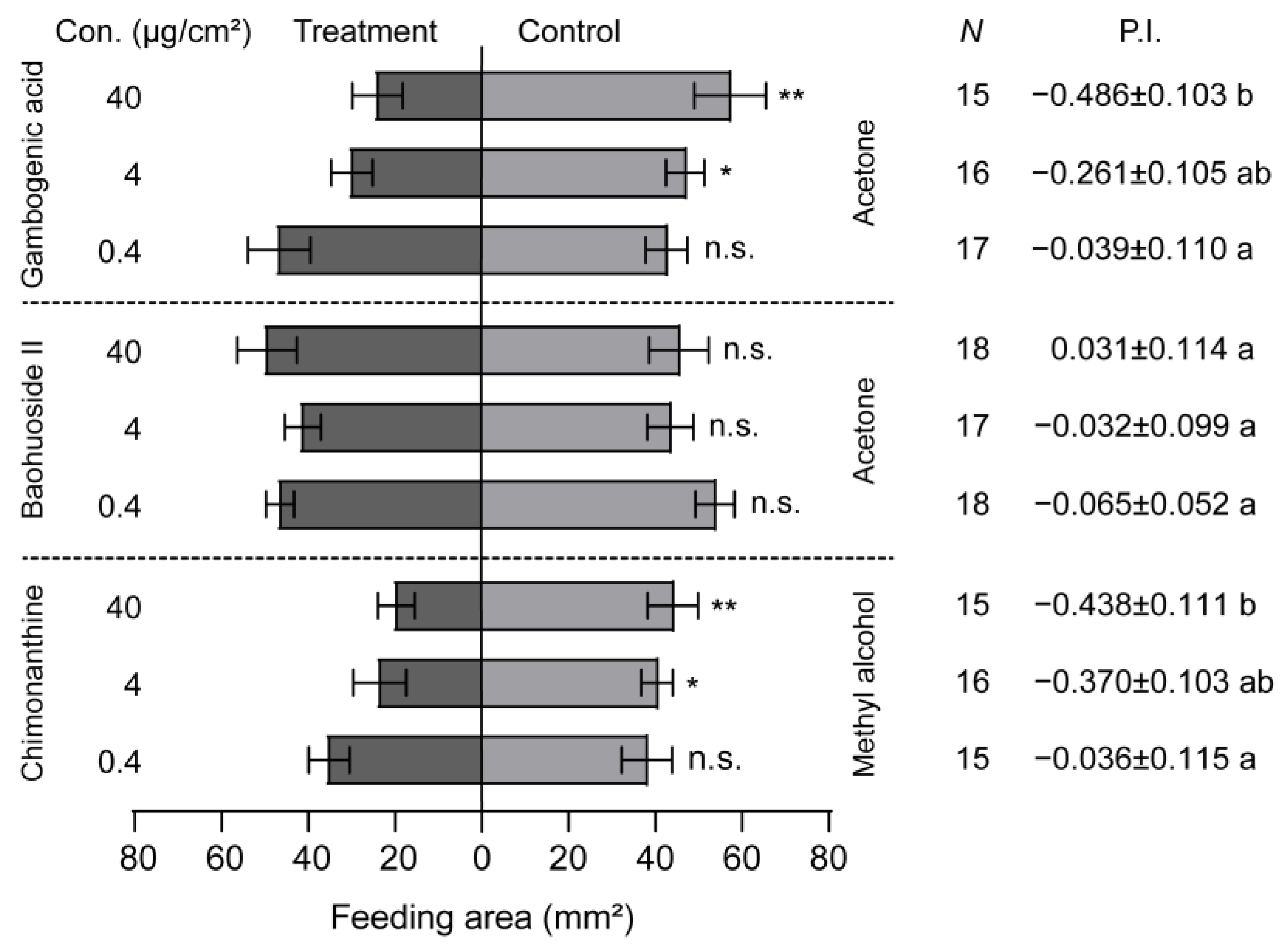
The larval feeding behavior was significantly inhibited by the presence of gambogenic acid and chimonanthine when gambogenic acid, baohuoside II, and chimonanthine were added to maize leaf disks (Figure 5). Additionally, the larval feeding preference index decreased as the concentration of these two compounds increased (Figure 5). However, baohuoside II had no inhibiting effect on the larval feeding, and the larval PI did not correlate with the concentration of baohuoside II (Figure 5). The inhibiting concentration of gambogenic acid and chimonanthine in FAW larvae started from 4 μg/cm2 (Figure 5). Compared with the larval PIs at the concentration of 40 μg/cm2, gambogenic acid was the most effective at inhibiting larval feeding, followed by chimonanthine (Figure 5).
