Potential Functions and Transmission Dynamics of Fungi Associated with Anoplophora glabripennis Across Different Life Stages, Between Sexes, and Between Habitats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Samples
2.2. DNA Extraction and Sequencing Analysis
2.3. Sequencing Data Analysis
3. Results
3.1. Diversity of Fungal Communities
3.2. Dominant Fungal Community Composition
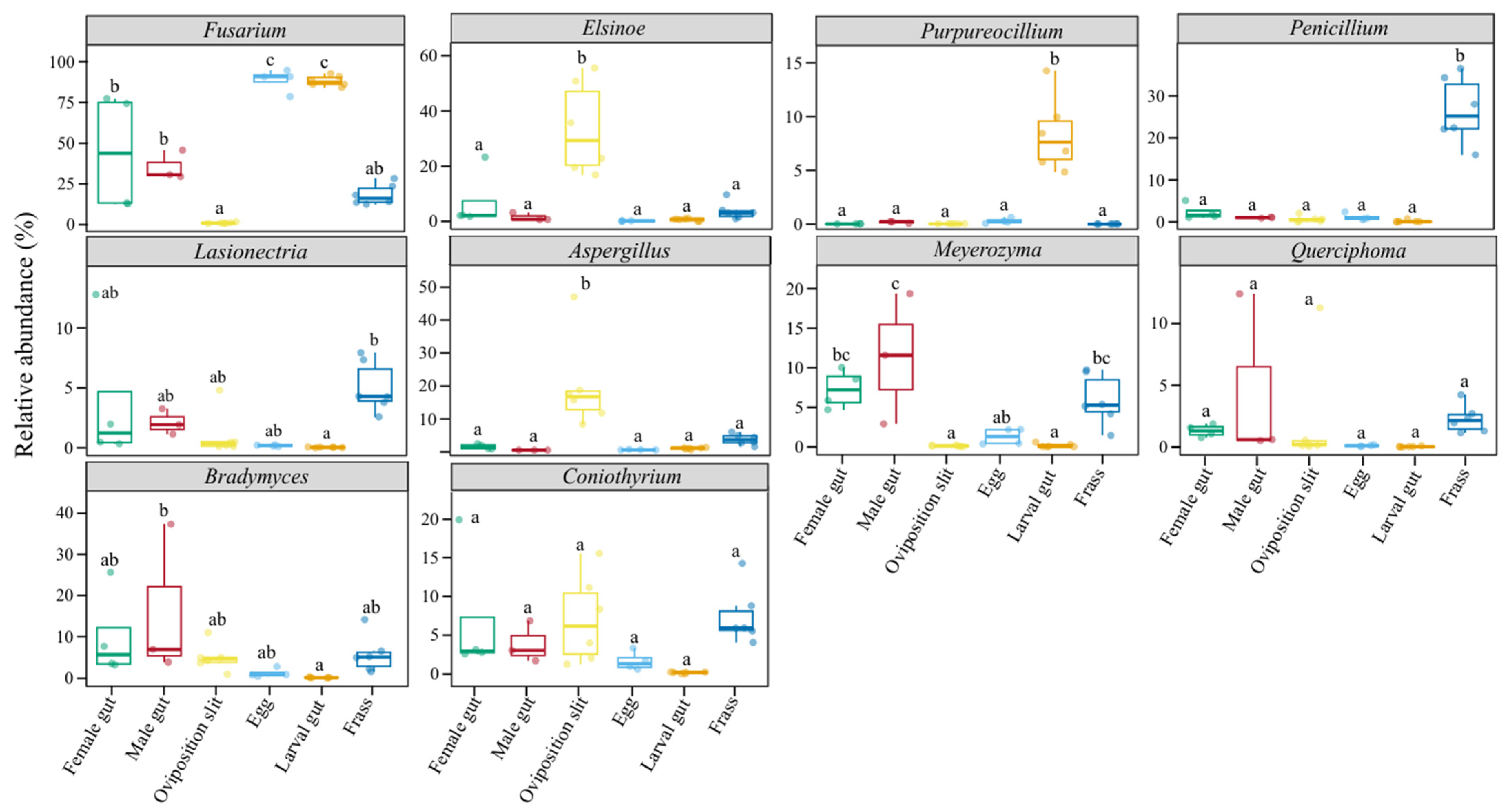
3.3. Differential Fungal Communities
3.4. Potential Functions of the Microbiome in Fungal Communities
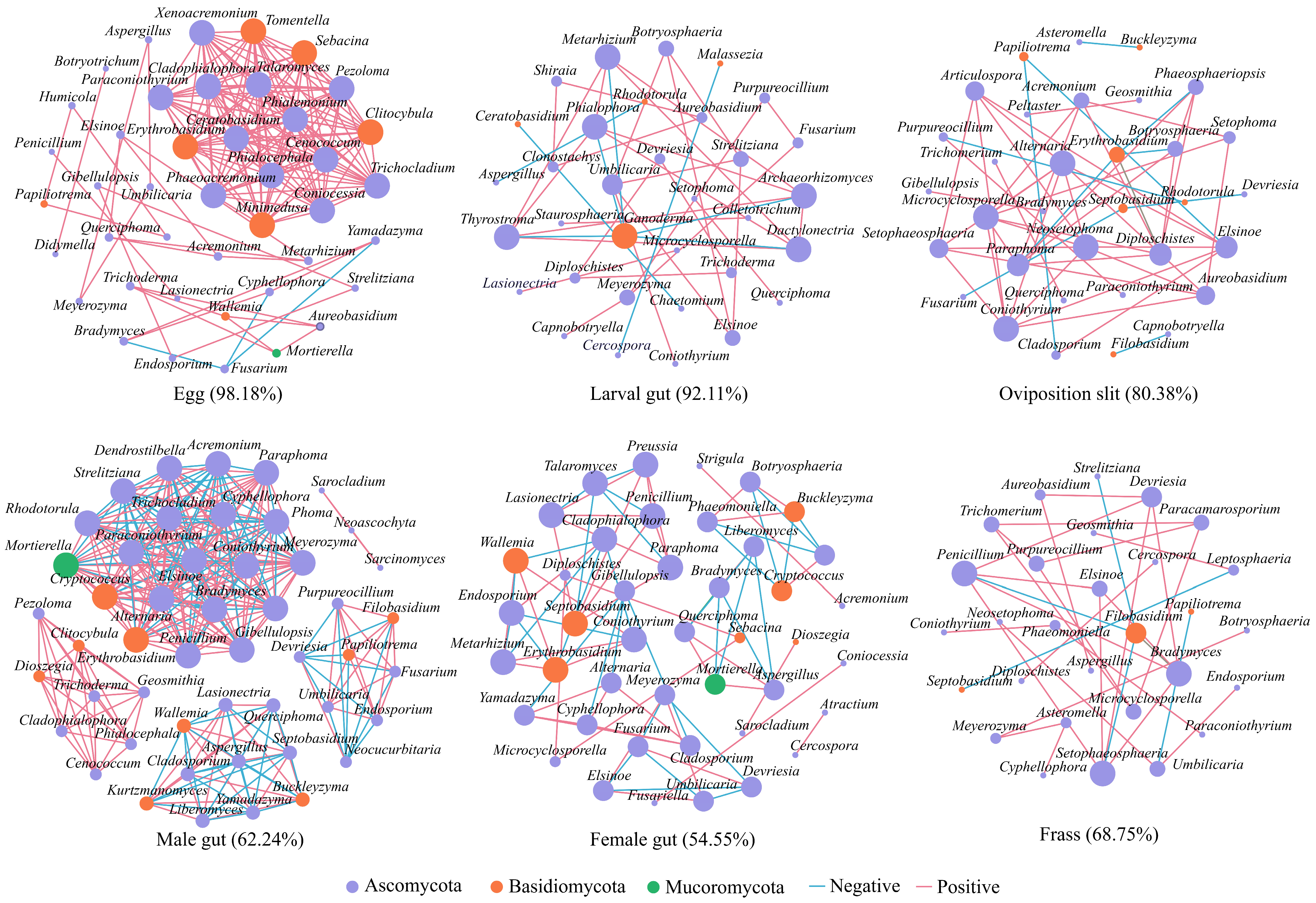
3.5. Fungal Interactions Revealed by Co-Occurrence Network
3.6. The Potential Transmission Processes of ALB Gut Fungi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Yang, Z.; Wang, S.; Li, W.; Shang, S.; Luo, Y.; Wang, L. Effects of Fusarium solani on the growth and development of Anoplophora glabripennis larvae. Microb. Ecol. 2023, 87, 23. [Google Scholar] [CrossRef]
- Ayres, M.P.; Wilkens, R.T.; Ruel, J.J.; Lombardero, M.J.; Vallery, E. Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi. Ecology 2000, 81, 2198. [Google Scholar] [CrossRef]
- Thompson, B.M.; Grebenok, R.J.; Behmer, S.T.; Gruner, D.S. Microbial symbionts shape the sterol profile of the xylem-feeding wood wasp, Sirex noctilio. J. Chem. Ecol. 2013, 39, 129–139. [Google Scholar] [CrossRef]
- Morales, J.J.; Zúñiga, G.; Villa, T.L.; Hernández, R.C. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae). Microb. Ecol. 2009, 58, 879–891. [Google Scholar] [CrossRef]
- Morales, J.J.; Vera, P.L.A.; García, D.A.; Vera, P.L.A.; García-Domínguez, A.; Martínez, R.E.; Zúñiga, G.; Hernández, R.C. Nitrogen-fixing and uricolytic bacteria associated with the gut of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytinae). Microb. Ecol. 2013, 66, 200–210. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Klepzig, K.D.; Raffa, K.F. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006, 31, 636–645. [Google Scholar] [CrossRef]
- Scott, J.J.; Oh, D.C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322, 63. [Google Scholar] [CrossRef]
- Adams, A.S.; Currie, C.R.; Cardoza, Y.; Klepzig, K.D.; Raffa, K.F. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. For. Res. 2009, 39, 1133–1147. [Google Scholar] [CrossRef]
- Adams, A.S.; Jordan, M.S.; Adams, S.M.; Suen, G.; Goodwin, L.A.; Davenport, K.W.; Currie, C.R.; Raffa, K.F. Cellulose-degrading bacteria associated with the invasive wood wasp Sirex noctilio. ISME J. 2011, 5, 1323–1331. [Google Scholar] [CrossRef]
- Therrien, J.; Mason, C.J.; Cale, J.A.; Adams, A.; Aukema, B.H.; Currie, C.R.; Raffa, K.F.; Erbilgin, N. Bacteria influence mountain pine beetle brood development through interactions with symbiotic and antagonistic fungi: Implications for climate-driven host range expansion. Oecologia 2015, 179, 467–485. [Google Scholar] [CrossRef]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S.; Bohlmann, J.; Raffa, K.F. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, L.; Xu, D.; Lou, Q.; Lu, M.; Sun, J. Does cryptic microbiota mitigate pine resistance to an invasive beetle-fungus complex? Implications for invasion potential. Sci. Rep. 2016, 6, 33110. [Google Scholar] [CrossRef]
