Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Plant Culture
2.2. Chemicals
2.3. Biological Assay
2.4. Sample Collection and Metabolite Extraction
2.5. UHPLC-MS/MS Analysis
2.6. Functional Assay of Candidate Compounds on S. frugiperda Larvae
2.7. Statistical Analysis
3. Results
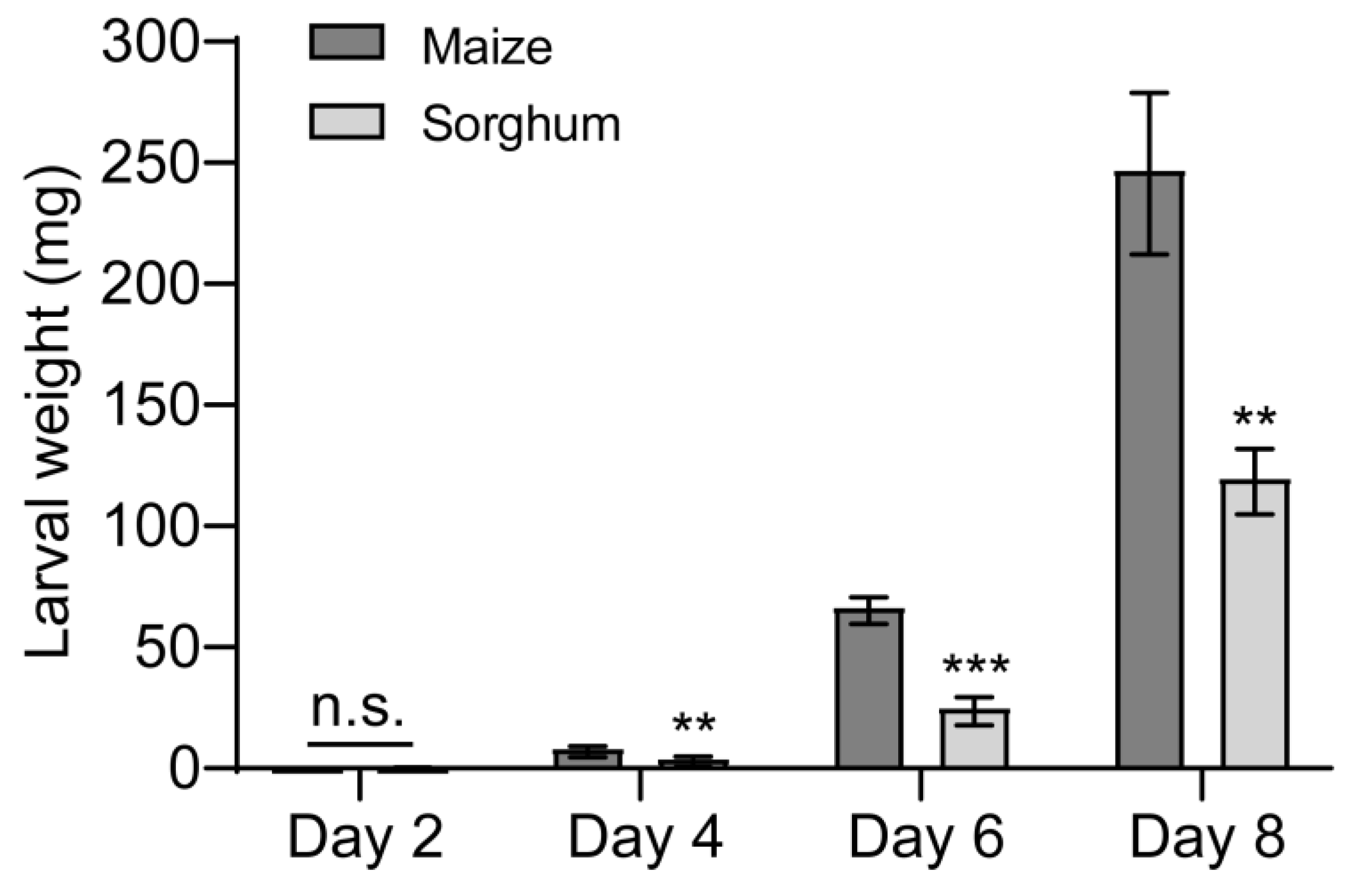
3.1. Larvae of S. frugiperda Prefer to Feed on Maize over Sorghum
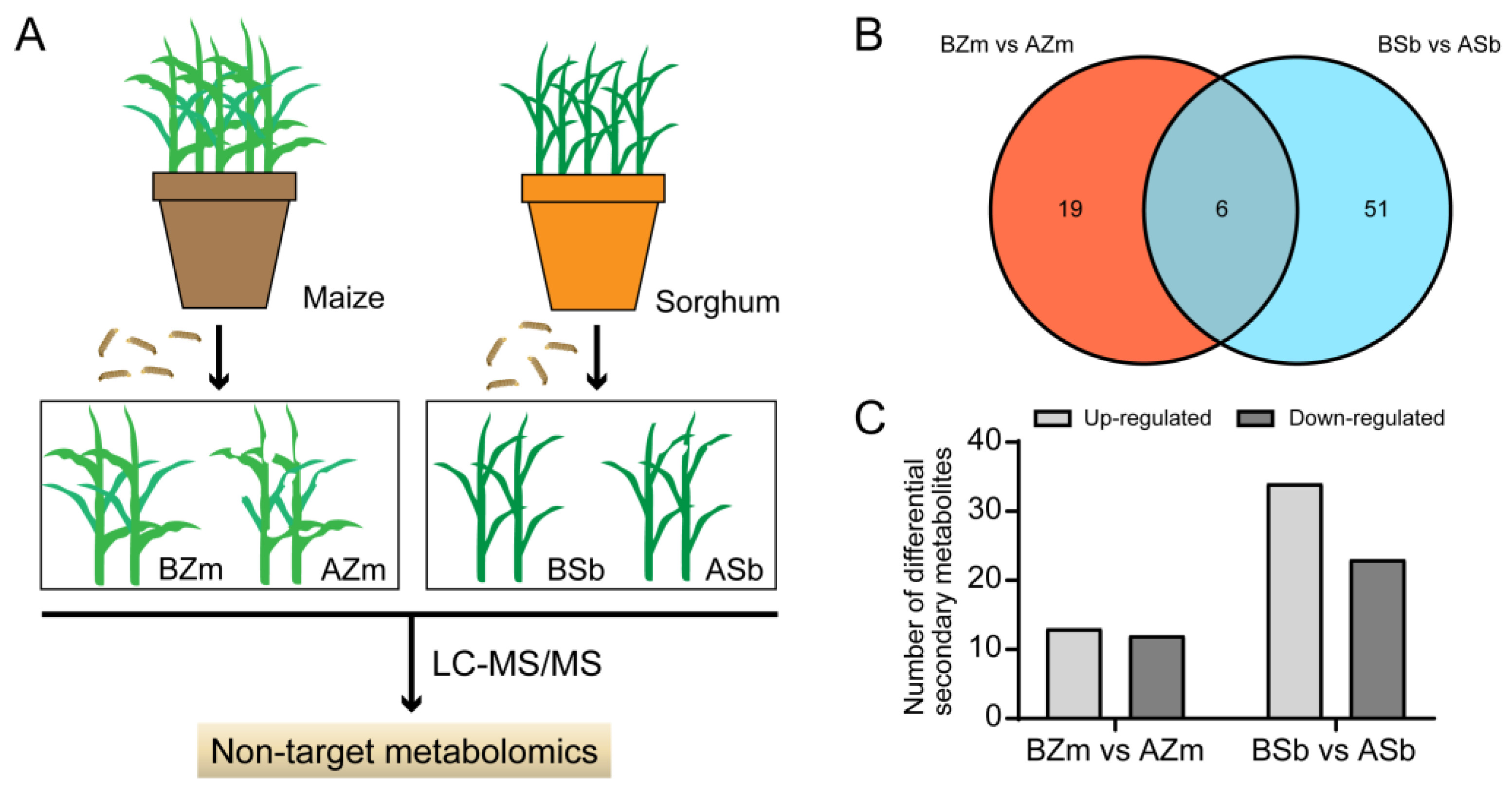
3.2. Screening of Differential Secondary Metabolites with Significant Changes in Maize and Sorghum
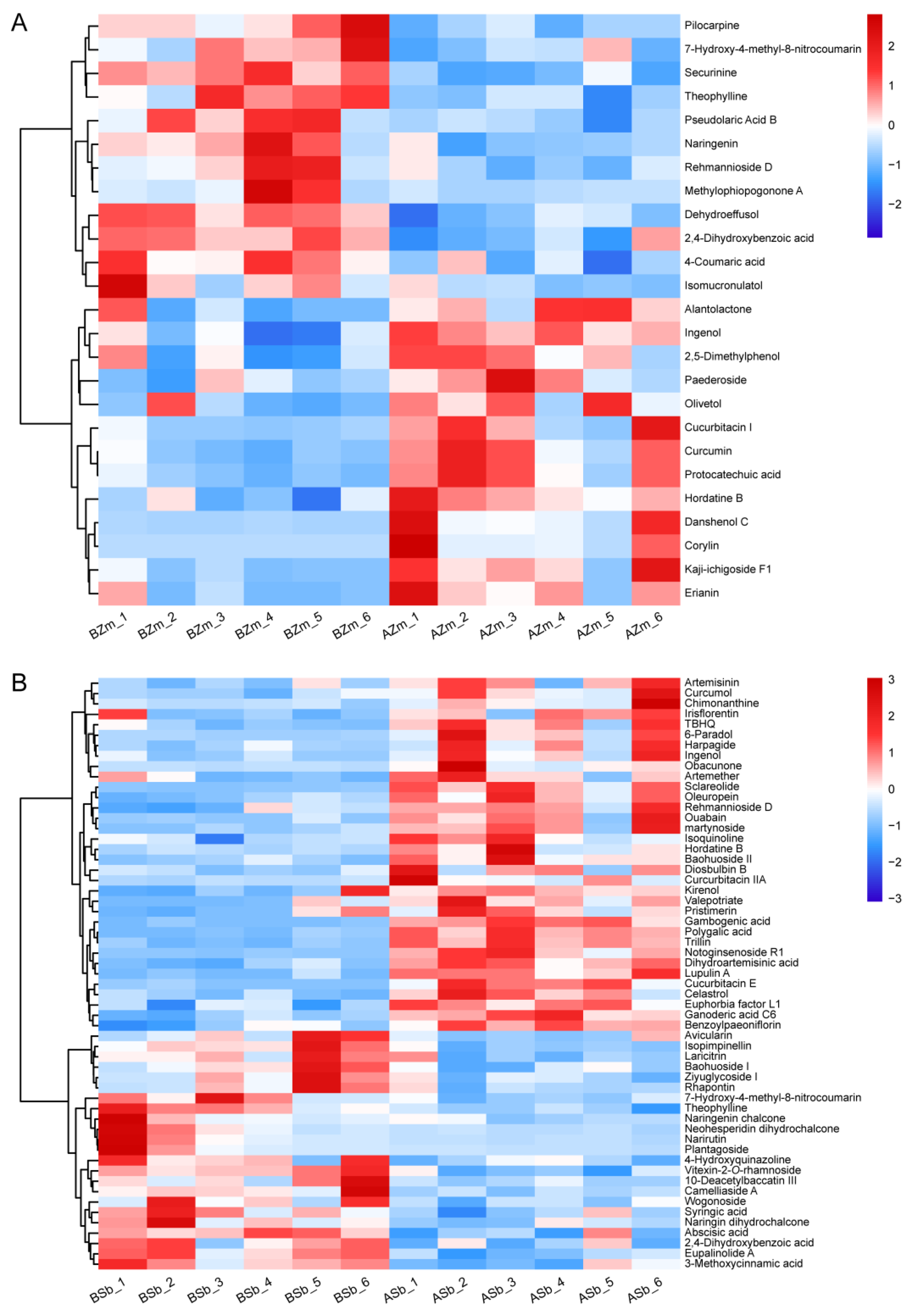
3.3. Change in the Abundance of Differential Secondary Metabolites in Maize and Sorghum Groups
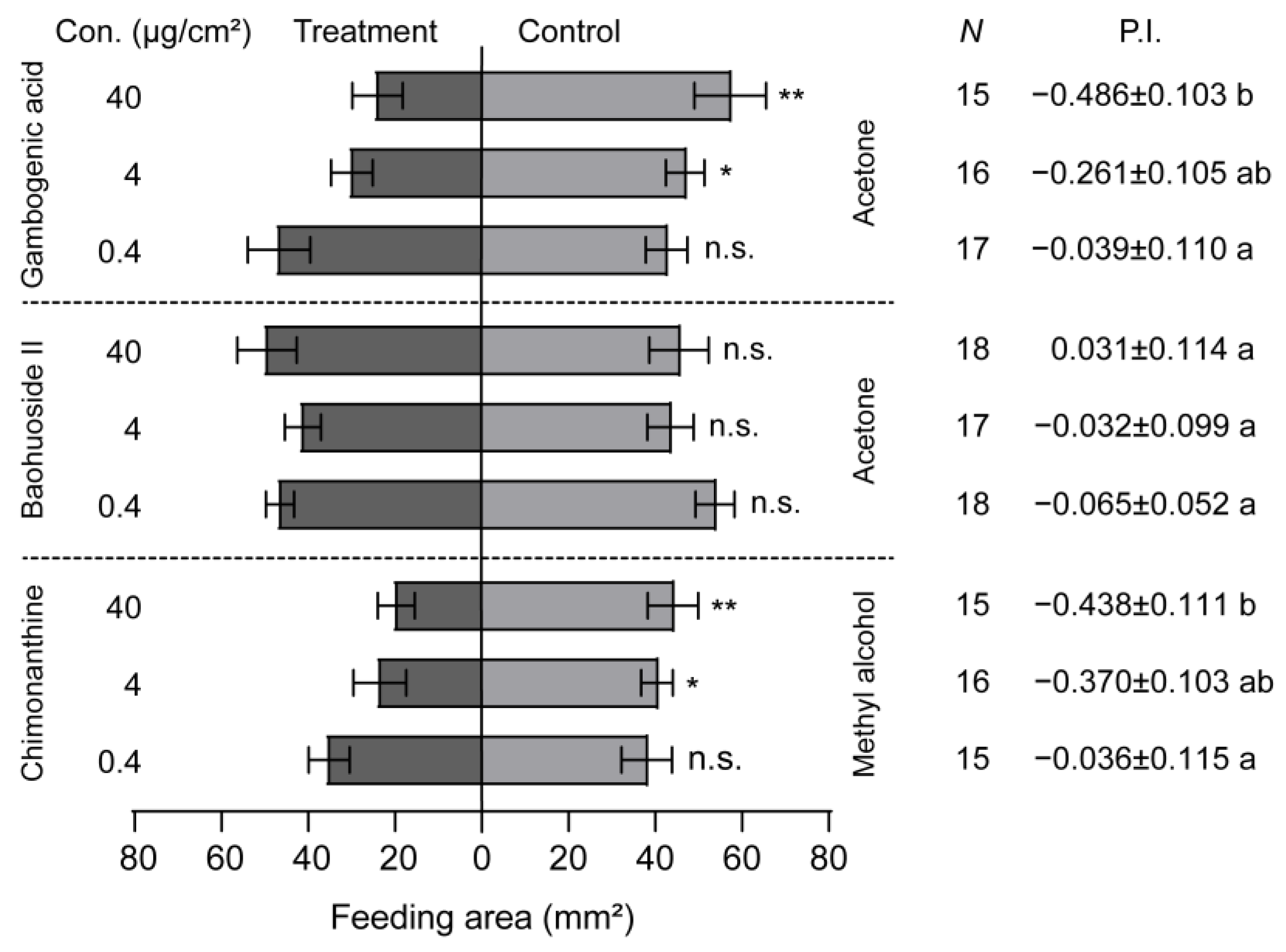
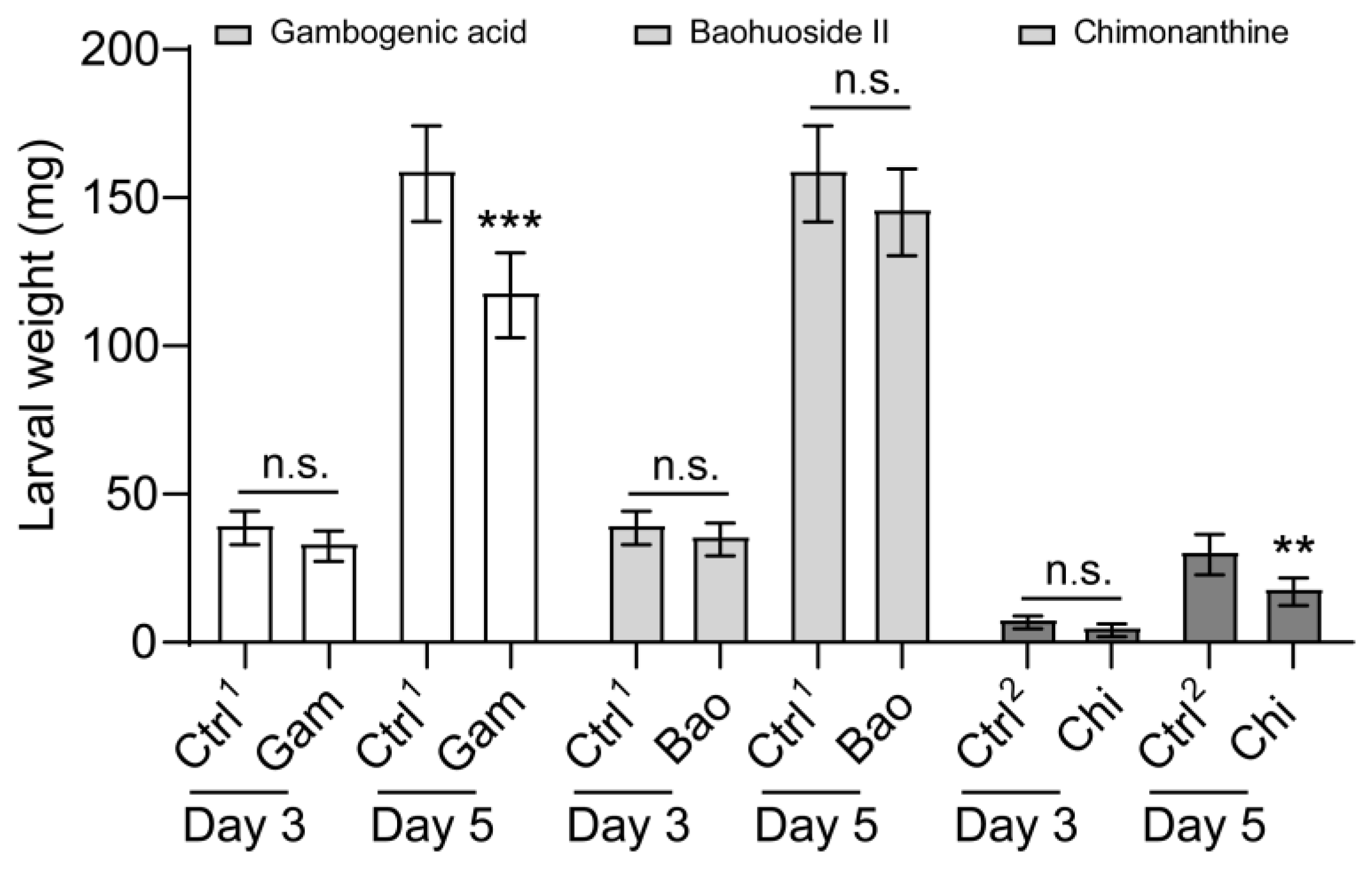
3.4. The Inhibiting Effect of Sorghum Defensive Compounds on the Larval Feeding and Growth of S. frugiperda
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraenkel, G.S. The raison d’Être of secondary plant substances. Science 1959, 129, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.A. P450s in plant-insect interactions. Biochim. Biophys. Acta 2011, 1814, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015; pp. 1–761. [Google Scholar]
- Razzaq, M.K.; Hina, A.; Abbasi, A.; Karikari, B.; Ashraf, H.J.; Mohiuddin, M.; Maqsood, S.; Maqsood, A.; Haq, I.U.; Xing, G.; et al. Molecular and genetic insights into secondary metabolic regulation underlying insect-pest resistance in legumes. Funct. Integr. Genomics 2023, 23, 217. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as insecticides in crop protection-A review of current research and future prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Murchie, A.K.; Smart, L.E.; Williams, I.H. Responses of Dasineura brassicae and its parasitoids Platygaster subuliformis and Omphale clypealis to field traps baited with organic isothiocyanates. J. Chem. Ecol. 1997, 23, 917–926. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Sachdev-Gupta, K.; Radke, C.D.; Renwick, J.A.A.; Dimock, M.B. Cardenolides from Erysimum cheiranthoides: Feeding deterrents to Pieris rapae larvae. J. Chem. Ecol. 1993, 19, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.; Stevenson, P.C. Effects of isoflavonoids from Cicer on larvae of Heliocoverpa armigera. J. Chem. Ecol. 2001, 27, 965–977. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Gershenzon, J.; Heckel, D.G. Insect attraction versus plant defense: Young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 2014, 9, e95766. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef]