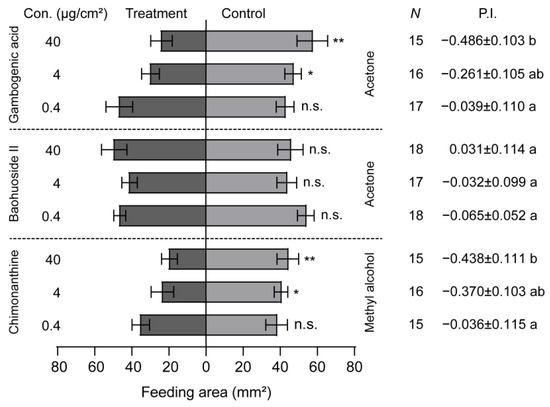
Figure 5.
Feeding responses of Spodoptera frugiperda larvae to three secondary compounds. The feeding preference of 5th-instar larvae of S. frugiperda to gambogenic acid, baohuoside II, and chimonanthine ranged from 0.4 to 40 μg/cm2. P.I., preference index. Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01. Different letters labeled indicate significant differences and n.s. indicates no significant difference (p > 0.05).
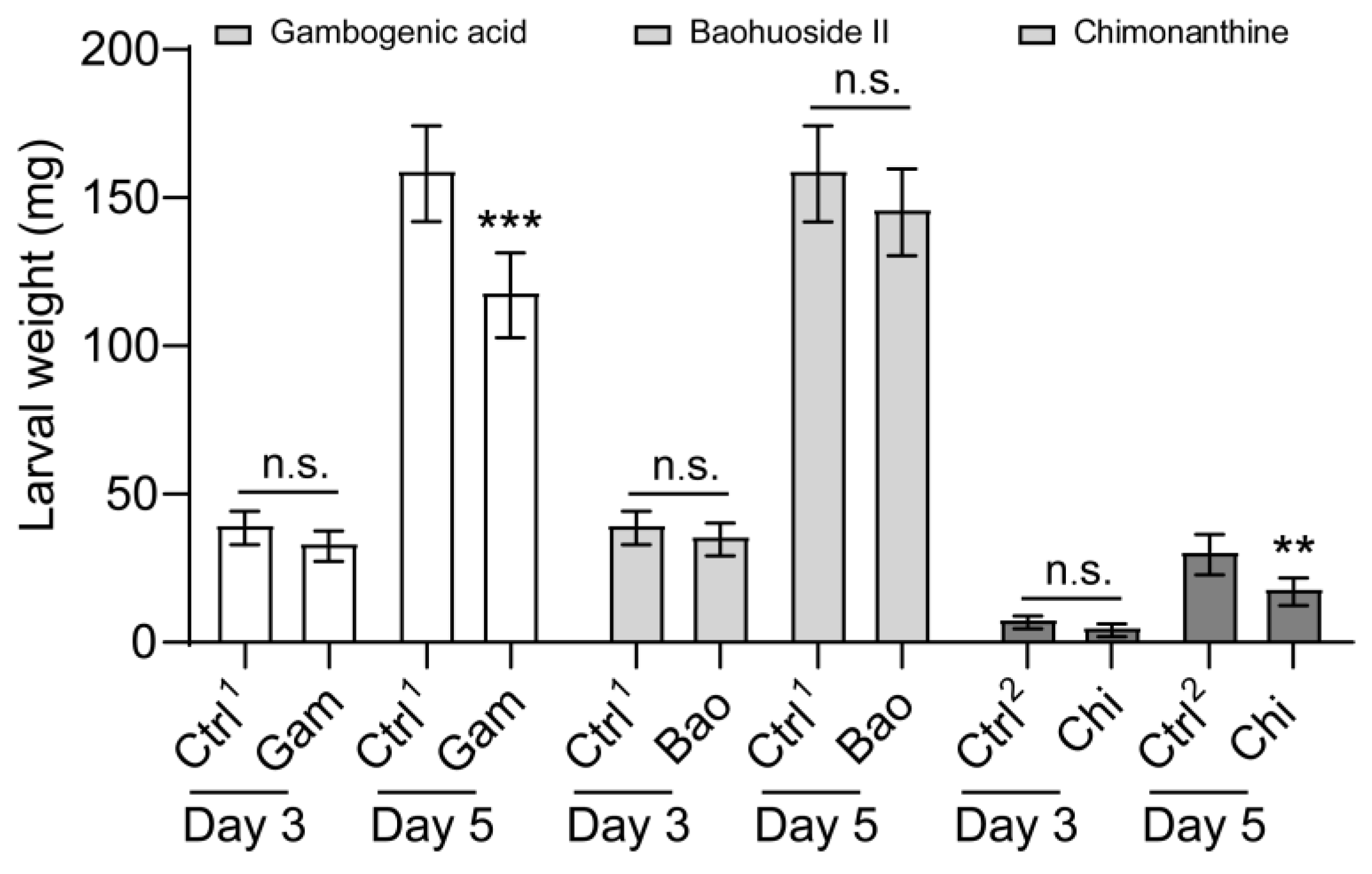
We also set out to determine the effect of gambogenic acid, baohuoside II, and chimonanthine on larval growth and development. Supplemental gambogenic acid and chimonanthine in the artificial diet severely impacted growth and development (Figure 6). Baohuoside II did not decrease FAW larva body weight (Figure 6). As the feeding time was prolonged, the inhibitory effects of gambogenic acid and chimonanthine on larval weight increased gradually, despite the body weight of the larvae fed on the chimonanthine-contained diet being lower than that of the larvae fed on the gambogenic acid and baohuoside II diet (Figure 6). After three days, the weight of larvae that ingested these compounds began to decrease, but the difference was not statistically