- Xu, L.; Lou, Q.; Cheng, C.; Lu, M.; Sun, J. Gut-Associated bacteria of Dendroctonus valens and their Involvement in verbenone production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; De Bruin, S.; Luckerhoff, L.; Van Logtestijn, R.S.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and Evolutionary determinants of bark beetle -fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- De Fine Licht, H.H.; Biedermann, P.H. Patterns of functional enzyme activity in fungus farming ambrosia beetles. Front. Zool. 2012, 9, 13. [Google Scholar] [CrossRef]
- Pons, I.; González Porras, M.Á.; Breitenbach, N.; Berger, J.; Hipp, K.; Salem, H. For the road: Calibrated maternal investment in light of extracellular symbiont transmission. Proc. R. Soc. B 2022, 289, 20220386. [Google Scholar] [CrossRef]
- Ganesan, R.; Janke, R.S.; Kaltenpoth, M.; Flórez, L.V. Colonization dynamics of a defensive insect ectosymbiont. Biol. Lett. 2023, 19, 20230100. [Google Scholar] [CrossRef]
- Tetreau, G.; Dhinaut, J.; Galinier, R.; Audant-Lacour, P.; Voisin, S.N.; Arafah, K.; Chogne, M.; Hilliou, F.; Bordes, A.; Sabarly, C.; et al. Deciphering the molecular mechanisms of mother-to-egg immune protection in the mealworm beetle Tenebrio molitor. PLoS Pathog. 2020, 16, e1008935. [Google Scholar] [CrossRef] [PubMed]
- Kyei-Poku, G.; Gauthier, D.; Schwarz, R.; van Frankenhuyzen, K. Morphology, molecular characteristics and prevalence of a Cystosporogenes species (Microsporidia) isolated from Agrilus anxius (Coleoptera: Buprestidae). J. Invertebr. Pathol. 2011, 107, 1–10. [Google Scholar] [CrossRef]
- Salem, H.; Bauer, E.; Kirsch, R.; Berasategui, A.; Cripps, M.; Weiss, B.; Koga, R.; Fukumori, K.; Vogel, H.; Fukatsu, T.; et al. Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell 2017, 171, 1520–1531. [Google Scholar] [CrossRef]
- Fukatsu, T.; Hosokawa, T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl. Environ. Microbiol. 2002, 62, 389–396. [Google Scholar] [CrossRef]
- Hosokawa, T.; Hironaka, M.; Mukai, H.; Inadomi, K.; Suzuki, N.; Fukatsu, T. Mothers never miss the moment: A fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim. Behav. 2012, 83, 293–300. [Google Scholar] [CrossRef]
- Salem, H.; Kreutzer, E.; Sudakaran, S.; Kaltenpoth, M. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae). Environ. Microbiol. 2012, 15, 1956–1968. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. Insect-microbe mutualism without vertical transmission: A stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 2007, 73, 4308–4316. [Google Scholar] [CrossRef]
- Mason, C.J.; Raffa, K.F. Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ. Entomol. 2014, 43, 595–604. [Google Scholar] [CrossRef]