- Kong, G.; Daud, M.K.; Zhu, S. Effects of pigment glands and gossypol on growth, development and insecticide-resistance of cotton bollworm (Heliothis armigera (Hübner)). Crop Prot. 2010, 29, 813–819. [Google Scholar] [CrossRef]
- Liu, J.; Benedict, C.R.; Stipanovic, R.D.; Bell, A.A. Purification and characterization of S-sdenosyl-L-methionine: Desoxyhemigossypol-6-O-methyltransferase from cotton plants. An enzyme capable of methylating the defense terpenoids of cotton. Plant Physiol. 1999, 121, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest. Sci. 2013, 87, 173–180. [Google Scholar] [CrossRef]
- Sambangi, P.; Rani, P.U. Physiological effects of resveratrol and coumaric acid on two major groundnut pests and their egg parasitoid behavior. Arch. Insect Biochem. Physiol. 2016, 91, 230–245. [Google Scholar] [CrossRef]
- Dixit, G.; Praveen, A.; Tripathi, T.; Yadav, V.K.; Verma, P.C. Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J. Asia Pac. Entomol. 2017, 20, 341–351. [Google Scholar] [CrossRef]
- Guo, J.; Qi, J.; He, K.; Wu, J.; Bai, S.; Zhang, T.; Zhao, J.; Wang, Z. The Asian corn borer Ostrinia furnacalis feeding increases the direct and indirect defence of mid-whorl stage commercial maize in the field. Plant Biotechnol. J. 2019, 17, 88–102. [Google Scholar] [CrossRef]
- Liu, M.; Hong, G.; Li, H.; Bing, X.; Chen, Y.; Jing, X.; Gershenzon, J.; Lou, Y.; Baldwin, I.T.; Li, R. Sakuranetin protects rice from brown planthopper attack by depleting its beneficial endosymbionts. Proc. Natl. Acad. Sci. USA 2023, 120, e2305007120. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Pashley, D.P.; Johnson, S.J.; Sparks, A.N. Genetic population structure of migratory moths: The fall armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1985, 78, 756–762. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.J.; Mo, B.T.; Guo, H.; Yang, J.; Tang, R.; Wang, C.Z. Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. 