significant (Figure 6). The larval weight was significantly lower than that of the control when they were reared on a diet containing gambogenic acid and chimonanthine for 5 days, respectively (Figure 6). The larvae fed gambogenic acid and chimonanthine experienced a reduction in body weight of 34.6% and 46.2%, respectively, in comparison to the controls. In addition, we also analyzed the net growth rate of larvae from day three to day five, and the net growth rate of larvae fed with gambogenic acid and chimonanthine was significantly lower than that of the control (Figure S1).
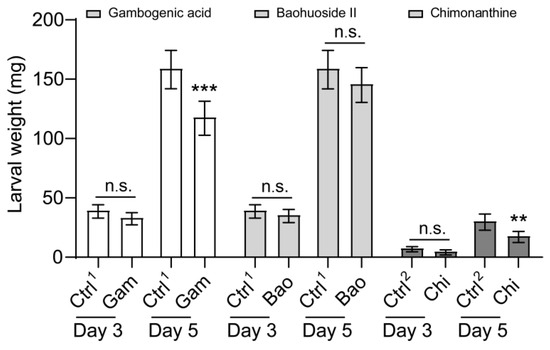
Figure 6.
Effect of three secondary compounds on the larval growth rate of Spodoptera frugiperda. The growth rate of 2nd-instar S. frugiperda larvae to gambogenic acid, baohuoside II, and chimonanthine on day 3 and day 5. Gam, gambogenic acid; Bao, baohuoside II; Chi, chimonanthine. Ctrl1, acetone; Ctrl2, methyl alcohol. Data are presented as mean ± SEM. The nonparametric test (Mann–Whitney U test) was used. ** p < 0.01, *** p < 0.001; n.s. indicates no significant difference between control and treatment (p > 0.05). The colors white, grey, and black represent gambogenic acid, baohuoside II, and chimonanthine, respectively.