- Mason, C.J.; Campbell, A.M.; Scully, E.D.; Hoover, K. Bacterial and fungal midgut community dynamics and transfer between mother and brood in the Asian longhorned beetle (Anoplophora glabripennis), an invasive xylophage. Microb. Ecol. 2019, 77, 230–242. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian longhorned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J. Integr. Pest. Manag. 2015, 6, 4. [Google Scholar] [CrossRef]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and management of exotic and endemic Asian longhorned beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Keena, M.A.; Sánchez, V. Reproductive behaviors of Anoplophora glabripennis (Coleoptera: Cerambycidae) in the Laboratory. J. Econ. Entomol. 2018, 111, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, R.K.; Duan, J.J.; Palli, S.R. Orally delivered dsRNA induces knockdown of target genes and mortality in the Asian long-horned beetle, Anoplophora glabripennis. Arch. Insect Biochem. Physiol. 2020, 104, e21679. [Google Scholar] [CrossRef]
- Liu, F.; Wickham, J.D.; Cao, Q.; Lu, M.; Sun, J. An invasive beetle-fungus complex is maintained by fungal nutritional-compensation mediated by bacterial volatiles. ISME J. 2020, 14, 2829–2842. [Google Scholar] [CrossRef]
- Lehenberger, M.; Foh, N.; Göttlein, A.; Six, D.; Biedermann, P.H.W. Nutrient-poor breeding substrates of ambrosia beetles are enriched with biologically important elements. Front. Microbiol. 2021, 12, 664542. [Google Scholar] [CrossRef]
- Geib, S.M.; Jimenez-Gasco, M.d.M.; Carlson, J.E.; Tien, M.; Hoover, K. Effect of host tree species on cellulase activity and bacterial community composition in the gut of larval Asian longhorned beetle. Environ. Entomol. 2009, 38, 686–699. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Hoover, K.; Tien, M.; Tringe, S.G.; Barry, K.W.; Glavina del Rio, T.; Chovatia, M.; Herr, J.R.; Carlson, J.E. Metagenomic profiling reveals lignocellulose degrading system in a microbial community associated with a wood-feeding beetle. PLoS ONE 2013, 8, e73827. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef]
- Ayayee, P.; Rosa, C.; Ferry, J.G.; Felton, G.; Saunders, M.; Hoover, K. Gut microbes contribute to nitrogen provisioning in a wood-feeding cerambycid. Environ. Entomol. 2014, 43, 903–912. [Google Scholar] [CrossRef]
- Ayayee, P.A.; Larsen, T.; Rosa, C.; Felton, G.W.; Ferry, J.G.; Hoover, K. Essential amino acid supplementation by gut microbes of a wood-feeding cerambycid. Environ. Entomol. 2016, 45, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiao, S.; Li, X.; Li, M. Bacterial and fungal gut communities of Agrilus mali at different developmental stages and fed different diets. Sci. Rep. 2018, 8, 15634. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Gao, J.; Hao, C.; Dai, L.; Chen, H. Biodiversity and activity of gut fungal communities across the life history of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Int. J. Mol. Sci. 2018, 19, 2010. [Google Scholar] [CrossRef]