2022, 29, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Ba, T.X.; Zhang, Y.H.; Zhang, Z.; Guan, D.D.; Li, C.C.; Ji, Z.Y.; Yin, X.T.; Zhang, A.H.; Tang, Q.B.; Liu, Y.H.; et al. The host preference and population life tables of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on maize and wheat. Plant Prot. 2020, 46, 17–23. [Google Scholar]
- Van den Berg, J.; du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Erb, M. Plant defenses against herbivory: Closing the fitness gap. Trends Plant Sci. 2017, 23, 187–194. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005; pp. 1–440. [Google Scholar]
- Diawara, M.M.; Wiseman, B.R.; Isenhour, D.J.; Hill, N.S. Panicle feeding resistance to Spodoptera frugiperda (Lepidoptera: Noctuidae) and its relationship to some chemical characteristics of sorghum accessions. Environ. Entomol. 1991, 20, 1393–1402. [Google Scholar] [CrossRef]
- Chatterjee, D.; Lesko, T.; Peiffer, M.; Elango, D.; Beuzelin, J.; Felton, G.W.; Chopra, S. Sorghum and maize flavonoids are detrimental to growth and survival of fall armyworm Spodoptera frugiperda. J. Pest. Sci. 2022, 96, 1551–1567. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef]
- Giongo, A.M.; Vendramim, J.D.; Freitas, S.D.; Silva, M.F. Toxicity of secondary metabolites from meliaceae against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2016, 45, 725–733. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Huang, Y.; Liu, L.; Cai, X.; Lin, J.; Shu, B. The effects of carvacrol on development and gene expression profiles in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2023, 195, 105539. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Vilela Bertolucci, S.K.; Carvalho, G.A. Toxicity of essential oils and pure compounds of Lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Prot. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Singh, S.; Kariyat, R.R. Exposure to polyphenol-rich purple corn pericarp extract restricts fall armyworm (Spodoptera frugiperda) growth. Plant Signal Behav. 2020, 15, 1784545. [Google Scholar] [CrossRef]