4. Discussion
For herbivorous insects, seeking suitable hosts is essential for the survival and reproduction of the population. Plant chemical defense plays an important role in plant host adaptation to herbivorous insects. The defensive mechanism of sorghum, an important graminaceous crop, to the polyphagous invasive insect the FAW remains enigmatic. Elucidating how sorghum defends itself against FAWs is of great significance for the effective prevention and control of FAW damage in sorghum planting. In this study, we found that FAW preferred maize, and feeding on sorghum resulted in a decrease in larval weight. FAW larval feeding caused the change in the response spectra of the secondary metabolites in maize and sorghum, according to non-target metabolomics, and these DSMs showed species-specific distribution traits. Bioassays demonstrated that gambogenic acid and chimonanthine, which are compounds specific to sorghum, had significant inhibitory effects on larval feeding and growth.
The host range and adaptability of insects are the result of coevolution between insects and host plants. The host preferences of different insect species have evolved from adaptations to host secondary metabolites [33]. For instance, H. armigera is a polyphagous insect that mostly feeds on cotton and tomato; however, H. armigera does not prefer to feed on Arabidopsis because the glucosinolate in Arabidopsis effectively prevents larval feeding [46,47,48]. P. rapae, a specialist crucifer insect, prefers cabbage and broccoli over Erysimum cheiranthoides for feeding and oviposition because Erysimum contains deterrents such as cardenolide, erysimoside, and erychroside [49,50,51]. In this study, we found that sorghum can reduce FAWs’ fitness, consistent with previous reports [52,53], suggesting sorghum may contain defensive compounds that can deter FAW larvae feeding or have a toxic effect on this insect. Thus, how sorghum evolves to use secondary metabolites to defend against these herbivorous insects is worthy of further study.
Chemical defenses represented by plant secondary compounds are the key barrier of plants against insects [10,54]. In response to insect attacks, the majority of plant chemical defenses are inducible [55]. The induction of these compounds has an adverse effect on insect feeding or survival. For example, the accumulation of stigmasterol and sakuranetin in rice induced by BPH [22,56], and the production of gallic acid, 4-cinnamic acid, p-coumaric acid, and salicylic acid in cotton induced by H. armigera and S. litura suppress larval feeding and growth [20], Therefore, utilizing plant secondary compounds to regulate insect behavior and development is one of the most important measures for developing green pest control strategies [8,46,55]. In this study, feeding by FAW larvae changed the abundance of secondary metabolites in maize and sorghum, and these DSMs displayed species-specific changes, which indicates that in plants, altering the abundance of their metabolites is a way to respond to insect attacks. We also found that the number of DSMs in maize is lower than in sorghum. This may be a factor contributing to the preference of FAW for feeding on maize. Despite this, there is currently no evidence for the effects of induced DSMs such as danshenol C, notoginsenoside R1, martynoside, corylin, gambogenic acid, baohuoside II and chimonanthine, on insects, some studies have found that these compounds are involved in regulating some diseases and health in humans and mice [57,58,59,60,61,62,63].
Numerous induced plant secondary metabolites are directly involved in plant defense against insects. Flavonoids and alkaloids are important barriers for plants to defend against insects [35,44,45]. The contents of flavonoids and alkaloids are negatively correlated with the growth and development and survival of insects, such as sakuranetin, schaftoside, and gramine against BPH [22,44,64,65], two alkaloids, α-chaconine and α-solanine, against Tecia solanivora [66], cedrelone and carvacrol against FAW [37,38], and quercetin against Cydia pomonella [67]. We found that gambogenic acid and chimonanthine have antifeedant activity for FAW larvae, but baohuoside II has no effect. Our findings are consistent with previous reports [12,68,69]. Four isoflavonoids (judaicin 7-O-glucoside, 2-methoxyjudaicin, judaicin, and maackiain) displayed significant antifeedant activity for H. armigera. Judaicin deters S. littoralis, and maackiain alone can deter S. frugiperda. These four compounds have no deterrent effect on Heliothis virescens and S. exigua [12]. Similarly, five pyrrolizidine alkaloids (senkirkine, senecionine, seneciphylline, monocrotaline, and retrorsine) significantly decreased the survival rate of Frankliniella occidentalis, but heliotrine did not. None of these alkaloids inhibited S. exigua or Mamestra brassicae larvae [69]. These results imply that plant secondary metabolites have different effects for each herbivorous insect, and these metabolites play an important role in the adaptation of insects to host plants [68]. The ingestion of secondary compounds adversely affects larval growth and development, as evidenced by reduced weight. We found that feeding on gambogenic acid and chimonanthine suppressed larval weight. These observations are similar to previous reports, such as sinigrin against H. armigera, and pinocembrin and camptothecin against FAW [43,70,71]. The weight of larvae fed with chimonanthine was lower than that of larvae fed with gambogenic acid and baohuoside II. This may be due to solvent differences because the weight of larvae that ingested methyl alcohol was also lower than that of acetone-fed larvae. Certainly, the decrease in weight can generally be attributed to the deterrent effect of secondary compounds and/or toxicity to larvae [35,72]. Some phenolic compounds in sorghum have been implicated in the resistance to FAWs and R. maidis [36,41]. Therefore, these results suggest that gambogenic acid and chimonanthine in sorghum induced by FAW larvae are involved in the defense of host plants against insects. However, the compounds involved in host defense and their function need to be further investigated. The functional identification of sorghum secondary compounds against FAW would be conducive to understanding the molecular basis of the coevolution between polyphagous insects and host plants.