- Guo, Q.; Yao, Z.; Cai, Z.; Bai, S.; Zhang, H. Gut fungal community and its probiotic effect on Bactrocera dorsalis. Insect Sci. 2022, 29, 1145–1158. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Delalibera, I., Jr.; Handelsman, J.; Klepzig, K.D.; Schloss, P.D.; Raffa, K.F. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle Dendroctonus frontalis Zimmermann. Environ. Entomol. 2006, 35, 1710–1717. [Google Scholar] [CrossRef]
- Delalibera, I.; Vasanthakumar, A.; Burwitz, B.J.; Schloss, P.D.; Klepzig, K.D.; Handelsman, J.; Raffa, K.F. Composition of the bacterial community in the gut of the pine engraver, Ips pini (Say) (Coleoptera) colonizing red pine. Symbiosis 2007, 43, 97–104. [Google Scholar]
- Aylward, F.O.; Suen, G.; Biedermann, P.H.; Adams, A.S.; Scott, J.J.; Malfatti, S.A.; Glavina del Rio, T.; Tringe, S.G.; Poulsen, M.; Raffa, K.F.; et al. Convergent bacterial microbiotas in the fungal agricultural systems of insects. Mbio 2014, 5, 10-1128. [Google Scholar] [CrossRef]
- Hulcr, J.; Adams, A.S.; Raffa, K.; Hofstetter, R.W.; Klepzig, K.D.; Currie, C.R. Presence and diversity of streptomyces in Dendroctonus and sympatric bark beetle galleries across North America. Microb. Ecol. 2011, 61, 759–768. [Google Scholar] [CrossRef]
- Scully, E.D.; Hoover, K.; Carlson, J.E.; Tien, M.; Geib, S.M. Midgut transcriptome profiling of Anoplophora glabripennis, a lignocellulose degrading cerambycid beetle. BMC Genom. 2013, 14, 850. [Google Scholar] [CrossRef]
- Mason, C.J.; Long, D.C.; Lindroth, R.L.; Hoover, K. Divergent host plant utilization by adults and offspring is related to intra-plant variation in chemical defences. J. Anim. Ecol. 2019, 88, 1789–1798. [Google Scholar] [CrossRef]
- Mueller, R.C.; Paula, F.S.; Mirza, B.S.; Rodrigues, J.L.; Nüsslein, K.; Bohannan, B.J. Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 2014, 8, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Mogouong, J.; Constant, P.; Legendre, P.; Guertin, C. The phyllosphere microbiome of host trees contributes more than leaf phytochemicals to variation in the Agrilus planipennis Fairmaire gut microbiome structure. Sci. Rep. 2021, 11, 15911. [Google Scholar] [CrossRef]
- Koski, T.M.; Zhang, B.; Mogouong, J.; Wang, H.L.; Chen, Z.Z.; Li, H.P.; Bushley, K.E.; Sun, J.H. Distinct metabolites affect the phloem fungal communities in ash trees (Fraxinus spp.) native and nonnative to the highly invasive emerald ash borer (AGRILUS PLANIPENNIS). Plant Cell Environ. 2024, 47, 4116–4134. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.R.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Amith, M.T.; Fujimoto, K.; Tao, C. NET-EXPO: A gephi plugin towards social network analysis of network exposure for unipartite and bipartite graphs. In HCI International 2019-Posters: 21st International Conference, HCII 2019, Orlando, FL, USA, July 26–31, 2019, Proceedings, Part III 21; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 1034, pp. 3–12. [Google Scholar]