- Grover, S.; Shinde, S.; Puri, H.; Palmer, N.; Sarath, G.; Sattler, S.E.; Louis, J. Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm. Front. Plant Sci. 2022, 13, 1019266. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xu, H.X.; Hu, Y.; Han, H.L.; Qian, J.N.; Yin, C.; Lü, Z.X. Growth development and reproduction of Spodoptera frugiperda reared on an artificial diet. Chin. J. Appl. Entomol. 2020, 57, 1341–1344. [Google Scholar]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest. Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhang, M.X.; Yu, J.Y.; Jin, Y.; Ling, B.; Du, J.P.; Li, G.H.; Qin, Q.M.; Cai, Q.N. Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants. PLoS ONE 2013, 8, e64026. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanikolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, P.C.; Zhang, S.S.; Yang, J.; Li, G.C.; Huang, L.Q.; Wang, C.Z. Functional analysis of a bitter gustatory receptor highly expressed in the larval maxillary galea of Helicoverpa armigera. PLoS Genet. 2022, 18, e1010455. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.P.; Zalucki, M.P. Understanding heliothine (Lepidoptera: Heliothinae) pests: What is a host plant? J. Econ. Entomol. 2014, 107, 881–896. [Google Scholar] [CrossRef]
- Huang, X.P.; Renwick, J.A.A.; Sachdev-Gupta, K. A chemical basis for differential acceptance of Erysimum cheiranthoides by two Pieris species. J. Chem. Ecol. 1993, 19, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Renwick, J.A.A.; Lopez, K. Experience-based food consumption by larvae of Pieris rapae: Addiction to glucosinolates? Entomol. Exp. Appl. 1999, 91, 51–58. [Google Scholar] [CrossRef]
- Renwick, J.A.A.; Radke, C.D. Chemical recognition of host plants for oviposition by the cabbage butterfly, Pieris rapae (Lepidoptera: Pieridae). Environ. Entomol. 1983, 12, 446–450. [Google Scholar] [CrossRef]
- Lv, L.; Xia, H.X.; Guo, L.; Chang, X.Q.; Wan, P.; Zhang, S. Effect of feeding Spodoptera frugiperda corn or sorghum on oviposition site selection and fitness. Chinese J. Appl. Entomol. 2022, 59, 542–550. [Google Scholar]