5. Conclusions
In summary, this study investigated the chemical defense mechanism of sorghum against S. frugiperda. Feeding sorghum reduced the fitness of FAWs. The sorghum ingested by the FAWs observably changed the response spectra of secondary metabolites. Gambogenic acid and chimonanthine were specifically present and induced in sorghum, and have a negative impact on FAW larval feeding behavior and larval growth. The current findings have significant implications for the identification of potential natural insecticidal compounds and provide a theoretical basis for the development of environmentally friendly strategies for pest control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16020218/s1, Figure S1. Effect of three secondary compounds on the relative growth rate of Spodoptera frugiperda larvae. The relative growth rate of 2nd instar larvae of S. frugiperda to gambogenic acid (A), baohuoside II (B), and chimonanthine (C) from day 3 to day 5. Data are presented as mean ± SEM. ** p < 0.01, *** p < 0.001; n.s. indicates no significant difference (p > 0.05). Table S1. Detailed classification of differential secondary metabolites in maize and sorghum groups.
Author Contributions
Conceptualization, J.-Y.Z. and J.Y.; methodology, J.-Y.Z., R.M., Y.W., H.W. and J.Y.; software, J.-Y.Z., J.H. and D.J.; validation, J.-Y.Z., Q.L., J.S., L.-Y.S., R.M., Y.W., H.W., J.H., Y.Z., D.J. and J.Y.; formal analysis, J.-Y.Z., Q.L., L.-Y.S., R.M., Y.Z. and J.Y.; investigation, J.-Y.Z., Q.L., J.S., L.-Y.S., J.H., Y.Z., D.J. and J.Y.; resources, J.-Y.Z., R.M. and J.Y.; data curation, J.-Y.Z., Q.L. and J.Y.; writing—original draft preparation, J.-Y.Z., Y.W., H.W. and J.Y.; writing—review and editing, J.-Y.Z., R.M., Y.W., Y.Z. and J.Y.; visualization, J.-Y.Z., Q.L., J.S., L.-Y.S., J.H., H.W., Y.Z., D.J. and J.Y.; supervision, J.-Y.Z. and J.Y.; project administration, J.-Y.Z. and J.Y.; funding acquisition, J.-Y.Z., R.M. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the special fund for Science and Technology Innovation Teams of Shanxi Province, grant number 202304051001006; the National Natural Science Foundation of China, grant number 32202391; the PhD Research Launch Project of Shanxi Agricultural University, grant number 2023BQ12; the Fundamental Research Program of Shanxi Province, grant number 202203021212419; the National Fund Cultivation Project of Sorghum Research Institute, Shanxi Agricultural University, grant number GLS-gp-202402; the Excellent Doctoral Award of Shanxi Province for Scientific Research, grant number SXBYKY2022095; and the Professor and PHD Working Station in Jinzhong National Agricultural Hi-tech Industries Demonstration Zone, grant number JZNGQBSGZZ001.