- Knights, D.; Kuczynski, J.; Charlson, E.S.; Zaneveld, J.; Mozer, M.C.; Collman, R.G.; Bushman, F.D.; Knight, R.; Kelley, S.T. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 2011, 8, 761–763. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Bin, X.; Wang, P.; Shen, Y.; Xiang, X.; Jafir, M.; Wan, X. Investigation of fungal community structure in the gut of the stag beetle Dorcus hopei (Coleoptera; Lucanidae): Comparisons among developmental stages. Microb. Ecol. 2024, 87, 70. [Google Scholar] [CrossRef] [PubMed]
- Veselská, T.; Švec, K.; Kostovčík, M.; Peral-Aranega, E.; Garcia-Fraile, P.; Křížková, B.; Havlíček, V.; Saati-Santamaría, Z.; Kolařík, M. Proportions of taxa belonging to the gut core microbiome change throughout the life cycle and season of the bark beetle Ips typographus. FEMS Microbiol. Ecol. 2023, 99, fiad072. [Google Scholar] [CrossRef]
- Geib, S.M.; Jimenez-Gasco, M.d.M.; Carlson, J.E.; Tien, M.; Jabbour, R.; Hoover, K. Microbial community profiling to investigate transmission of bacteria between life stages of the wood-boring beetle, Anoplophora glabripennis. Microb. Ecol. 2009, 58, 199–211. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, bacterial, and archaeal diversity in the digestive tract of several beetle larvae (Coleoptera). Biomed Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef]
- Grünwald, S.; Pilhofer, M.; Höll, W. Microbial associations in gut systems of wood- and bark-inhabiting longhorned beetles (Coleoptera: Cerambycidae). Syst. Appl. Microbiol. 2010, 33, 25–34. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Steiger, S. Unearthing carrion beetles’ microbiome: Characterization of bacterial and fungal hindgut communities across the Silphidae. Mol. Ecol. 2014, 23, 1251–1267. [Google Scholar] [CrossRef]
- Scully, E.D.; Hoover, K.; Carlson, J.; Tien, M.; Geib, S.M. Proteomic analysis of Fusarium solani isolated from the Asian longhorned beetle, Anoplophora glabripennis. PLoS ONE 2012, 7, e32990. [Google Scholar] [CrossRef]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; O’Donnell, K.; Homma, Y.; Lattanzi, A.R. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex—F. virguliforme in North America and F. tucumaniae in South America. Mycologia 2003, 95, 660–684. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; O’Donnell, K.; Scandiani, M. Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme. Mycoscience 2005, 46, 162–183. [Google Scholar] [CrossRef]
- Kolander, T.M.; Bienapfl, J.C.; Kurle, J.E.; Malvick, D.K. Symptomatic and asymptomatic host range of Fusarium virguliforme, the causal agent of soybean sudden death syndrome. Plant Dis. 2012, 96, 1148–1153. [Google Scholar] [CrossRef]
- Romberg, M.K.; Davis, R.M. Host range and phylogeny of Fusarium solani f. sp. eumartii from potato and tomato in California. Plant Dis. 2007, 91, 585–592. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Masuya, H.; Kajimura, H. Novel symbiotic association between Euwallacea ambrosia beetle and Fusarium fungus on fig trees in Japan. Front. Microbiol. 2021, 12, 725210. [Google Scholar] [CrossRef]