- He, L.; Zhao, S.; Gao, X.; Wu, K. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agric. 2021, 20, 804–814. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Guo, J.; Du, B.; He, G.; Zhang, Y.; Chen, R.; Li, J. Lipid profiles reveal different responses to brown planthopper infestation for pest susceptible and resistant rice plants. Metabolomics 2018, 14, 120. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, L.; Feng, J.; Han, Z.; Huang, C.; Xie, F.; Li, Y.; Luo, X.; Wang, Q.; He, J.; et al. Molecular mechanism of Danshenol C in reversing peritoneal fibrosis: Novel network pharmacological analysis and biological validation. BMC Complement. Med. Ther. 2023, 23, 361. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yang, W.; Hu, S.; Wu, Y.; Zhao, B.; Hu, H.; Du, S. Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des. Devel Ther. 2020, 14, 551–565. [Google Scholar] [CrossRef]
- Hong, M.; Du, Y.; Chen, D.; Shi, Y.; Hu, M.; Tang, K.; Hong, Z.; Meng, X.; Xu, W.; Wu, G.; et al. Martynoside rescues 5-fluorouracil-impaired ribosome biogenesis by stabilizing RPL27A. Sci. Bull. 2023, 68, 1662–1677. [Google Scholar] [CrossRef]
- Wang, T.H.; Tseng, W.C.; Leu, Y.L.; Chen, C.Y.; Lee, W.C.; Chi, Y.C.; Cheng, S.F.; Lai, C.Y.; Kuo, C.H.; Yang, S.L.; et al. The flavonoid corylin exhibits lifespan extension properties in mouse. Nat. Commun. 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, L.; Wu, C.; Hu, Y.; Guo, F.; Ren, Q.; Ma, L.; Fu, P. Gambogenic acid alleviates kidney fibrosis via epigenetic inhibition of EZH2 to regulate Smad7-dependent mechanism. Phytomedicine 2022, 106, 154390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Zhao, B.S.; Zhang, J.X.; Yang, S.; Fan, C.L.; Li, P. Effect of 2″-O-rhamnosyl lcariside II, baohuoside I and baohuoside II in herba epimedii on cytotoxicity indices in HL-7702 and HepG2 Cells. Molecules 2019, 24, 1263. [Google Scholar]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014, 68, 539–549. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Chevallier, O.P.; Xu, Y.; Kamolsukyeunyong, W.; Nookaew, I.; Somboon, T.; Toojinda, T.; Vanavichit, A.; Goodacre, R.; Elliott, C.T.; et al. Global metabolite profiles of rice brown planthopper-resistant traits reveal potential secondary metabolites for both constitutive and inducible defenses. Metabolomics 2019, 15, 151. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kimmins, F.M.; Grayer, R.J.; Raveendranath, S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lugens. Entomol. Exp. Appl. 1996, 80, 246–249. [Google Scholar] [CrossRef]