Data Availability Statement
The data presented in this study are available in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the assistance of colleagues in the Biosafety and Biocontrol Laboratory at Shanxi Agricultural University and thank Shanghai Tengyun Biotechnology Co., Ltd. for developing the Hiplot Pro platform.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fraenkel, G.S. The raison d’Être of secondary plant substances. Science 1959, 129, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.A. P450s in plant-insect interactions. Biochim. Biophys. Acta 2011, 1814, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015; pp. 1–761. [Google Scholar]
- Razzaq, M.K.; Hina, A.; Abbasi, A.; Karikari, B.; Ashraf, H.J.; Mohiuddin, M.; Maqsood, S.; Maqsood, A.; Haq, I.U.; Xing, G.; et al. Molecular and genetic insights into secondary metabolic regulation underlying insect-pest resistance in legumes. Funct. Integr. Genomics 2023, 23, 217. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as insecticides in crop protection-A review of current research and future prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Murchie, A.K.; Smart, L.E.; Williams, I.H. Responses of Dasineura brassicae and its parasitoids Platygaster subuliformis and Omphale clypealis to field traps baited with organic isothiocyanates. J. Chem. Ecol. 1997, 23, 917–926. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Sachdev-Gupta, K.; Radke, C.D.; Renwick, J.A.A.; Dimock, M.B. Cardenolides from Erysimum cheiranthoides: Feeding deterrents to Pieris rapae larvae. J. Chem. Ecol. 1993, 19, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.; Stevenson, P.C. Effects of isoflavonoids from Cicer on larvae of Heliocoverpa armigera. J. Chem. Ecol. 2001, 27, 965–977. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Gershenzon, J.; Heckel, D.G. Insect attraction versus plant defense: Young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 2014, 9, e95766. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef]
- Kong, G.; Daud, M.K.; Zhu, S. Effects of pigment glands and gossypol on growth, development and insecticide-resistance of cotton bollworm (Heliothis armigera (Hübner)). Crop Prot. 2010, 29, 813–819. [Google Scholar] [CrossRef]
- Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Bell, A.A. Purification and characterization of S-sdenosyl-L-methionine: Desoxyhemigossypol-6-O-methyltransferase from cotton plants. An enzyme capable of methylating the defense terpenoids of cotton. Plant Physiol. 1999, 121, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest. Sci. 2013, 87, 173–180. [Google Scholar] [CrossRef]
- Sambangi, P.; Rani, P.U. Physiological effects of resveratrol and coumaric acid on two major groundnut pests and their egg parasitoid behavior. Arch. Insect Biochem. Physiol. 2016, 91, 230–245. [Google Scholar] [CrossRef]
- Dixit, G.; Praveen, A.; Tripathi, T.; Yadav, V.K.; Verma, P.C. Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J. Asia Pac. Entomol. 2017, 20, 341–351. [Google Scholar] [CrossRef]
- Guo, J.; Qi, J.; He, K.; Wu, J.; Bai, S.; Zhang, T.; Zhao, J.; Wang, Z. The Asian corn borer Ostrinia furnacalis feeding increases the direct and indirect defence of mid-whorl stage commercial maize in the field. Plant Biotechnol. J. 2019, 17, 88–102. [Google Scholar] [CrossRef]
- Liu, M.; Hong, G.; Li, H.; Bing, X.; Chen, Y.; Jing, X.; Gershenzon, J.; Lou, Y.; Baldwin, I.T.; Li, R. Sakuranetin protects rice from brown planthopper attack by depleting its beneficial endosymbionts. Proc. Natl. Acad. Sci. USA 2023, 120, e2305007120. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Pashley, D.P.; Johnson, S.J.; Sparks, A.N. Genetic population structure of migratory moths: The fall armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1985, 78, 756–762. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.J.; Mo, B.T.; Guo, H.; Yang, J.; Tang, R.; Wang, C.Z. Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. 2022, 29, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Ba, T.X.; Zhang, Y.H.; Zhang, Z.; Guan, D.D.; Li, C.C.; Ji, Z.Y.; Yin, X.T.; Zhang, A.H.; Tang, Q.B.; Liu, Y.H.; et al. The host preference and population life tables of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on maize and wheat. Plant Prot. 2020, 46, 17–23. [Google Scholar]