- Osborn, R.K.; Ordóñez, M.E.; Cognato, A.I. Ecuadorian Coptoborus beetles harbor Fusarium and Graphium fungi previously associated with Euwallacea ambrosia beetles. Mycologia 2002, 114, 487–500. [Google Scholar] [CrossRef]
- Aoki, T.; Kasson, M.T.; Berger, M.C.; Freeman, S.; Geiser, D.M.; O’Donnell, K. Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern U.S. and typification of F. ambrosium. Fungal Syst. Evol. 2018, 1, 23–39. [Google Scholar] [CrossRef]
- Brune, A.; Dietrich, C. The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Wang, X.; Wang, G.; Shang, S.; Dou, Z.P.; Luo, Y. Gut lignocellulose activity and microbiota in Asian longhorned beetle and their predicted contribution to larval nutrition. Front. Microbiol. 2022, 13, 899865. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.Q.; Duong, T.A.; Wingfield, B.D.; Barnes, I.; Durán, A.; Wingfield, M.J. Characterisation of the mating-type loci in species of Elsinoe causing scab diseases. Fungal Biol. 2023, 127, 1484–1490. [Google Scholar] [CrossRef]
- Fan, X.L.; Barreto, R.W.; Groenewald, J.Z.; Bezerra, J.D.; Pereira, O.L.; Cheewangkoon, R.; Mostert, L.; Tian, C.M.; Crous, P.W. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Gañán-Betancur, L.; Gazis, R. Genome sequence resource of the avocado scab pathogen Elsinoe perseae. Microbiol. Resour. Announc. 2023, 12, e0019023. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, K.; Coetzee, M.P.; Hoffmeister, D. Secrets of the subterranean pathosystem of Armillaria. Mol. Plant Pathol. 2011, 12, 515–534. [Google Scholar] [CrossRef]
- Paudyal, D.P.; Hyun, J.W. Physical changes in satsuma mandarin leaf after infection of Elsinoë fawcettii causing citrus scab disease. Plant Pathol. J. 2015, 31, 421–427. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, H.; Ma, X.; Cheng, Q. Elsinoë australis causing spot anthracnose on poplar in China. Plant Dis. 2020, 104, 2202–2209. [Google Scholar] [CrossRef]
- Van Heerden, A.; Pham, N.Q.; Wingfield, B.D.; Wingfield, M.J.; Muro Abad, J.I.; Durán, A.; Wilken, P.M. LAMP assay to detect Elsinoë necatrix, an important Eucalyptus shoot and leaf pathogen. Plant Dis. 2024, 108, 2731–2739. [Google Scholar] [CrossRef]
- Schott, J.; Rakei, J.; Remus-Emsermann, M.; Johnston, P.; Mbedi, S.; Sparmann, S.; Hilker, M.; Paniagua Voirol, L.R. Microbial associates of the elm leaf beetle: Uncovering the absence of resident bacteria and the influence of fungi on insect performance. Appl. Environ. Microbiol. 2024, 90, e0105723. [Google Scholar] [CrossRef]
- Ramírez-Camejo, L.A.; Zuluaga-Montero, A.; Lázaro-Escudero, M.; Hernández-Kendall, V.; Bayman, P. Phylogeography of the cosmopolitan fungus Aspergillus flavus: Is everything everywhere? Fungal Biol. 2012, 116, 452–463. [Google Scholar] [CrossRef]