- Karlsson, M.F.; Birgersson, G.; Witzgall, P.; Lekfeldt, J.D.; Nimal Punyasiri, P.A.; Bengtsson, M. Guatemalan potato moth Tecia solanivora distinguish odour profiles from qualitatively different potatoes Solanum tuberosum L. Phytochemistry 2013, 85, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Bai, B.; Chen, G.M.; Wang, Y.Q.; Hu, C.; Liu, X.F.; Gao, P.; Li, Y.T.; Fu, N.X.; Yang, X.Q. Secondary metabolites in host pears defense against two fruit borers and cytochrome-P450-mediated counter-defense. iScience 2024, 27, 109518. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant Defense and Insect Adaptation with Reference to Secondary Metabolites. In Co-Evolution of Secondary Metabolites Reference Series in Phytochemistry, 1st ed.; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 795–822. [Google Scholar]
- Macel, M.; Bruinsma, M.; Dijkstra, S.M.; Ooijendijk, T.; Niemeyer, H.M.; Klinkhamer, P.G. Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J. Chem. Ecol. 2005, 31, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, A.R.; Hulagabali, C.V.; Adhav, A.S.; Joshi, R.S. Mechanistic insight in potential dual role of sinigrin against Helicoverpa armigera. Phytochemistry 2018, 145, 121–127. [Google Scholar] [CrossRef]
- Yu, X.; Xie, X.; Liu, C.; Huang, Y.; Hu, H.; Zeng, J.; Shu, B.; Zhang, J. Impact of camptothecin exposures on the development and larval midgut metabolomic profiles of Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 205, 106136. [Google Scholar] [CrossRef] [PubMed]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.R. Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef]

| Class | Name | RT (min) | BZm vs. AZm | BSb vs. ASb | ||
|---|---|---|---|---|---|---|
| FC | VIP | FC | VIP | |||
| Terpenes | Diosbulbin B | 5.168 | – | – | 1.45 | 1.23 |
| Danshenol C | 5.267 | 12.58 | 3.04 | – | – | |
| Artemisinin | 5.568 | – | – | 1.54 | 1.25 | |
| Paederoside | 5.571 | 1.78 | 1.43 | – | – | |
| Obacunone | 6.017 | – | – | 3.92 | 2.09 | |
| Eupalinolide A | 6.168 | – | – | 0.40 | 1.40 | |
| Ouabain | 6.293 | – | – | 2.91 | 1.16 | |
| Artemether | 6.309 | – | – | 1.70 | 1.69 | |
| Valepotriate | 6.375 | – | – | 2.83 | 2.73 | |