- Van den Berg, J.; du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Erb, M. Plant defenses against herbivory: Closing the fitness gap. Trends Plant Sci. 2017, 23, 187–194. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005; pp. 1–440. [Google Scholar]
- Diawara, M.M.; Wiseman, B.R.; Isenhour, D.J.; Hill, N.S. Panicle feeding resistance to Spodoptera frugiperda (Lepidoptera: Noctuidae) and its relationship to some chemical characteristics of sorghum accessions. Environ. Entomol. 1991, 20, 1393–1402. [Google Scholar] [CrossRef]
- Chatterjee, D.; Lesko, T.; Peiffer, M.; Elango, D.; Beuzelin, J.; Felton, G.W.; Chopra, S. Sorghum and maize flavonoids are detrimental to growth and survival of fall armyworm Spodoptera frugiperda. J. Pest. Sci. 2022, 96, 1551–1567. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef]
- Giongo, A.M.; Vendramim, J.D.; Freitas, S.D.; Silva, M.F. Toxicity of secondary metabolites from meliaceae against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2016, 45, 725–733. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Huang, Y.; Liu, L.; Cai, X.; Lin, J.; Shu, B. The effects of carvacrol on development and gene expression profiles in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 195, 105539. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Vilela Bertolucci, S.K.; Carvalho, G.A. Toxicity of essential oils and pure compounds of Lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Prot. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Singh, S.; Kariyat, R.R. Exposure to polyphenol-rich purple corn pericarp extract restricts fall armyworm (Spodoptera frugiperda) growth. Plant Signal Behav. 2020, 15, 1784545. [Google Scholar] [CrossRef]
- Grover, S.; Shinde, S.; Puri, H.; Palmer, N.; Sarath, G.; Sattler, S.E.; Louis, J. Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm. Front. Plant Sci. 2022, 13, 1019266. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xu, H.X.; Hu, Y.; Han, H.L.; Qian, J.N.; Yin, C.; Lü, Z.X. Growth development and reproduction of Spodoptera frugiperda reared on an artificial diet. Chin. J. Appl. Entomol. 2020, 57, 1341–1344. [Google Scholar]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest. Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhang, M.X.; Yu, J.Y.; Jin, Y.; Ling, B.; Du, J.P.; Li, G.H.; Qin, Q.M.; Cai, Q.N. Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants. PLoS ONE 2013, 8, e64026. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanikolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, P.C.; Zhang, S.S.; Yang, J.; Li, G.C.; Huang, L.Q.; Wang, C.Z. Functional analysis of a bitter gustatory receptor highly expressed in the larval maxillary galea of Helicoverpa armigera. PLoS Genet. 2022, 18, e1010455. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.P.; Zalucki, M.P. Understanding heliothine (Lepidoptera: Heliothinae) pests: What is a host plant? J. Econ. Entomol. 2014, 107, 881–896. [Google Scholar] [CrossRef]
- Huang, X.P.; Renwick, J.A.A.; Sachdev-Gupta, K. A chemical basis for differential acceptance of Erysimum cheiranthoides by two Pieris species. J. Chem. Ecol. 1993, 19, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Renwick, J.A.A.; Lopez, K. Experience-based food consumption by larvae of Pieris rapae: Addiction to glucosinolates? Entomol. Exp. Appl. 1999, 91, 51–58. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Radke, C.D. Chemical recognition of host plants for oviposition by the cabbage butterfly, Pieris rapae (Lepidoptera: Pieridae). Environ. Entomol. 1983, 12, 446–450. [Google Scholar] [CrossRef]
- Lv, L.; Xia, H.X.; Guo, L.; Chang, X.Q.; Wan, P.; Zhang, S. Effect of feeding Spodoptera frugiperda corn or sorghum on oviposition site selection and fitness. Chinese J. Appl. Entomol. 2022, 59, 542–550. [Google Scholar]