- Wu, S.; Wu, J.; Wang, Y.; Qu, Y.; He, Y.; Wang, J.; Cheng, J.; Zhang, L.; Cheng, C. Discovery of entomopathogenic fungi across geographical regions in southern China on pine sawyer beetle Monochamus alternatus and implication for multi-pathogen vectoring potential of this beetle. Front. Plant Sci. 2022, 13, 1061520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, Y.; Wang, F. Hydrostatic pressure is the universal key driver of microbial evolution in the deep ocean and beyond. Environ. Microbiol. Rep. 2021, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Delalibera, I.; Handelsman, J.; Raffa, K.F. Contrasts in cellulolytic activities of gut microorganisms between the wood borer, Saperda vestita (Coleoptera: Cerambycidae), and the bark beetles, Ips pini and Dendroctonus frontalis (Coleoptera: Curculionidae). Environ. Entomol. 2005, 34, 541–547. [Google Scholar] [CrossRef]
- Paludo, C.R.; Menezes, C.; Silva-Junior, E.A.; Vollet-Neto, A.; Andrade-Dominguez, A.; Pishchany, G.; Khadempour, L.; do Nascimento, F.S.; Currie, C.R.; Kolter, R.; et al. Stingless bee larvae require fungal steroid to pupate. Sci. Rep. 2018, 8, 1122. [Google Scholar] [CrossRef]
- Schott, J.; Fuchs, B.; Böttcher, C.; Hilker, M. Responses to larval herbivory in the phenylpropanoid pathway of Ulmus minor are boosted by prior insect egg deposition. Planta 2021, 255, 16. [Google Scholar] [CrossRef]
- Schott, J.; Jantzen, F.; Hilker, M. Elm tree defences against a specialist herbivore are moderately primed by an infestation in the previous season. Tree Physiol. 2023, 43, 1218–1232. [Google Scholar] [CrossRef]
- Austel, N.; Eilers, E.J.; Meiners, T.; Hilker, M. Elm leaves ‘warned’ by insect egg deposition reduce survival of hatching larvae by a shift in their quantitative leaf metabolite pattern. Plant Cell Environ. 2016, 39, 366–376. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Li, G.H.; Li, C.Q.; Wang, S.L.; Pan, X.R.; Zhang, K.Q.; Zhao, P.J. Identification of nematicidal metabolites from Purpureocillium lavendulum. Microorganisms 2022, 10, 1343. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; de Lima Neto, R.G.; da Costa, G.L.; de Moraes Borba, C.; Oliveira, M.M.E. Purpureocillium lilacinum an emergent pathogen: Antifungal susceptibility of environmental and clinical strains. Lett. Appl. Microbiol. 2022, 75, 45–50. [Google Scholar] [CrossRef]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef]
- Baron, N.C.; de Souza Pollo, A.Z.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Tamura, T.; Shimizu, K. Draft genome sequence of Purpureocillium takamizusanense, a potential bioinsecticide. Microbiol. Resour. Announc. 2022, 11, e0026822. [Google Scholar] [CrossRef] [PubMed]
- Mashilingi, S.K.; Zhang, H.; Chen, W.; Vaissière, B.E.; Garibaldi, L.A.; An, J. Temporal trends in pollination deficits and its potential impacts on Chinese agriculture. J. Econ. Entomol. 2021, 114, 1431–1440. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar]