| Pseudolaric acid B | 6.421 | 0.58 | 1.29 | – | – | |
| Dehydroeffusol | 6.478 | 0.41 | 1.13 | – | – | |
| Curcumol | 6.871 | – | – | 1.72 | 1.75 | |
| Harpagide | 6.986 | – | – | 3.31 | 2.28 | |
| Kaji-ichigoside F1 | 7.558 | 4.93 | 1.70 | – | – | |
| Kirenol | 7.592 | – | – | 1.65 | 1.40 | |
| Sclareolide | 7.625 | – | – | 4.77 | 3.12 | |
| Ingenol | 7.641 | 1.73 | 1.04 | 2.18 | 1.10 | |
| Cucurbitacin I | 7.973 | 3.41 | 2.05 | – | – | |
| Polygalic acid | 8.356 | – | – | 2.01 | 1.96 | |
| Trillin | 8.383 | – | – | 1.96 | 1.80 | |
| Euphorbia factor L1 | 8.436 | – | – | 2.38 | 1.60 | |
| Curcurbitacin IIA | 8.561 | – | – | 3.18 | 1.76 | |
| Dihydroartemisinic acid | 8.624 | – | – | 1.84 | 2.74 | |
| 10-Deacetylbaccatin III | 8.810 | – | – | 0.22 | 1.27 | |
| Ziyuglycoside I | 9.082 | – | – | 0.49 | 1.31 | |
| Ganoderic acid C6 | 9.197 | – | – | 2.88 | 1.30 | |
| Oleuropein | 9.233 | – | – | 3.12 | 1.72 | |
| Rehmannioside D | 9.304 | 0.57 | 1.46 | 1.73 | 1.41 | |
| Benzoylpaeoniflorin | 9.334 | – | – | 1.56 | 1.02 | |
| Cucurbitacin E | 9.430 | – | – | 5.35 | 2.58 | |
| Notoginsenoside R1 | 9.684 | – | – | 12.89 | 2.74 | |
| Abscisic acid | 9.771 | – | – | 0.72 | 1.58 | |
| Alantolactone | 10.079 | 1.49 | 1.39 | – | – | |
| Celastrol | 10.769 | – | – | 2.91 | 2.17 | |
| Pristimerin | 11.149 | – | – | 2.03 | 1.45 | |
| Simple phenols | 7-Hydroxy-4-methyl-8- nitrocoumarin | 1.527 | 0.46 | 1.46 | 0.32 | 2.11 |
| Isopimpinellin | 2.197 | – | – | 0.34 | 2.19 | |
| Syringic acid | 5.118 | – | – | 0.57 | 1.17 | |
| 4-Coumaric acid | 5.241 | 0.54 | 2.00 | – | – | |
| 2,4-Dihydroxybenzoic acid | 5.386 | 0.46 | 2.40 | 0.51 | 1.73 | |
| 3-Methoxycinnamic acid | 5.429 | – | – | 0.70 | 1.86 | |
| 2,5-Dimethylphenol | 5.828 | 1.34 | 1.34 | – | – | |
| Rhapontin | 6.028 | – | – | 0.52 | 1.22 | |
| 6-Paradol | 6.467 | – | – | 5.22 | 1.46 | |
| Erianin | 6.483 | 2.12 | 1.71 | – | – | |
| Lupulin A | 6.854 | – | – | 4.94 | 3.04 | |
| Protocatechuic acid | 7.359 | 1.70 | 2.29 | – | – | |
| TBHQ | 7.507 | – | – | 2.99 | 1.35 | |
| Curcumin | 7.546 | 1.65 | 1.63 | – | – | |
| Olivetol | 7.556 | 1.66 | 1.51 | – | – | |
| Martynoside | 8.463 | – | – | 6.44 | 2.21 | |
| Flavonoids | Corylin | 4.783 | 29.11 | 2.90 | – | – |