- He, L.; Zhao, S.; Gao, X.; Wu, K. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agric. 2021, 20, 804–814. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Guo, J.; Du, B.; He, G.; Zhang, Y.; Chen, R.; Li, J. Lipid profiles reveal different responses to brown planthopper infestation for pest susceptible and resistant rice plants. Metabolomics 2018, 14, 120. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, L.; Feng, J.; Han, Z.; Huang, C.; Xie, F.; Li, Y.; Luo, X.; Wang, Q.; He, J.; et al. Molecular mechanism of Danshenol C in reversing peritoneal fibrosis: Novel network pharmacological analysis and biological validation. BMC Complement. Med. Ther. 2023, 23, 361. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yang, W.; Hu, S.; Wu, Y.; Zhao, B.; Hu, H.; Du, S. Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des. Devel Ther. 2020, 14, 551–565. [Google Scholar] [CrossRef]
- Hong, M.; Du, Y.; Chen, D.; Shi, Y.; Hu, M.; Tang, K.; Hong, Z.; Meng, X.; Xu, W.; Wu, G.; et al. Martynoside rescues 5-fluorouracil-impaired ribosome biogenesis by stabilizing RPL27A. Sci. Bull. 2023, 68, 1662–1677. [Google Scholar] [CrossRef]
- Wang, T.H.; Tseng, W.C.; Leu, Y.L.; Chen, C.Y.; Lee, W.C.; Chi, Y.C.; Cheng, S.F.; Lai, C.Y.; Kuo, C.H.; Yang, S.L.; et al. The flavonoid corylin exhibits lifespan extension properties in mouse. Nat. Commun. 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, L.; Wu, C.; Hu, Y.; Guo, F.; Ren, Q.; Ma, L.; Fu, P. Gambogenic acid alleviates kidney fibrosis via epigenetic inhibition of EZH2 to regulate Smad7-dependent mechanism. Phytomedicine 2022, 106, 154390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Zhao, B.S.; Zhang, J.X.; Yang, S.; Fan, C.L.; Li, P. Effect of 2″-O-rhamnosyl lcariside II, baohuoside I and baohuoside II in herba epimedii on cytotoxicity indices in HL-7702 and HepG2 Cells. Molecules 2019, 24, 1263. [Google Scholar]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014, 68, 539–549. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Chevallier, O.P.; Xu, Y.; Kamolsukyeunyong, W.; Nookaew, I.; Somboon, T.; Toojinda, T.; Vanavichit, A.; Goodacre, R.; Elliott, C.T.; et al. Global metabolite profiles of rice brown planthopper-resistant traits reveal potential secondary metabolites for both constitutive and inducible defenses. Metabolomics 2019, 15, 151. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kimmins, F.M.; Grayer, R.J.; Raveendranath, S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lugens. Entomol. Exp. Appl. 1996, 80, 246–249. [Google Scholar] [CrossRef]
- Karlsson, M.F.; Birgersson, G.; Witzgall, P.; Lekfeldt, J.D.; Nimal Punyasiri, P.A.; Bengtsson, M. Guatemalan potato moth Tecia solanivora distinguish odour profiles from qualitatively different potatoes Solanum tuberosum L. Phytochemistry 2013, 85, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Bai, B.; Chen, G.M.; Wang, Y.Q.; Hu, C.; Liu, X.F.; Gao, P.; Li, Y.T.; Fu, N.X.; Yang, X.Q. Secondary metabolites in host pears defense against two fruit borers and cytochrome-P450-mediated counter-defense. iScience 2024, 27, 109518. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant Defense and Insect Adaptation with Reference to Secondary Metabolites. In Co-Evolution of Secondary Metabolites Reference Series in Phytochemistry, 1st ed.; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 795–822. [Google Scholar]
- Macel, M.; Bruinsma, M.; Dijkstra, S.M.; Ooijendijk, T.; Niemeyer, H.M.; Klinkhamer, P.G. Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J. Chem. Ecol. 2005, 31, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, A.R.; Hulagabali, C.V.; Adhav, A.S.; Joshi, R.S. Mechanistic insight in potential dual role of sinigrin against Helicoverpa armigera. Phytochemistry 2018, 145, 121–127. [Google Scholar] [CrossRef]
- Yu, X.; Xie, X.; Liu, C.; Huang, Y.; Hu, H.; Zeng, J.; Shu, B.; Zhang, J. Impact of camptothecin exposures on the development and larval midgut metabolomic profiles of Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 205, 106136. [Google Scholar] [CrossRef] [PubMed]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.R. Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).