- Panyasiri, C.; Supothina, S.; Veeranondha, S.; Chanthaket, R.; Boonruangprapa, T.; Vichai, V. Control efficacy of entomopathogenic fungus Purpureocillium lilacinum against Chili Thrips (Scirtothrips dorsalis) on Chili Plant. Insects 2022, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Hoffmann, A.A.; Xing, K.; Ma, C.S. Life stages of an aphid living under similar thermal conditions differ in thermal performance. J. Insect Physiol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Berasategui, A.; Breitenbach, N.; García-Lozano, M.; Pons, I.; Sailer, B.; Lanz, C.; Rodríguez, V.; Hipp, K.; Ziemert, N.; Windsor, D.; et al. The leaf beetle Chelymorpha alternans propagates a plant pathogen in exchange for pupal protection. Curr. Biol. 2022, 32, 4114–4127. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Teetor-Barsch, G.H.; Roberts, D.W. Entomogenous Fusarium species. Mycopathologia 1983, 84, 3–16. [Google Scholar] [CrossRef]
- Grove, J.F.; Pople, M. The insecticidal activity of beauvericin and the enniatin complex. Mycopathologia 1980, 70, 103–105. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, K.D.; Bok, J.W.; Ye, R.; Miley, G.P.; Verdan, M.H.; Velk, T.; Chen, C.; Yang, K.; Robey, M.T.; Gao, P.; et al. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat. Chem. Biol. 2017, 13, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Fusarium euwallaceae sp. nov.—A symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 2013, 105, 1595–1606. [Google Scholar] [CrossRef]
- Geib, S.M.; Scully, E.D.; Jimenez-Gasco, M.M.; Carlson, J.E.; Tien, M.; Hoover, K. Phylogenetic analysis of Fusarium solani associated with the Asian longhorned beetle, Anoplophora glabripennis. Insects 2012, 3, 141–160. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Jia, Y.; Li, Y.; Geng, S.; Yu, Y.; Wang, Z.; Wang, X.; Fu, N.; Zeng, J.; Su, X.; et al. Potential Functions and Transmission Dynamics of Fungi Associated with Anoplophora glabripennis Across Different Life Stages, Between Sexes, and Between Habitats. Insects 2025, 16, 273. https://doi.org/10.3390/insects16030273
Liu Q, Jia Y, Li Y, Geng S, Yu Y, Wang Z, Wang X, Fu N, Zeng J, Su X, et al. Potential Functions and Transmission Dynamics of Fungi Associated with Anoplophora glabripennis Across Different Life Stages, Between Sexes, and Between Habitats. Insects. 2025; 16(3):273. https://doi.org/10.3390/insects16030273
Chicago/Turabian StyleLiu, Qing, Yuanting Jia, Yishuo Li, Shilong Geng, Yanqi Yu, Zhangyan Wang, Xinru Wang, Ningning Fu, Jianyong Zeng, Xiaoyu Su, and et al. 2025. "Potential Functions and Transmission Dynamics of Fungi Associated with Anoplophora glabripennis Across Different Life Stages, Between Sexes, and Between Habitats" Insects 16, no. 3: 273. https://doi.org/10.3390/insects16030273
APA StyleLiu, Q., Jia, Y., Li, Y., Geng, S., Yu, Y., Wang, Z., Wang, X., Fu, N., Zeng, J., Su, X., Li, H., & Wang, H. (2025). Potential Functions and Transmission Dynamics of Fungi Associated with Anoplophora glabripennis Across Different Life Stages, Between Sexes, and Between Habitats. Insects, 16(3), 273. https://doi.org/10.3390/insects16030273