| Laricitrin | 4.959 | – | – | 0.57 | 1.45 | |
| Naringin dihydrochalcone | 5.211 | – | – | 0.39 | 2.36 | |
| Methylophiopogonone A | 5.217 | 0.14 | 1.55 | – | – | |
| Wogonoside | 5.338 | – | – | 0.48 | 1.37 | |
| Camelliaside A | 5.399 | – | – | 0.53 | 2.50 | |
| Naringenin | 5.495 | 0.46 | 1.62 | – | – | |
| Narirutin | 5.717 | – | – | 0.15 | 1.16 | |
| Isomucronulatol | 5.729 | 0.60 | 1.96 | – | – | |
| Irisflorentin | 5.794 | – | – | 2.10 | 1.06 | |
| Plantagoside | 5.842 | – | – | 0.09 | 1.07 | |
| Naringenin chalcone | 5.872 | – | – | 0.41 | 1.89 | |
| Neohesperidin dihydrochalcone | 5.955 | – | – | 0.16 | 1.09 | |
| Baohuoside I | 6.314 | – | – | 0.66 | 1.19 | |
| Avicularin | 6.337 | – | – | 0.33 | 1.45 | |
| Gambogenic acid | 8.842 | – | – | 6.73 | 2.65 | |
| Baohuoside II | 8.982 | – | – | 3.55 | 2.27 | |
| Vitexin-2-O-rhamnoside | 9.484 | – | – | 0.44 | 1.52 | |
| Alkaloids | Securinine | 1.322 | 0.24 | 2.46 | – | – |
| Theophylline | 1.347 | 0.48 | 1.69 | 0.36 | 2.10 | |
| 4-Hydroxyquinazoline | 3.670 | – | – | 0.43 | 1.37 | |
| Pilocarpine | 4.972 | 0.42 | 1.20 | – | – | |
| Isoquinoline | 5.480 | – | – | 1.62 | 1.26 | |
| Chimonanthine | 6.057 | – | – | 3.57 | 2.01 | |
| Hordatine B | 8.288 | 1.97 | 1.32 | 4.08 | 1.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.-Y.; Lu, Q.; Sun, J.; Sun, L.-Y.; Ma, R.; Wang, Y.; Hu, J.; Wang, H.; Zhang, Y.; Jia, D.; et al. Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack. Insects 2025, 16, 218. https://doi.org/10.3390/insects16020218
Zhao J-Y, Lu Q, Sun J, Sun L-Y, Ma R, Wang Y, Hu J, Wang H, Zhang Y, Jia D, et al. Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack. Insects. 2025; 16(2):218. https://doi.org/10.3390/insects16020218
Chicago/Turabian StyleZhao, Juan-Ying, Qi Lu, Jiang Sun, Li-Yuan Sun, Ruiyan Ma, Yuanxin Wang, Jun Hu, Huiyan Wang, Yizhong Zhang, Dong Jia, and et al. 2025. "Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack" Insects 16, no. 2: 218. https://doi.org/10.3390/insects16020218
APA StyleZhao, J.-Y., Lu, Q., Sun, J., Sun, L.-Y., Ma, R., Wang, Y., Hu, J., Wang, H., Zhang, Y., Jia, D., & Yang, J. (2025). Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack. Insects, 16(2), 218. https://doi.org/10.3390/insects16020218






